Malaria is a devastating disease that accounts for nearly one million deaths every year. Caused by parasites of the genus Plasmodium, malaria transmission requires the bite of a mosquito to transmit the parasite from one host to another. Thus, understanding the parasite life cycle in the mosquito host is of prime importance. In PNAS, Ramphul et al. (1) show that a protein, Pfs47, expressed in the mosquito stages of the malaria parasite can disrupt mosquito JNK signaling to render parasites “invisible” to the mosquito immune system, and in this way increase its survival and chances of transmission.
A number of components (vertebrate factors, mosquito microbiota, and mosquito immune responses) contribute to a pronounced bottleneck in malaria parasite numbers in the mosquito host (2). As such, the success of ookinete invasion and subsequent oocyst formation are critical determinants of malaria transmission. In a landmark study by Collins et al. (3), a refractory strain of Anopheles gambiae was selected that displayed resistance to multiple Plasmodium species, indicating that there was a genetic basis for mosquito refractoriness to malaria parasites. Importantly, when parasites of different geographic origins were used, it was shown that Plasmodium falciparum isolates from Africa were more successful at bypassing encapsulation responses in African mosquitoes than New World or Asian parasite isolates (3), suggesting that malaria parasites have adapted to their natural mosquito hosts to increase survival and transmission.
In support of this hypothesis, recent evidence using a refractory L3-5 line of A. gambiae suggest that some strains of P. falciparum strains can evade the mosquito complement-like system (4). Silencing of thioester-containing protein 1 (TEP1), a complement-like protein required for parasite lysis and melanization, had no effect on the intensity of infection of African P. falciparum isolates, yet proved integral to limiting New World (Brazilian) P. falciparum isolates (4). A genetic screen involving a cross between the African and the Brazilian parasites led to the identification of the Pfs47 gene encoding an ookinete protein that allows African isolates of P. falciparum to evade the mosquito complement-like system (5). Disruption of the Pfs47 locus led to parasite recognition by the A. gambiae immune system, similar to recognition of the Brazilian isolate (5). Together, these studies suggest that genetic divergence of P. falciparum Pfs47 alleles directly contributes to parasite survival in the mosquito, yet the manner by which Pfs47 confers immune evasion in A. gambiae has yet to be fully explored.
Recent work has shown that midgut epithelial nitration is an essential step for Plasmodium recognition by the mosquito immune system (6). Triggered by ookinete invasion, heme peroxidase 2 (HPX2) and NAPDH oxidase 5 (NOX5) are critical enzymes required for the mosquito nitration response, in this way “marking” invading parasites for subsequent TEP1-mediated complement-like elimination (6). Previous work suggests that the African Pfs47 allele reduces HPX2 and NOX5 expression and consequent reduced TEP1 recognition, providing the basis of its immune-evasion phenotype (5). The JNK pathway has been shown to regulate HPX2 and NOX5 transcription (7), yet a direct connection between the JNK pathway and Pfs47 had yet to be established.
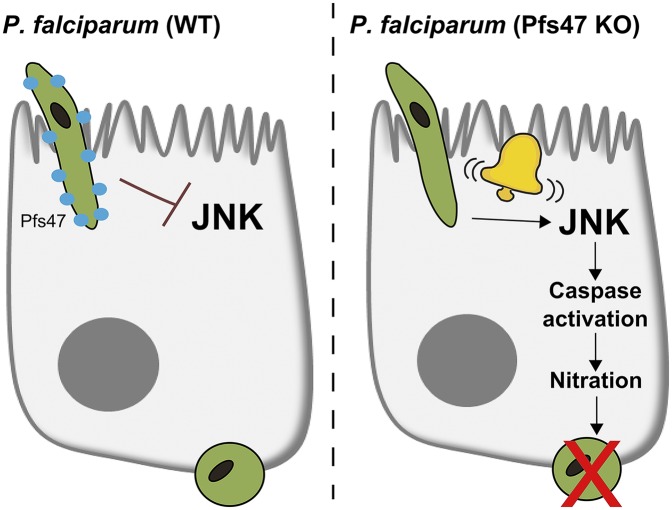
The article by Ramphul et al. in PNAS demonstrates that Pfs47 mediates suppression of the mosquito JNK signaling and anti-Plasmodium immunity (1). To establish this connection, gene-silencing experiments were performed using NF54 WT (African Pfs47 allele “compatible” with the African mosquito) or Pfs47 knockout (KO) parasites. When A. gambiae were infected with NF54 WT parasites, the silencing of JNK pathway components had no effect on the intensity or prevalence of infection, suggesting that the JNK pathway does not actively participate in limiting wild-type parasite survival (1). In contrast, similar experiments with the Pfs47 KO parasite indicate that parasite survival in the mosquito is modulated by JNK signaling (1), inferring that a single parasite protein (Pfs47) can significantly alter mosquito immune responses by suppressing JNK signaling (Fig. 1).
Fig. 1.
Role of parasite Pfs47 in evasion from mosquito JNK-mediated immune responses. Ookinete invasion by NF54 (WT) P. falciparum parasites results in the suppression of JNK activation, ultimately leading to increased parasite survival. In the absence of Pfs47 (Pfs47 KO), ookinete invasion activates the JNK pathway. Downstream events including caspase activation and epithelial nitration effectively “mark” invading parasites for recognition and lysis by the mosquito complement-like system.
In addition, Ramphul et al. further compare gene expression in mosquitoes infected with the NF54 WT and Pfs47 KO parasites to provide evidence of a direct link between JNK signaling, apoptosis, and midgut epithelial nitration (1). In the absence of Pfs47, JNK signaling promotes cell death mediated by the induction of forkhead box O, caspase (CASP)-L1, and several downstream effector caspases, including CASP-S2 (1). When CASP-S2 is silenced, the activation of HPX2 and NOX5 transcripts is attenuated, demonstrating that cell death initiated by caspase activation is required for midgut epithelial nitration (1). Taken together, these data suggest that in the absence of Pfs47, ookinete invasion activates the JNK pathway and downstream signaling events that lead to increased caspase activity, epithelial nitration, immune recognition, and ultimately parasite death (Fig. 1).
These findings provide significant new insight into the molecular mechanisms that accompany Plasmodium ookinete invasion. Not only do Ramphul et al. (1) demonstrate that a parasite protein, Pfs47, can modulate immune recognition and signaling in its mosquito host, but they also integrate existing knowledge of ookinete midgut invasion to provide a more cohesive understanding of the mosquito immune responses to Plasmodium. Observations that ookinete invasion promotes cell death (8) and epithelial nitration (6, 9) have been made previously, yet Ramphul et al. (1) now formally connect these mosquito responses. This added information provides a better understanding of JNK signaling and the cascade of events that lead to parasite immune recognition by the mosquito complement-like system.
Several questions regarding the function of Pfs47 have yet to be explored. Pfs47 is a member of a conserved family of Plasmodium proteins characterized by the presence of multiple copies of 6-cysteine (6-cys) domains, and initially characterized for its localization to the parasite surface following female gamete activation (10). Also present on the surface of ookinetes (5), the expression of Pfs47 can have significant effects on parasite infection as early as 12 h postblood feeding, well before midgut invasion. In addition, Pfs47 dramatically alters midgut transcriptional responses to ookinete invasion (1). Likely mediated in part by evading JNK
As shown by Ramphul et al., Pfs47 is an important determinant of success of parasite development in the mosquito host.
activation, these results suggest that Pfs47 can modulate mosquito responses both before and after ookinete invasion. What remains unclear is how this parasite protein can so dramatically influence responses in the mosquito host. What is the molecular connection between Pfs47 and suppression of the JNK pathway? Pfs47 remains relatively uncharacterized, with little information regarding its function or biochemical properties. It is unclear if Pfs47 is secreted or has an enzymatic activity that disrupts JNK signaling, properties that may argue for an active role in immune evasion. It is also possible that the role of Pfs47 is passive, either in masking immune recognition of parasite surface proteins, or by serving as a ligand for an alternate ookinete invasion pathway. As shown by Ramphul et al. (1), Pfs47 is an important determinant of success of parasite development in the mosquito host. We may now look forward to future reports detailing how Pfs47 mediates immune evasion by malaria parasites in more detail.
Footnotes
The authors declare no conflict of interest.
See companion article on page 1273.
References
- 1.Ramphul UN, Garver LS, Molina-Cruz A, Canepa GE, Barillas-Mury C. Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proc Natl Acad Sci USA. 2015;112:1273–1280. doi: 10.1073/pnas.1423586112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith RC, Vega-Rodríguez J, Jacobs-Lorena M. The Plasmodium bottleneck: Malaria parasite losses in the mosquito vector. Mem Inst Oswaldo Cruz. 2014;109(5):644–661. doi: 10.1590/0074-0276130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins FH, et al. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234(4776):607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- 4.Molina-Cruz A, et al. Some strains of Plasmodium falciparum, a human malaria parasite, evade the complement-like system of Anopheles gambiae mosquitoes. Proc Natl Acad Sci USA. 2012;109(28):E1957–E1962. doi: 10.1073/pnas.1121183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina-Cruz A, et al. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science. 2013;340(6135):984–987. doi: 10.1126/science.1235264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira GdeA, Lieberman J, Barillas-Mury C. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science. 2012;335(6070):856–859. doi: 10.1126/science.1209678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garver LS, de Almeida Oliveira G, Barillas-Mury C. The JNK pathway is a key mediator of Anopheles gambiae antiplasmodial immunity. PLoS Pathog. 2013;9(9):e1003622. doi: 10.1371/journal.ppat.1003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: The time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000;19(22):6030–6040. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S, Gupta L, Han YS, Barillas-Mury C. Inducible peroxidases mediate nitration of anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J Biol Chem. 2004;279(51):53475–53482. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- 10.van Schaijk BCL, et al. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol Biochem Parasitol. 2006;149(2):216–222. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]