Significance
People often consider the well-being of others. However, they are more likely to be generous toward individuals they feel close to than to those they only meet sporadically. Using neuroimaging tools, we show that the decline in generosity across social distance is realized by the interplay of two brain structures—the ventromedial prefrontal cortex coding the relative appeal of a selfish or a generous option, and the temporoparietal junction modulating appeal signals of the generous outcome, depending on social distance between participant and beneficiary. Based on these findings, we developed a biologically plausible model explaining social discounting in particular, and prosocial behavior in general. Our study opens up new avenues to understand and tackle frictions arising in social networks.
Keywords: social discounting, prosocial choice, fMRI, connectivity, neuroeconomics
Abstract
Most people are generous, but not toward everyone alike: generosity usually declines with social distance between individuals, a phenomenon called social discounting. Despite the pervasiveness of social discounting, social distance between actors has been surprisingly neglected in economic theory and neuroscientific research. We used functional magnetic resonance imaging (fMRI) to study the neural basis of this process to understand the neural underpinnings of social decision making. Participants chose between selfish and generous alternatives, yielding either a large reward for the participant alone, or smaller rewards for the participant and another individual at a particular social distance. We found that generous choices engaged the temporoparietal junction (TPJ). In particular, the TPJ activity was scaled to the social-distance–dependent conflict between selfish and generous motives during prosocial choice, consistent with ideas that the TPJ promotes generosity by facilitating overcoming egoism bias. Based on functional coupling data, we propose and provide evidence for a biologically plausible neural model according to which the TPJ supports social discounting by modulating basic neural value signals in the ventromedial prefrontal cortex to incorporate social-distance–dependent other-regarding preferences into an otherwise exclusively own-reward value representation.
Prosociality is one of the most fundamental qualities of all human societies. Without the ability to take other people’s interests into account, human relationships would disintegrate and societies would malfunction. It has been widely demonstrated in laboratory and field experiments that individuals consider the welfare of others in their decisions and the consequences a decision has on them (1–3). Although almost all of us behave prosocially at times, it is clear that people are not equally generous to everyone alike. Rather, generosity decreases as a function of the closeness of the relationship between two individuals (2, 4). However, it is currently unknown how social distance contributes to the decision process on a neural level. In the present study, we set out to address this question.
Our first aim was to investigate the systematic influence of social-distance–dependent levels of generosity on neural activation. This was investigated using a social discounting experiment adapted to the functional magnetic resonance imaging (fMRI) environment (1). We measured blood oxygen level-dependent (BOLD) responses while subjects made choices between selfish and generous rewards for themselves and for other people that varied in social distance. Choosing selfishly yielded a payoff only for the subject, whereas making a generous choice resulted in a lower payoff for the subject coupled with a reward for another person at a specific social distance (Fig. 1). Next, based on the individual choices, we reconstructed the social-distance–dependent other-regarding utility (ORU), that is, the value participants attached to increasing the wealth of another person at a given social distance.
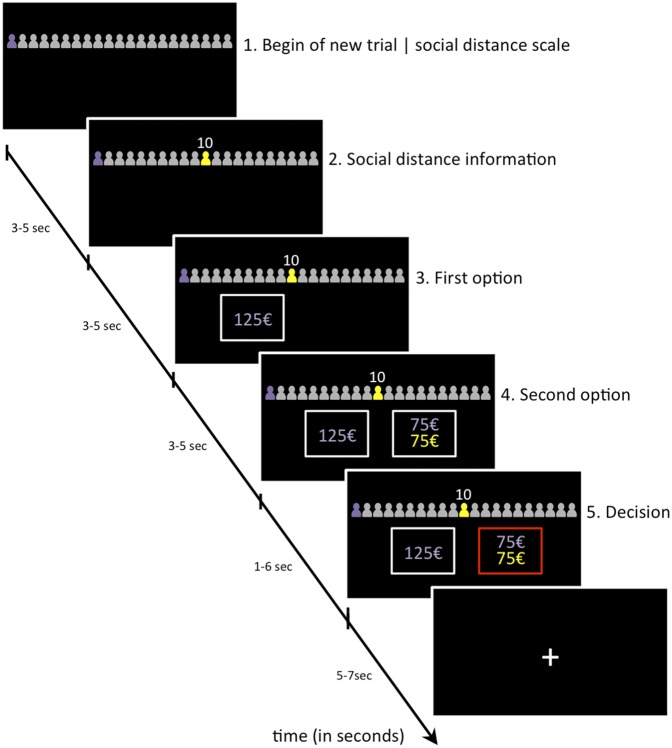
Fig. 1.
Participants received task-relevant information sequentially. First, social distance information was given on a scale consisting of 101 icons (100 icons representing 100 social distance levels plus one icon, shown in purple on the left end, representing the participant himself). The social distance information for a specific trial was indicated by a yellow icon and, additionally, presented numerically as a number on top of the yellow icon (here: social distance 10). Participants chose between a selfish (here: €125 only for themselves) and a generous option (here: €75 for the participant and €75 for a recipient on the specific social distance). The generous and selfish options were then presented sequentially and in random order. All ISIs had a mean duration of 4 s (jittered by ±1 s). Participants indicated their preference during the decision period within a maximum time frame of 6 s. The trials were separated using a fixation cross with a mean ITI of 6 s (jittered by ±1 s). Note that this figure has been adjusted for illustration purposes; stimulus size and screen format are not to scale with the presentation dimensions used during fMRI scanning. In addition, the figure displays only 21 icons, instead of 101 icons shown during scanning, to facilitate perceptibility.
We then asked which brain regions showed activity that correlated with the difference between other- and self-regarding utilities. Our paradigm was designed so that the degree of generosity varied systematically as a function of social distance while objective economic outcome parameters—own- and other-person payoffs—were kept constant. This allowed us to identify the neural correlates of social-distance–dependent other-regarding preferences independent of objective payoffs.
We hypothesized, based on existing literature (5–10), that own-reward values are represented in the brain’s valuation system, specifically in the ventromedial prefrontal cortex (VMPFC). Furthermore, changes in other-regarding value would recruit areas associated with theory of mind (ToM) and altruistic choice, such as the temporoparietal junction (TPJ) (11, 12). Should this be the case, this would show that social distance is indeed systematically integrated into the neural underpinnings of the decision process.
Our second aim was to investigate the role of the TPJ in prosocial behavior in more detail. To this end, we tested the predictions of two competing ideas on the role of the TPJ during prosocial choice in general, and social discounting in particular. Previous research showed that this region is involved in tasks requiring the ability to represent and understand others’ perspectives (12, 13) and in social and selfish decisions (14–17). Thus, the TPJ’s implication in prosocial choice, perspective taking, empathizing, and ToM suggests that it plays a role in putting oneself in someone else’s shoes. In other words, the TPJ may encode the other-regarding value participants attach to increasing the well-being of another person. Individuals empathize more with people they feel close to than with more distant others. Therefore, if this hypothesis is true, TPJ activation should correlate positively with the social-distance–dependent ORU. This view of the role of the TPJ is challenged by more recent studies postulating that TPJ activation solves the conflict between generous and selfish motives (16). According to this hypothesis, to make a generous decision, the putatively natural bias to maximize own-payoff needs to be overcome (18). If the TPJ enables overcoming egoism bias, activation should be high when the temptation to be selfish is high (i.e., large social distance and/or large selfish reward) and low when there is little conflict between selfish and generous motives (i.e., small social distance and/or relatively small selfish reward).
Our results confirmed the latter hypothesis according to which the TPJ plays a role in overcoming the default response of maximizing one’s own profit and thus behaving selfishly, rather than in representing other-regarding value. We also asked how the brain implements generous decisions. Specifically, we propose that the TPJ facilitates generous decisions by modulating basic reward signals in the VMPFC, incorporating other-regarding preference signals into an otherwise exclusive own-reward value representation, thus computing the subjective value for a social reward. Thus, the TPJ supports prosocial choice by shaping neural value signals in the VMPFC whenever the temptation to be selfish needs to be overcome; the stronger the temptation to be selfish, the more the TPJ up-regulates VMPFC activity in favor of generous choices.
A mechanistic model that integrates these data makes two predictions for which we provide empirical support: first, VMPFC activity should be higher during generous than selfish decisions. Second, connectivity between the TPJ and the VMPFC should be stronger during generous than during selfish decisions. Together, our findings suggest that prosocial decisions arise from a refined interplay between the VMPFC and the TPJ. In particular, value signals in the VMPFC are orchestrated by the TPJ according to the social distance between the decision maker and the recipient of generous decisions.
Results
Behavioral Results.
We inferred social-discounting parameters based on the participants’ individual choices and used the obtained social discount functions to econometrically reconstruct the social-distance–dependent ORU for each individual (1). To this end, we first determined, for each social distance level, the point at which a participant was indifferent between the selfish (yielding a larger reward for the participant) and the generous alternative (yielding a smaller reward for the participant plus a reward for the other person) using logistic regression. The difference in reward magnitudes for the participant between the two alternatives at the indifference points represented the amount of money a subject was willing to forego to increase the wealth of another person at a given social distance, and could be construed as a social premium equivalent to the utility of increasing the other person’s well-being. For example, if a participant was indifferent between €125 own-reward and €75 own-reward and €75 for a person at social distance 1, this participant was willing to forego €50 (the social premium) to increase the wealth of the other person by €75. Subsequently, we fit a standard hyperbolic model (2) (Materials and Methods) to the individual social-distance–dependent social premiums with the parameters k [Median (Mdn) = 0.078] and V (Mdn = 74.15). As expected, the magnitude of the social premium subjects were willing to pay for someone else’s benefit declined with increasing social distance. The standard hyperbolic model captured the individual discounting behavior well (mean r2 = 0.72, SD = 0.242; Fig. 2 shows the median social premiums together with the best-fitting hyperbolic function).
Fig. 2.
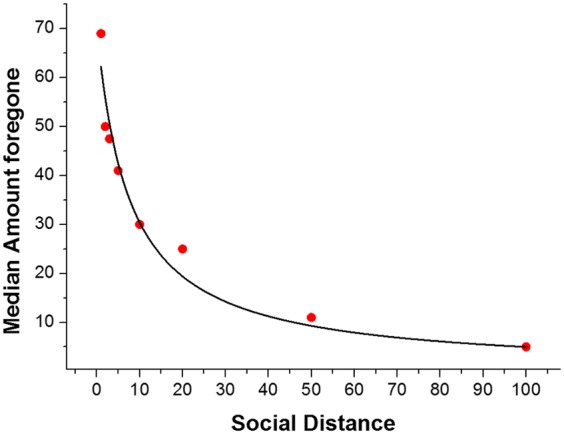
We determined, for social distance levels 1, 2, 3, 5, 10, 20, 50, and 100, the individual payoff magnitudes at which a participant was indifferent between the selfish (yielding a larger reward for the participant) and the generous alternative (yielding a smaller reward for the participant plus a reward for the other person). The amount foregone, i.e., the difference in own-reward magnitude between the selfish and generous option at indifference point, indicates the willingness to sacrifice a reward to give to another person at a specific social distance. The amount foregone can be interpreted as a social premium that reflects the utility a participant attaches to increasing a recipient’s payoff. A standard hyperbolic model was fit to the individual social-distance–dependent amounts foregone to reconstruct the participant’s ORU function. The figure shows the best-fitting hyperbolic function to the median amounts foregone across all participants.
These findings replicated those of previous studies on social discounting (1, 2), suggesting that the scanner environment did not substantially affect discounting behavior compared with studies carried out in more natural surroundings. The obtained individual hyperbolic fits served as estimates of the decline in other-regarding value across social distance and were used to estimate the individual ORUs, which corresponded to the hyperbolic fit plus the sure €75 (constant, within-subject) for the participant herself.
Neural Mechanisms of Social Discounting.
Social discounting is the consequence of the social-distance–dependent balance between generous and selfish motives.
The fundamental premise in our study is that, in essence, a prosocial decision results from the balance between generous and selfish motives. Due to social discounting, the balance between generous and selfish motives is increasingly tilted toward selfishness as social distance increases. With this premise in mind, we tested the hypothesis that the TPJ is involved in orchestrating the balance between generous and selfish motives across social distance. We also hypothesized that the TPJ would perform this function together with classical value coding regions such as the VMPFC (8, 19).
Neural value signals in the valuation network.
First, we investigated the neural correlates of selfish rewards. To identify brain regions associated with own-reward value coding, we examined neural activity during the decision period of selfish decisions only. For this analysis, we concentrated on the VMPFC and asked whether BOLD activity in the VMPFC covaried with selfish reward magnitude, thus with the value of the selfish decision. We used a region of interest (ROI) based on a metaanalysis [−2, 40, −4] (20), which suggests this part of the VMPFC plays a role in value processing. Using a 6-mm sphere around the ROI, we found significant correlations within the VMPFC [−6, 41, −5; t(22) = 3.10, P = 0.017, small-volume (SV) familywise error (FWE) corrected; Fig. S1 and Table S1].
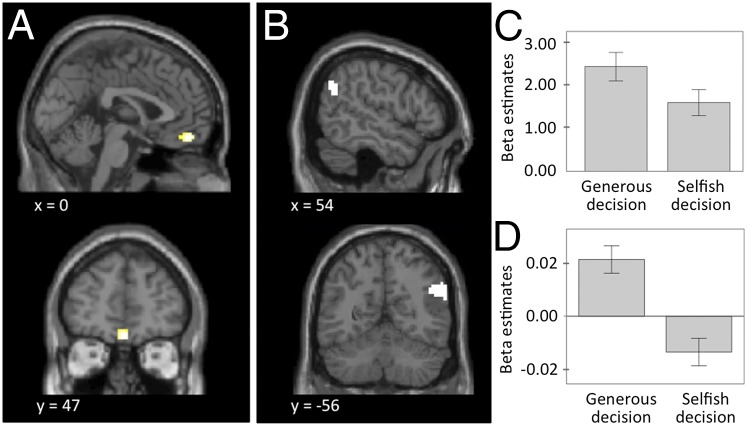
Next, we also included generous decisions in our analysis. Interestingly, we found that activity in the VMPFC was significantly higher during generous than during selfish choices [0, 47, −20; t(22) = 4.21, P = 0.028, whole-brain FWE corrected; Fig. 3A and Table S2]. Thus, in line with other findings (21), the VMPFC coded not only the own-reward value of a selfish choice but also generosity in addition to own-reward value, possibly reflecting the satisfaction derived from increasing someone else’s wealth (21). Generous decisions also elicited stronger responses than selfish decisions in the right [60, −58, 31; t(22) = 5.15, P < 0.001, whole-brain FWE corrected; Table S2] and left TPJ [−24, −79, 52, t(22) = 4.51, P = 0.002, whole-brain FWE corrected]. This section of TPJ has previously been shown to be associated with ToM and altruistic choice (11). We used these brain areas as ROIs in all subsequent analyses to further characterize their contribution to social discounting.
Fig. 3.
Brain activations during social discounting. (A) BOLD responses in the VMPFC were stronger during generous than during selfish decisions [0, 47, −20; t(22) = 5.47, P = 0.028, whole-brain FWE corrected; displayed at P < 0.005, uncorrected, k ≥ 10 voxel]. (B) Generous decisions elicited activation in the posterior part of the rTPJ. (C) Beta estimates within the rTPJ . The rTPJ was more activated during generous than selfish decisions. (D) Activity in the rTPJ was more strongly modulated by the temptation to be selfish during generous than selfish decisions. Error bars indicate ±1 SE.
Generous decisions recruit the TPJ to resist the temptation to be selfish.
Our design allowed us to shed light on two competing hypotheses about the role of the TPJ in social decision making. If the TPJ was important for encoding the social-distance–dependent value participants derive from increasing someone else’s well-being, we would expect a positive correlation between TPJ activity and the ORU. To test the first hypothesis, we searched for brain regions whose activity correlated with the social-distance–dependent other-regarding value, using the individual ORUs as parametric regressors at decision onset. Inconsistent with our predictions, the parametric analysis revealed no activation in the TPJ, even at very liberal thresholds (P < 0.1, uncorrected, Table S3; see SI Materials and Methods and Figs. S2 and S3 for more analyses).
Next, we tested the second hypothesis, that the TPJ is associated with overcoming egoism bias. We reasoned that the temptation to make a selfish choice should be stronger, the larger the utility of the own-reward relative to the social-distance–dependent ORU. By extension, the stronger the temptation to be selfish, the more effort should have been exerted to overcome this temptation when a generous decision had been taken. Thus, we hypothesized that activity in brain regions important for overcoming egoism bias would scale to the difference between own-reward and other-regarding values when a generous choice is revealed. We therefore searched for BOLD signals that correlated with the difference between own-reward and other-regarding values during generous decisions. This analysis revealed, among others, activation in the right parietal cortex, expanding into the parietal part of the TPJ (rTPJ) [42, −79, 46; t(22) = 5.55, P = 0.019, whole-brain FWE corrected; Table S4]. A conjunction analysis, revealing the strict intersection between this contrast and the contrast yielding generosity-related activations confirmed that this was indeed the same region as the one engaged during generous decision making. Additionally, a ROI analysis using a mask for the parietal subsection of rTPJ (22), an area known to be involved in social cognition, confirmed that it was activity in this “social” part of the TPJ that correlated with the parametric modulation of the temptation to be selfish during generous decisions (P = 0.032, SV FWE corrected; Fig. 3B).
Importantly, according to the second hypothesis, the rTPJ should be active when the conflict between selfishness and generosity is resolved in favor of the latter (i.e., in generous choices), whereas activity should be less when selfish choices are made. In support of this idea, rTPJ activation survived when contrasting the difference in own-reward and ORU during generous against selfish decisions, suggesting that the rTPJ was more active during generous (i.e., when the temptation to be selfish had been overcome) than during selfish choices [42, −79, 46; t(22) = 7.10, P < 0.001, whole-brain FWE corrected; Table S5]. Thus, rTPJ activation correlated with the difference between own-reward values and ORU when generous choices were made, but not with ORU in general, irrespective of the actual decisions taken. Although the lack of evidence in favor of the first hypothesis of TPJ function is not evidence against ToM, it is worth noting that rTPJ activity was in fact positively correlated with the difference between own-reward and other-regarding value. In other words, rTPJ activity was negatively correlated with ORU alone, which is difficult to reconcile with the ToM-based hypothesis. Conversely, our results are in line with the hypothesis of overcoming egoism bias and are therefore more consistent with the idea that the rTPJ facilitates generous choice whenever a conflict between egoistic and selfish motives needs to be resolved.
The TPJ was functionally connected with the VMPFC when egoism bias was overcome.
So far, our data have shown that the VMPFC encoded the value of own-reward during selfish decisions, and both the VMPFC and the TPJ were more engaged during generous than during selfish choices. Furthermore, the rTPJ activation pattern was consistent with the idea that the rTPJ facilitates prosocial choice by overcoming the temptation to maximize own-payoff. We next asked how a social-distance–dependent prosocial choice is implemented in the brain. We propose a model of generous choice according to which the VMPFC encodes goal values. We hypothesize that the TPJ suppresses egoism bias by modulating basic value signals in the VMPFC to incorporate other-regarding preferences into an otherwise exclusively own-reward value representation.
If our hypothesis was true, functional connectivity between the rTPJ and the VMPFC should be higher during generous than during selfish decisions. Our idea was inspired by recent models of self-control (8, 22) according to which basic value representations in the VMPFC are modulated by superordinate brain regions encoding higher-order considerations, such as long-term goals or other-regarding preferences. Thereby, these higher-order factors are incorporated into basic valuation signals of the VMPFC. Adapting this approach to social decision making, we propose that the rTPJ modulates activation in the valuation network and orchestrates social decision making in favor of other-regarding instead of individual preferences.
To test the hypothesis, we conducted a psychophysiological interaction (PPI) analysis to identify which brain regions were functionally more strongly connected with the rTPJ during generous than during selfish decisions. To this end, we placed a seed (Fig. 4A) in the individual peak activations in the right TPJ cluster associated with overcoming egoism bias. The PPI analysis identified functional connectivity between the rTPJ and the VMPFC [t(14) = 6.61, P = 0.031, whole-brain FWE corrected; Fig. 4B and Table S6]. A conjunction analysis confirmed that the activated VMPFC cluster was indeed the same region as the VMPFC ROI that coded own-reward value during selfish decisions. Thus, compared with selfish decisions, the TPJ increased functional connectivity with regions associated with own-reward processing during generous decisions.
Fig. 4.
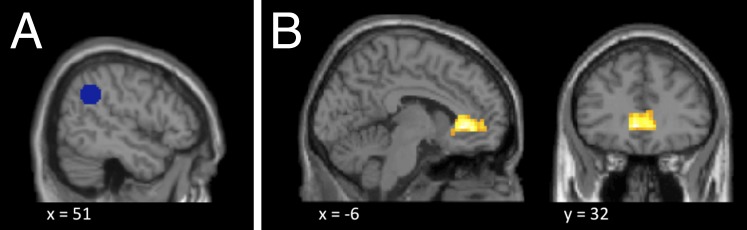
(A) ROI in the rTPJ (51, −49, 34; 10-mm sphere) as the seed region for the PPI. (B) Positive functional connectivity of the rTPJ with VMPFC during generous decisions. The PPI analysis revealed that connectivity between the VMPFC and the rTPJ was stronger during prosocial than selfish choices [t(14) = 6.61, P = 0.031, whole-brain FWE corrected; displayed at P < 0.005, uncorrected, k ≥ 10 voxel].
Discussion
To function well in our society, it is important to share resources with others. The closer our interaction partners are to us, the more likely we are to be generous toward them. Thus, the social context in which a decision is made strongly affects how the information is processed, making it essential for our brain to be able to encode such social context factors (23). However, neuroeconomic theories have so far neglected social distance in models of decision making. The current experiment investigated the neural correlates of social discounting and aimed to provide support for a neural model of social distance-dependent generous decision making. Generosity requires overcoming egoistic motives (1, 2, 16, 24), and the temptation to be selfish grows with increasing own-reward magnitude, but also with increasing social distance. We were able to demonstrate that a region associated with ToM, social cognition, and decision making, the TPJ, is involved in this process (24). However, contrary to the predictions of the first, ToM-based, hypothesis, we find no evidence that the TPJ computes other-regarding value. Instead, we propose that the TPJ facilitates overcoming egoistic motives to maximize own-payoff during generous decisions by modulating basic value signals in the VMPFC through integrating other-regarding preferences into an otherwise exclusively own-reward value representation (24, 25). Thus, the stronger the social-distance–dependent temptation to be selfish, the more the TPJ is engaged and VMPFC-value signals become up-regulated to facilitate a generous decision.
On a behavioral level, we replicated existing findings on social discounting, confirming that generosity declines hyperbolically across social distance, with individuals being more willing to forego a reward for recipients at close social distances. Hyperbolic social discounting has been observed in diverse locations, populations, cultures, as well as under different experimental conditions and with various methods of implementing social distance and eliciting social preferences (1, 2, 26–28). This suggests that a hyperbolic discount function is an accurate, valid, and useful description of social-discounting behavior, even though it is likely that there is a large range of individual motives underlying the actual decisions during social discounting.
To reveal the neural mechanisms underlying social discounting, we first identified regions that code value signals. When participants made selfish choices, activity in the VMPFC reflected own-reward value, replicating findings on valuation processes from a multitude of studies (5, 7–9, 29). Additionally, we found that the VMPFC was more active during generous than during selfish choices, even though selfish decisions yielded higher payoffs for the participants. This is in line with studies that postulated that VMPFC activity represents the extra value obtained from charitable giving (21). Thus, it is likely that the increased activation during generous choices reflected the additional satisfaction derived from sharing a reward during generous decision making.
Our results suggest that the TPJ is important for overriding selfish impulses during prosocial decisions. Note that recent research on TPJ function suggests that it is not a monolithic structure that supports one single cognitive function, but is more likely to be composed of anatomically and functionally distinct subdivisions that may subserve different computational roles such as value, salience, and ToM (11, 24, 25, 30, 31). Although we cannot rule out that our subjects used ToM or other mechanisms to make their decisions, it is possible that we found no evidence in favor of the first, ToM-based hypothesis simply because we did not explicitly elicit ToM cognition. Thus, we are not rejecting the wealth of evidence from previous work relating the TPJ to ToM and mentalizing. Instead, we complement existing literature by lending support to the idea that subparts of the TPJ have the additional role of overcoming egoism bias and thus facilitating prosocial choice. Future research should study the TPJ in more detail and map the putative different cognitive functions to its different subdivisions.
In summary, the present findings provide insights into social decision making. We were able to characterize the role of the TPJ and proposed a neural model of prosocial choice. Our data identify the TPJ as the core component in overcoming egoism bias. This finding has a significant impact on neuroeconomic theory that has so far neglected the effect of social distance on prosocial decision making. Having shown that social distance is an important component of an individual’s decision-making process, it should be integrated into future models of decision making. Furthermore, using social discounting to understand the influence of social factors and individual differences in generosity and other-regarding behavior opens up new opportunities to evaluate psychopathologic decision-making and antisocial behavior in more detail.
Materials and Methods
Participants.
Twenty-seven subjects (meanage = 25.03; 14 men) were tested. Participants received a €10 show-up fee and an additional amount depending on their decisions in the experiment (own-reward: €7.50 to €16.50; other reward: €2.50 or €7.50), determined by a random draw of one of the trials. All subjects were native German speakers. Participants had no history of psychiatric or neurological disorders. Written consent was obtained. The study was approved by the ethics committee of the University of Bonn. Subjects were acquired using the subject database of the Life&Brain Centre, University Hospital Bonn.
Stimuli and Task.
The experimental paradigm was adapted from a cross-cultural study on social discounting (1). During the preparation phase, participants received verbal and written instructions for the tasks they carried out during the experiment. Participants started with a self-representation task in which they specified closeness to people in their social environment (1). Using a 20-point scale (1 = very close; 20 = not close), participants were asked to rate their closeness to the following people: mother, father, siblings, grandparents, family, kin, best friend, circle of friends, colleagues, neighbors, acquaintances, partner, children, and stranger. In case some of these people did not exist in a participant’s social environment, the corresponding trial was skipped. This task was aimed at getting subjects used to the idea of social distance and to think about their social network.
In the fMRI scanner, social distance was transformed into a scale consisting of 100 icons (Fig. 1). Participants were informed that the purple icon at the left end of the scale represents themselves and the yellow icon stands for a specific person in their social environment. For example, if the yellow icon is directly next to the purple one (social distance 1), this shows the person they feel closest to, e.g., mother or partner. If the yellow icon is at social distance 50 (the middle of the scale), this symbolizes an acquaintance, whereas at social distance 100, it would represent the most socially distant, but emotionally neutral person, such as a stranger. Before entering the scanner, participants were asked to choose and write down names of representatives from their social environment, one for each of the following social distances: 1, 2, 3, 5, 10, and 20. We also included social distance levels 50 and 100 in the experiment; however, as these distance levels represent remote acquaintances or strangers, subjects were not required to indicate a name. Thus, eight social distances were included. We used a network-based approach, according to which one might mentally assign more than one person to a particular social distance (32). However, in the experiment, participants were asked to choose just one person for each distance. The network-based approach is important because we assume that an individuals’ social network is adaptive and might change within days. For example, bad experience with a friend might result in a readjustment of the perceived social distance to that friend. Furthermore, as negative emotions can interfere with prosocial behavior, subjects were explicitly asked to only include individuals they did not have a negative attitude toward (33–35). Subjects did not indicate any problems understanding the scale and the idea of social distance.
In the scanner, subjects were asked to make 160 decisions involving the eight social distances (Fig. 1). The task in each trial was to think about the person previously chosen for the specific social distance relevant to the actual trial. Each of the 160 trials started with the presentation of the scale indicating the relevant social distance followed by the generous and selfish options with a mean interstimulus interval (ISI) of 4 s (jittered: ±1 s) and a mean intertrial interval (ITI) of 6 s (jittered: ±1 s). The temporal and spatial ordering of the selfish and generous option presentation was pseudorandomized. For the specific social distance, subjects had to choose between these two alternatives. The selfish alternative always yielded a large reward for the participant alone, whereas the generous option yielded a smaller reward for the participant and an additional reward for the person at the indicated social distance. The selfish reward varied between €75 and €165, changing in increments of €10, resulting in 10 selfish alternatives. The generous option was a fixed reward of €75 for the participant and €75 for the other person (high other-reward trials), or €25 for the other person, respectively (low other-reward trials). The presentation order of social distances, selfish and generous alternatives was fully randomized. Subsequent to the experimental part in the scanner, subjects were asked to name the people they assigned to the specific social distances again, serving as a manipulation check, and indicate demographic information.
After completing the last questionnaire, subjects received their payment. In addition to the €10 show-up fee, a randomly chosen trial was paid out. Depending on whether the participant chose the selfish or the generous alternative in that specific trial, she received 10% of the selfish or the generous reward, respectively. The money for the selfish option was paid directly to the participant, and for the generous option subjects were asked to indicate the address of the other person. If the randomly chosen trial was about a person at social distance 50 or 100, a random person on the campus of the University of Düsseldorf received the reward. Thus, the experiment was fully incentive compatible, did not include deception, and met the experimental standards of behavioral economics.
Social Discount Function.
Because we aimed to quantify the degree of generosity as a function of social distance, we estimated the amount of money a participant was willing to forego to benefit a specific other at a given social distance (2). We first determined, for each social distance level, the point at which a participant was indifferent between the selfish and the generous alternatives, using logistic regression as described above. The decision maker switched from being generous to being selfish as selfish rewards increased. If the decision maker switched from generous to selfish decisions between a selfish reward of €135 and €145, the indifference point would be determined to be €140, thus a 50% probability of choosing generous and 50% of choosing selfish. We interpreted the amount foregone (the indifference point minus the €75 the subject would certainly get if he chose the generous option) as a social premium the participant was willing to pay to benefit the other. We fit the following standard hyperbolic model to the individual, social-distance–dependent social premiums (2):
| [1] |
(hyperbolic discount function), where v symbolizes the magnitude of a reward received by another person at social distance D. The parameter V refers to the social premium a subject is willing to pay in exchange for endowing another person with reward v. Thus, V can be interpreted as the socially discounted ORU of improving the wealth of another individual at social distance D. V is equal to the self-regarding utility at social distance D = 0, thus the intercept with the y axis, and determines the height of the social discount function. The degree of discounting is described by the parameter k, which indicates the steepness and shape of the curve (1, 2). The individual hyperbolic fits were used to estimate individual ORUs.
fMRI Data Acquisition and Preprocessing.
Scanning was performed on a 3-tesla Trio Scanner (Siemens) using an eight-channel head coil. Functional data were acquired using echo-planar imaging sequences with a repetition time of 2.5 s, an echo time of 30 ms, and a flip angle of 90°. Each volume comprised 37 slices acquired in an axial orientation covering all of the brain, including midbrain, but sparing parts of the cerebellum. The presentation of the task and recording of behavioral responses were performed using Presentation software, version 14.9 (Neurobehavioral Systems). Subjects saw the experiment via video goggles (Nordic NeuroLab) and gave their responses by response grips (Nordic NeuroLab) using their index fingers of both hands.
Neural data of 23 participants were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK) software. The results are visualized using the xjview toolbox. Three subjects had to be excluded due to extreme head movements during the experiment (>4-mm translation, >4° rotation). One subject had to be excluded who made exclusively selfish decisions, even when being generous did not involve any reduction in own-reward, as the aim of this study was to investigate prosocial behavior. Indeed, no social discount function can be fit to the data when a participant shows no variation in ORU.
The following preprocessing steps were carried out: slice timing correction, motion correction, segmentation using the T1-weighted image, linear trend removal, high-pass temporal filtering with a filter size of 128 s, spatial smoothing using a Gaussian kernel with FWHM of 8 mm, spatial segmentation, and spatial normalization by coregistering the functional with the individual structural data and then transforming it into the Montreal Neurological Institute (MNI) space.
General Linear Model.
We regressed fMRI time series onto two separate general linear models (GLMs). With the first GLM, we aimed to identify brain regions whose activity correlated with selfish reward magnitude and the econometrically reconstructed, social-distance–dependent ORU. In the second GLM, we searched for neural activity correlating with the relative value, i.e., the social-distance–dependent difference between the selfish value and ORU.
For both GLMs, we defined the following five onset regressors: (i) onset of the social distance information at the beginning of each trial, (ii) onset of the generous option, (iii) onset of the selfish option, (iv) onset of the button press when deciding generous, and (v) onset of the button press when deciding selfish. We modeled BOLD responses at these onsets as stick functions. For the first GLM, we used the following three parametric modulators to assess brain activation (36): (i) the social distance level during the onset of the social distance information (onset regressor i); (ii) the econometrically reconstructed ORU, given a generous choice (onset regressor iv); and (iii) the selfish reward magnitude given a selfish choice (onset regressor v). To obtain commensurability, ORU and selfish reward magnitudes were transformed and normalized to a common scale for all analyses.
In the second GLM, we used the following parametric modulators: (i) the social distance level during the onset of the social distance information; (ii) the difference between own-reward magnitude and ORU, given a generous decision (i.e., the strength of the temptation to choose selfish, given a generous decision); and (iii) the difference between own-reward magnitude and ORU, given a selfish decision. Both GLMs additionally included six movement regressors of no interest, three for translational movements (x, y, z) and three for rotation movements (pitch, roll, yaw). All regressors were convolved with the canonical hemodynamic response function. For each event, onset regressor parameter estimates were obtained and contrast images of each of the parameters against zero were generated. Furthermore, we obtained contrast images of “deciding generous vs. deciding selfish.” The obtained images were transferred to a second-level random-effects analysis using one-sample t tests on the single-subject contrasts. We performed whole-brain corrections for multiple comparisons at the cluster level. For all of the main contrasts reported in Results and in the figures, the individual voxel threshold was set to P > 0.005 with a minimal cluster extent of k ≥ 10 voxel (37). Results are reported using the MNI coordinate system.
PPIs.
We performed a whole-brain PPI analysis with the TPJ as seed region (8, 22, 38). The location of the TPJ seed ROI was based on a 10-mm sphere (39) around the peak activation within the conjunction between the contrasts of generous vs. selfish decisions (first GLM), and the parametric modulation of the temptation to be selfish (second GLM; 51, −49, 34; rTPJ; Fig. 4A). We computed individual average time series within a 4-mm sphere surrounding (39) the individual subject peak activations within the TPJ seed ROI. Seven participants had to be excluded from the PPI analysis because they did not show any individual activation above threshold in the TPJ ROI at P < 0.05, uncorrected. This exclusion criterion is the standard for identifying the location of corresponding activations in individual subjects as needed to extract time courses for connectivity analyses (39–42). We created two PPI regressors by computing an interaction regressor between the normalized time series and the respective condition, i.e., one regressor for generous and one for selfish decisions.
Second, we estimated a GLM with the following regressors: (i) a physiological regressor (i.e., the entire time series of the seed region over the whole experiment), (ii) a psychological regressor for the onset of the generous choices, (iii) the PPI regressor for the generous choices, (iv) a psychological regressor for the onset of the selfish choices, and (v) a PPI regressor for the selfish choices. The onset and PPI regressors were convolved with a canonical form of the hemodynamic response. The model also included the six motion parameters as regressors of no interest.
In a third step, to identify regions whose connectivity was higher during generous than during selfish choices, single-subject contrasts were calculated for the contrast between the PPI regressors, i.e., the contrast between the PPI regressor of the generous compared with the PPI regressor of the selfish choices. Then a second-level analysis was performed by calculating a one-sample t test on the single-subject contrast coefficients. We then identified voxels with significantly higher connectivity difference during generous compared with selfish choices.
Supplementary Material
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft (DFG) Grants KA 2675/4 (to T.K. and Z.H.) and We 4427/3-1 (to B.W.) and from the Swiss National Science Foundation (Schweizerische Nationalfonds) Grants PP00P1_128574 and PP00P1_150739 (to P.N.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.R.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414715112/-/DCSupplemental.
References
- 1.Strombach T, et al. Charity begins at home: Cultural differences in social discounting and generosity. J Behav Decis Making. 2014;27(3):235–245. [Google Scholar]
- 2.Jones B, Rachlin H. Social discounting. Psychol Sci. 2006;17(4):283–286. doi: 10.1111/j.1467-9280.2006.01699.x. [DOI] [PubMed] [Google Scholar]
- 3.Fehr E, Schmidt KM. A theory of fairness, competition, and cooperation. Q J Econ. 1999;114(3):817–868. [Google Scholar]
- 4.Goeree JK, McConnell MA, Mitchell T, Tromp T, Yariv L. The 1/d law of giving. Am Econ J Microecon. 2010;2(1):183–203. [Google Scholar]
- 5.Bartra O, McGuire JT, Kable JW. The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fareri DS, Niznikiewicz MA, Lee VK, Delgado MR. Social network modulation of reward-related signals. J Neurosci. 2012;32(26):9045–9052. doi: 10.1523/JNEUROSCI.0610-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 8.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 9.Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28(22):5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schurz M, Radua J, Aichhorn M, Richlan F, Perner J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind.”. Neuroimage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 13.Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Soc Cogn Affect Neurosci. 2006;1(3):229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Quervain DJ-F, et al. The neural basis of altruistic punishment. Science. 2004;305(5688):1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- 15.Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. J Neurosci. 2009;29(7):2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morishima Y, Schunk D, Bruhin A, Ruff CC, Fehr E. Linking brain structure and activation in temporoparietal junction to explain the neurobiology of human altruism. Neuron. 2012;75(1):73–79. doi: 10.1016/j.neuron.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Carter RM, Bowling DL, Reeck C, Huettel SA. A distinct role of the temporal-parietal junction in predicting socially guided decisions. Science. 2012;337(6090):109–111. doi: 10.1126/science.1219681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314(5800):829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- 19.Kenning P, Plassmann H. Neuroeconomics: An overview from an economic perspective. Brain Res Bull. 2005;67(5):343–354. doi: 10.1016/j.brainresbull.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci. 2014;9(9):1289–1302. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316(5831):1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- 22.Crockett MJ, et al. Restricting temptations: Neural mechanisms of precommitment. Neuron. 2013;79(2):391–401. doi: 10.1016/j.neuron.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rushworth MF, Kolling N, Sallet J, Mars RB. Valuation and decision-making in frontal cortex: One or many serial or parallel systems? Curr Opin Neurobiol. 2012;22(6):946–955. doi: 10.1016/j.conb.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Declerck CH, Boone C, Emonds G. When do people cooperate? The neuroeconomics of prosocial decision making. Brain Cogn. 2013;81(1):95–117. doi: 10.1016/j.bandc.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Smith DV, Clithero JA, Boltuck SE, Huettel SA. Functional connectivity with ventromedial prefrontal cortex reflects subjective value for social rewards. Soc Cogn Affect Neurosci. 2014;9(12):2017–2025. doi: 10.1093/scan/nsu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones BA, Rachlin H. Delay, probability, and social discounting in a public goods game. J Exp Anal Behav. 2009;91(1):61–73. doi: 10.1901/jeab.2009.91-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rachlin H, Jones BA. Altruism among relatives and non-relatives. Behav Processes. 2008;79(2):120–123. doi: 10.1016/j.beproc.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rachlin H, Jones BA. Social discounting and delay discounting. J Behav Decis Making. 2008;21(1):29–43. [Google Scholar]
- 29.Kurniawan IT, Guitart-Masip M, Dayan P, Dolan RJ. Effort and valuation in the brain: The effects of anticipation and execution. J Neurosci. 2013;33(14):6160–6169. doi: 10.1523/JNEUROSCI.4777-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahnt T, Park SQ, Haynes J-D, Tobler PN. Disentangling neural representations of value and salience in the human brain. Proc Natl Acad Sci USA. 2014;111(13):5000–5005. doi: 10.1073/pnas.1320189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahnt T, Tobler PN. Salience signals in the right temporoparietal junction facilitate value-based decisions. J Neurosci. 2013;33(3):863–869. doi: 10.1523/JNEUROSCI.3531-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison F, Sciberras J, James R. Strength of social tie predicts cooperative investment in a human social network. PLoS One. 2011;6(3):e18338. doi: 10.1371/journal.pone.0018338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bechara A. The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55(1):30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Lerner JS, Tiedens LZ. Portrait of the angry decision maker: How appraisal tendencies shape anger's influence on cognition. J Behav Decis Making. 2006;19(2):115–137. [Google Scholar]
- 35.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300(5626):1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 36.Büchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8(2):140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: Re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 39.Eickhoff SB, et al. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bzdok D, et al. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage. 2013;81:381–392. doi: 10.1016/j.neuroimage.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heim S, et al. Effective connectivity of the left BA 44, BA 45, and inferior temporal gyrus during lexical and phonological decisions identified with DCM. Hum Brain Mapp. 2009;30(2):392–402. doi: 10.1002/hbm.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007;1133(1):136–144. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.