Significance
Metamorphosis is an important biological process by which animals alter their body structures to become sexually mature adults. We discovered that tyramine signaling through the β3-octopamine receptor plays an essential role in producing the steroid hormone ecdysone, which is critical for metamorphosis. Based on our observations, we propose that monoamine signaling acts downstream of a body size checkpoint that allows metamorphosis to occur only when a critical body weight is attained during larval development and nutrients are sufficiently abundant. This work also provides a new perspective on an evolutionarily conserved monoaminergic regulation of steroid hormone production during developmental transitions such as metamorphosis. This study provides a new understanding of how metamorphosis is coordinately regulated by nutritional conditions and developmental timing.
Keywords: Drosophila, ecdysone, monoamine, prothoracic gland, metamorphosis
Abstract
In Drosophila, pulsed production of the steroid hormone ecdysone plays a pivotal role in developmental transitions such as metamorphosis. Ecdysone production is regulated in the prothoracic gland (PG) by prothoracicotropic hormone (PTTH) and insulin-like peptides (Ilps). Here, we show that monoaminergic autocrine regulation of ecdysone biosynthesis in the PG is essential for metamorphosis. PG-specific knockdown of a monoamine G protein-coupled receptor, β3-octopamine receptor (Octβ3R), resulted in arrested metamorphosis due to lack of ecdysone. Knockdown of tyramine biosynthesis genes expressed in the PG caused similar defects in ecdysone production and metamorphosis. Moreover, PTTH and Ilps signaling were impaired by Octβ3R knockdown in the PG, and activation of these signaling pathways rescued the defect in metamorphosis. Thus, monoaminergic autocrine signaling in the PG regulates ecdysone biogenesis in a coordinated fashion on activation by PTTH and Ilps. We propose that monoaminergic autocrine signaling acts downstream of a body size checkpoint that allows metamorphosis to occur when nutrients are sufficiently abundant.
In many animal species, the developmental transition is a well-known biological process in which the organism alters its body morphology and physiology to proceed from the juvenile growth stage to the adult reproductive stage. For example, in mammals, puberty causes a drastic change from adolescent to adulthood, whereas in insects, metamorphosis initiates alteration of body structures to produce sexually mature adults, a process accompanied by changes in habitat and behavior. These developmental transitions are primarily regulated by steroid hormones, production of which is regulated coordinately by developmental timing and nutritional conditions (1–3). How these processes are precisely regulated in response to developmental and environmental cues is a longstanding question in biology.
In holometabolous insects, the steroid hormone ecdysone plays a pivotal role in metamorphosis. In Drosophila, metamorphic development from the third-instar larva into the adult, through the prepupa and pupa, initiates 90–96 h after hatching (hAH) at 25 °C under a standard culture condition (4). At the onset of the larval–prepupal transition, ecdysone is produced in the prothoracic gland (PG) and then converted into its active form, 20-hydroxyecdysone (20E), in the peripheral organs. The activities of 20E terminate larval development and growth and initiates metamorphosis (5). Ecdysone biosynthesis is regulated in the PG by neuropeptides, enabling modulation of the timing of 20E pulses during development (2–4). The best-known stimulator of ecdysone biosynthesis is prothoracicotropic hormone (PTTH), which is produced by neurons in the CNS. PTTH activates the receptor tyrosine kinase Torso in the PG to stimulate expression of ecdysone biosynthetic genes through the Ras85D/Raf/MAPK kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway (6, 7). Insulin-like peptides (Ilps), members of another class of neuron-derived factors, activate PI3K in the PG, resulting in production of ecdysone biosynthetic proteins (8–11). The Activin/transforming growth factor-β (TGF-β) signaling pathway is also required in the PG for the expression of PTTH and Ilps receptors, although to date it remains unclear which organ produces the ligand that acts on the PG (12).
In addition to these neuropeptides, the larval–prepupal transition is modulated by environmental cues such as nutritional conditions that influence larval body size. For example, at 56 hAH, early third-instar larvae attain the minimal viable weight (MVW), at which sufficient nutrition is stored in larvae to ensure their survival through metamorphosis (2, 13, 14). After attaining MVW, larvae pass another checkpoint, critical weight (CW), defined as the minimum larval size at which starvation no longer delays the larval–prepupal transition (2, 13, 14). In Drosophila, both checkpoints occur almost simultaneously, making it difficult to distinguish them (2). However, CW is regarded as a body size checkpoint that initiates metamorphosis and is therefore believed to ultimately modulate ecdysone production in the PG. However, its downstream effectors and signaling pathway remain elusive.
Based on data obtained in Manduca and Bombyx (15, 16), a G protein-coupled receptor (GPCR) has long been postulated to be essential for ecdysone biosynthesis in the PG. However, this GPCR and its ligand have not yet been identified. Here we show that monoaminergic autocrine signaling through a GPCR, β3-octopamine receptor (Octβ3R), plays an essential role in ecdysone biosynthesis to execute the larval–prepupal transition. Octβ3R is also required for activation of PTTH and Ilps signaling. We propose that this autocrine system acts downstream of the CW checkpoint to allow the larval–prepupal transition. We speculate that monoamines play an evolutionarily conserved role in the regulation of steroid hormone production during developmental transitions.
Results and Discussion
Octβ3R Is Required for Ecdysone Biosynthesis to Execute the Larval–Prepupal Transition.
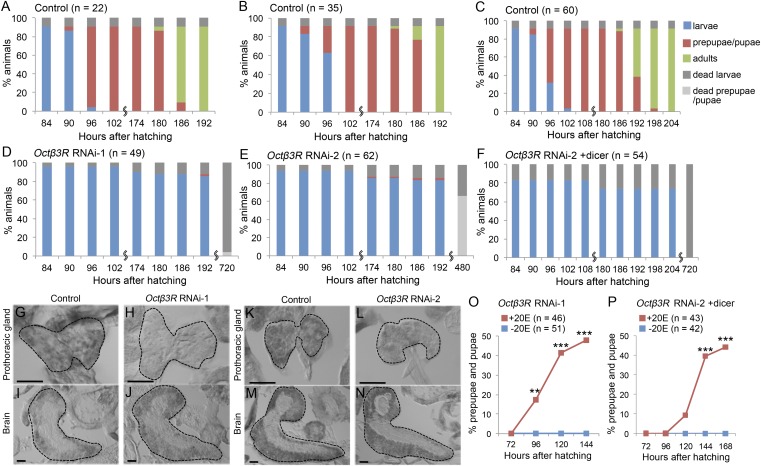
We previously reported that the GPCR Octβ3R is expressed in multiple larval tissues, including the PG (17). To determine whether Octβ3R is involved in ecdysone biosynthesis and metamorphosis, we used RNAi to knock down Octβ3R function specifically in the PG, using the Gal4-upstream activation sequence (UAS) system. Two different UAS-Octβ3RRNAi constructs targeting distinct regions of the Octβ3R mRNA (Octβ3RRNAi-1 and Octβ3RRNAi-2) (Fig. S1) were used to knock down Octβ3R in the PG with the help of a phantom (phm)-22-Gal4 driver (7). Strikingly, larvae expressing Octβ3RRNAi in the PG never developed into adult flies, and 96% of phm>Octβ3RRNAi-1 animals (Fig. 1 A and D) and 34% of phm>Octβ3RRNAi-2 animals (Fig. 1 B and E) arrested at the larval stage. When UAS-dicer2 was introduced into phm>Octβ3RRNAi-2 larvae (phm>Octβ3RRNAi-2+dicer2) to increase RNAi activity, all of these animals arrested at the larval stage (Fig. 1 C and F). Using in situ hybridization, we confirmed a significant reduction in the Octβ3R mRNA levels in the PG of knockdown animals (Fig. 1 H and L) relative to control larvae (Fig. 1 G and K). These data suggest that Octβ3R expression in the PG is essential for executing the larval–prepupal transition in metamorphosis.
Fig. 1.
PG-specific Octβ3R knockdown causes arrest at the larval–prepupal transition due to reduced levels of ecdysone. (A–F) Developmental profiles for control [UAS-Octβ3RRNAi-1 (A), UAS-Octβ3RRNAi-2 (B), and phm > dicer2 (C)] and PG-specific Octβ3R knockdown animals [phm > Octβ3RRNAi-1 (D), phm > Octβ3RRNAi-2 (E), and phm > Octβ3RRNAi-2+dicer2 (F)]. Color bars represent the proportions of larvae (blue), prepupae/pupae (red), adults (green), dead larvae (dark gray), and dead prepupae/pupae (light gray), expressed as percentages, at representative stages [hours after hatching (hAH)]. n, the number of animals examined. (G–N) Section in situ hybridization of the PG (G, H, K, and L) and the brain (I, J, M, and N) of control [UAS-Octβ3RRNAi-1 (G and I), UAS-Octβ3RRNAi-2 (K and M)] and PG-specific Octβ3R knockdown larvae [phm > Octβ3RRNAi-1 (H and J), and phm > Octβ3RRNAi-2 (L and N)] at 72 hAH, using an antisense RNA probe for Octβ3R. PGs and brains are outlined by dashed lines. (Scale bars, 50 μm.) Octβ3R signal was reduced in the PG of Octβ3R knockdown larvae (H and L), whereas it was unaffected in the brain (J and N). (O and P) Percentages of larvae that developed to prepupae/pupae at the representative stages. PG-specific Octβ3R knockdown larvae [phm > Octβ3RRNAi-1 (O) and phm > Octβ3RRNAi-2+dicer2 (P)] were cultured in media supplemented with 1 mg/mL 20E (red) or without 20E (blue) from 48 hAH. n, the number of animals examined. Significance was calculated using the χ2 test (**P < 0.01; ***P < 0.001).
Because a similar defect in the larval–prepupal transition occurs in ecdysone-deficient larvae (12), we hypothesized that the Octβ3R knockdown phenotype was due to lack of ecdysone production. Consistent with this idea, the 20E titer was much lower in phm>Octβ3RRNAi-1 larvae than in control larvae just before the larval–prepupal transition (90 hAH) (Fig. S2). Moreover, administration of 20E by feeding rescued the defect in the larval–prepupal transition caused by Octβ3R knockdown. When phm>Octβ3RRNAi-1 and phm>Octβ3RRNAi-2+dicer2 larvae were cultured on media containing 20E (1 mg/mL) from 48 hAH onward, approximately half of them developed to the prepupal stage, compared with only 2–3% of larvae not fed 20E (Fig. 1 O and P). Thus, PG-specific loss of Octβ3R activity causes an arrest in the larval–prepupal transition due to lack of ecdysone.
Octβ3R Function Is Required for Proper Expression of Ecdysone Biosynthetic Genes.
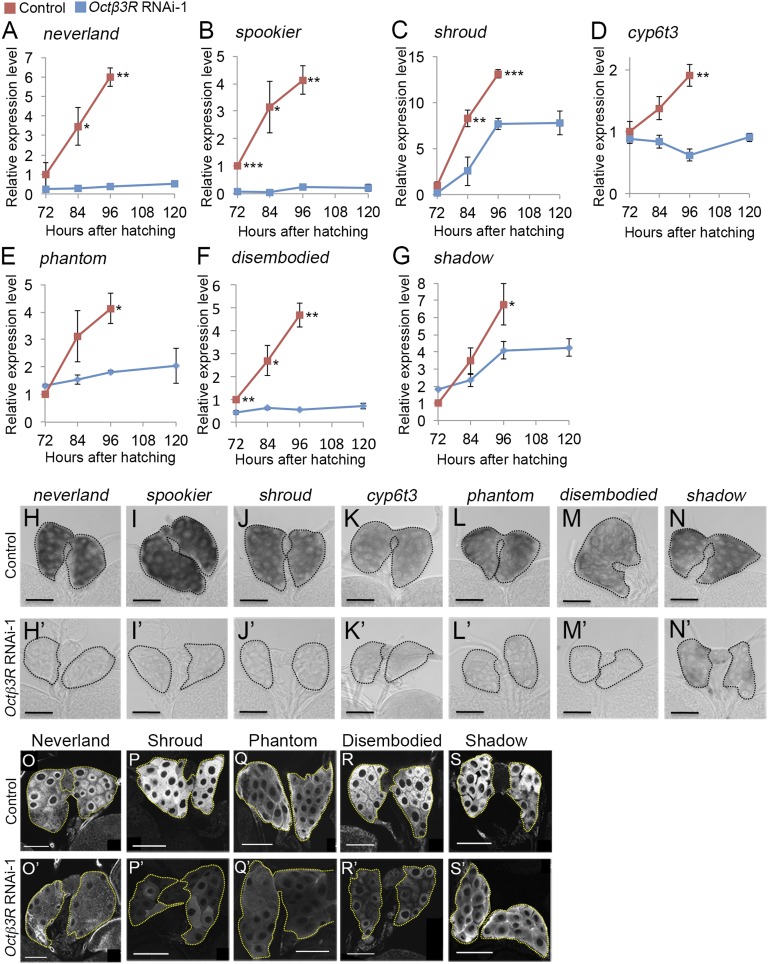
Ecdysone is synthesized in the PG from dietary cholesterol through the action of seven ecdysone biosynthetic genes (neverland, spookier, shroud, Cyp6t3, phantom, disembodied, and shadow) (5, 18–20). We therefore performed quantitative RT-PCR (qPCR) to investigate whether loss of Octβ3R function affects expression of these genes in the PG. In control larvae, expression of these genes increased dramatically between 72 and 96 hAH, when the larval–prepupal transition occurs (Fig. 2 A–G and Fig. S3 A–G). By contrast, in phm>Octβ3RRNAi-1 and phm>Octβ3RRNAi-2+dicer2 larvae, the expression of all of these genes was significantly reduced relative to control larvae at 96 hAH (Fig. 2 A–G and Fig. S3 A–G). We confirmed the reduced expression of ecdysone biosynthetic genes in the PG by in situ hybridization (Fig. 2 H′–N′ and Fig. S3 H′–N′). Furthermore, immunostaining revealed that Neverland, Shroud, Phantom, Disembodied, and Shadow protein levels were reduced in the PG of phm>Octβ3RRNAi-1 and phm>Octβ3RRNAi-2+dicer2 larvae (Fig. 3 O′–S′ and Fig. S3 O′–S′). Taken together, these data show that Octβ3R function is required in the PG for proper expression of ecdysone biosynthetic genes.
Fig. 2.
Expression of ecdysone biosynthetic genes is significantly reduced by Octβ3R knockdown. (A–G) Whole-body expression of ecdysone biosynthetic genes in control [UAS-Octβ3RRNAi-1 (red)] and PG-specific Octβ3R knockdown larvae [phm > Octβ3RRNAi-1 (blue)] was measured using qPCR. Relative expression levels in gene expression were calculated at the representative stages, and the average values of triplicate data sets are shown with SEs. Significance was calculated using Student t test (*P < 0.05; **P < 0.01; ***P < 0.001). (H–N and H′–N′) Expression of ecdysone biosynthetic genes in the PG of control [UAS-Octβ3RRNAi-1 (H–N)] and PG-specific Octβ3R knockdown larvae [phm > Octβ3RRNAi-1 (H′–N′)] at 96 hAH. Whole-mount in situ hybridization was performed using antisense probes for the neverland (H and H′), spookier (I and I′), shroud (J and J′), Cyp6t3 (K and K′), phantom (L and L′), disembodied (M and M′), and shadow transcripts (N and N′). PGs are outlined by dashed lines. (Scale bars, 50 μm.) (O–S and O′–S′) Expression of ecdysone biosynthetic enzymes in the PG of control [UAS-Octβ3RRNAi-1 (O–S)] and PG-specific Octβ3R knockdown larvae [phm > Octβ3RRNAi-1 (O′–S′)] at 96 hAH. Immunostaining was performed using antibodies against Neverland (O and O′), Shroud (P and P′), Phantom (Q and Q′), Disembodied (R and R′), and Shadow proteins (S and S′). PGs are outlined by dashed lines. (Scale bars, 50 μm.)
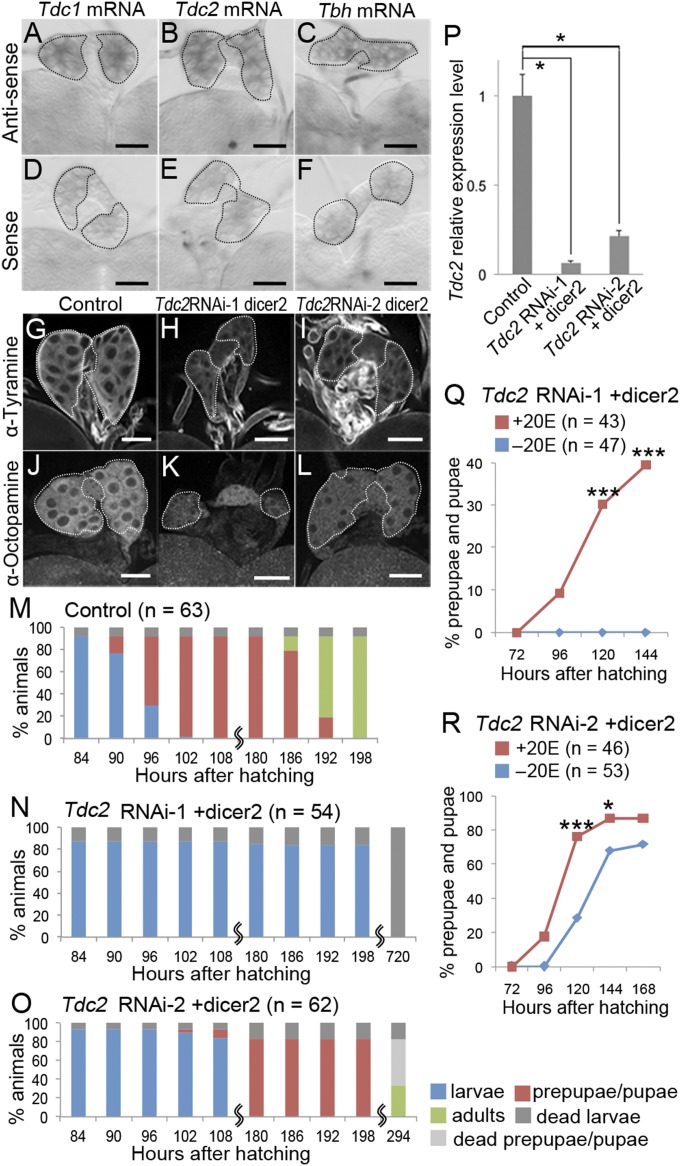
Fig. 3.
PG-specific Tdc2 knockdown causes defects in the larval–prepupal transition due to reduced levels of ecdysone. (A–F) Expression of the Tdc1, Tdc2, and Tbh genes in the PG at 72 hAH. Whole-mount in situ hybridization with antisense (A–C) and sense RNA probes (D–F) for Tdc1 (A and D), Tdc2 (B and E), and Tbh transcripts (C and F) were performed. The stage of the larvae (Oregon-R) from which the PG was obtained and the concentration (400 ng/mL) of the probes used for hybridization were the same for the antisense and sense experiments. PGs are outlined by dashed lines. (Scale bars, 50 μm.) (G–L) The distribution of tyramine and octopamine in the PG of control [phm > dicer2 (G and J)] and PG-specific Tdc2 knockdown larvae [phm > Tdc2RNAi-1+dicer2 (H and K) and phm > Tdc2RNAi-2+dicer2 (I and L)] at 72 hAH. Immunostaining was performed with antibodies against tyramine (G–I) and octopamine (J–L). PGs are outlined by dashed lines. (Scale bars, 50 μm.) (M–O) Developmental profiles for control [phm > dicer2 (M)] and PG-specific Tdc2 knockdown animals [phm > Tdc2RNAi-1+dicer2 (N) and phm > Tdc2RNAi-2+dicer2 (O)]. Color bars are described in the legend for Fig. 1 A–F. n, the number of animals examined. (P) Expression of Tdc2 in the RG of control (phm > dicer2) and PG-specific Tdc2 knockdown larvae (phm > Tdc2RNAi-1+dicer2 and phm > Tdc2RNAi-2+dicer2) were measured at 72 hAH using qPCR. Relative expression level in gene expression was calculated, and the average values of triplicate data sets and SEs are shown. Significance was calculated using the Student t test (*P < 0.05). (Q and R) Percentages of larvae that developed into prepupae/pupae at representative stages. PG-specific Tdc2 knockdown larvae [phm > Tdc2RNAi-1+dicer2 (Q) and phm > Tdc2RNAi-2+dicer2 (R)] were cultured in media supplemented with 1 mg/mL 20E (red) or without 20E (blue) from 48 hAH onward. n, the number of animals examined. Significance was calculated using the χ2 test (*P < 0.05; ***P < 0.001).
Tyramine Produced in the PG Regulates Ecdysone Biosynthesis.
Octβ3R is thought to be activated by octopamine and tyramine binding (21). Octopamine is synthesized from tyramine by tyramine β-hydroxylase (Tbh) (22), and tyramine is synthesized from tyrosine by tyrosine decarboxylase (Tdc) (22). In Drosophila, two Tdc genes (Tdc1 and Tdc2) and one Tbh gene have been identified (23, 24), and all of them are expressed in the larval CNS (23, 24). We found that Tdc1, Tdc2, and Tbh were also expressed in the PG (Fig. 3 A–F). Furthermore, we detected octopamine and tyramine in the PG by immunostaining (Fig. 3 G and J and Fig. S4 A and C). Thus, octopamine and/or tyramine synthesized in the PG may activate Octβ3R in an autocrine manner to induce ecdysone production.
To test this, we generated PG-specific knockdowns of Tdc1, Tdc2, and Tbh. To knock down Tdc2, two constructs targeting distinct regions of the Tdc2 transcript (Tdc2RNAi-1 and Tdc2RNAi-2) (Fig. S1) were expressed along with dicer2 in the PG under the control of the phm-22-Gal4 driver (phm > Tdc2RNAi-1+dicer2 and phm > Tdc2RNAi-2+dicer2). All phm > Tdc2RNAi-1+dicer2 larvae arrested at the larval stage (Fig. 3N), and phm > Tdc2RNAi-2+dicer2 larvae were significantly delayed at the larval–prepupal transition, relative to control animals (Fig. 3 M and O). Tdc2 mRNA level was reduced in the ring gland (RG) containing the PG in both sets of knockdown animals, as demonstrated by qPCR (Fig. 3P). Moreover, octopamine and tyramine production in the PG was impaired by Tdc2 knockdown (Fig. 3 G–L). By contrast, Tdc1 knockdown (phm > Tdc1RNAi+dicer2) caused only a subtle delay in the larval–prepupal transition (Fig. S4 J and G) and had no detectable effect on octopamine or tyramine production (Fig. S4 B and D). These results suggest that Tdc2 is the predominant Tdc regulating octopamine and tyramine biosynthesis in the PG and the larval–prepupal transition. Contrary to our findings, a null mutation in Tdc2 does not affect metamorphosis, and these mutant flies are viable (23). Thus, PG-specific knockdown causes a stronger phenotype than complete loss of Tdc2 activity in whole animals. A similar situation has been reported in regulation of metamorphosis by Activin signaling (12). These phenomena can be explained by a model in which some compensatory changes in other mutant tissues rescue the PG-specific knockdown phenotype in null-mutant animals.
PG-specific Tdc2 knockdown caused a reduction in larval 20E concentration (Fig. S2B). Therefore, we next examined whether feeding 20E to Tdc2 knockdown larvae would rescue the larval–prepupal transition defect. To this end, we cultured phm > Tdc2RNAi-1+dicer2 and phm > Tdc2RNAi-2+dicer2 larvae in media with or without 20E (1 mg/mL) from 48 hAH onward. Approximately 40% of the 20E-fed phm > Tdc2RNAi-1+dicer2 larvae developed to the prepupal stage, whereas none of those larvae grown on control media progressed beyond the larval stage (Fig. 3Q). Furthermore, the delay in the larval–prepupal transition in phm > Tdc2RNAi-2+dicer2 larvae was rescued by 20E feeding (Fig. 3R). These results indicate that the defect in the larval–prepupal transition in Tdc2 knockdown animals results from a lack of 20E production. Thus, octopamine/tyramine synthesized in the PG appears to activate Octβ3R in an autocrine manner to execute the larval–prepupal transition by regulating ecdysone production.
To determine which Octβ3R ligand is responsible for this autocrine signaling, we knocked down Tbh in the PG to prevent conversion of tyramine into octopamine. To knock down Tbh, two constructs targeting distinct regions of the Tbh transcript (TbhRNAi-1 and TbhRNAi-2) (Fig. S1) were expressed along with dicer2 under the control of phm-22-Gal4 (phm > TbhRNAi-1+dicer2 and phm > TbhRNAi-2+dicer2) (Fig. S4 K and L). Although the Tbh knockdown caused a reduction in octopamine production in the PG (Fig. S4 E and F), these larvae did not exhibit any obvious defects in the larval–prepupal transition or subsequent metamorphosis (Fig. S4 H and I). These data suggest that tyramine, rather than octopamine, is the Octβ3R ligand that activates ecdysone production in the PG.
Octβ3R Is Required for Activation of Ilps and PTTH Signaling.
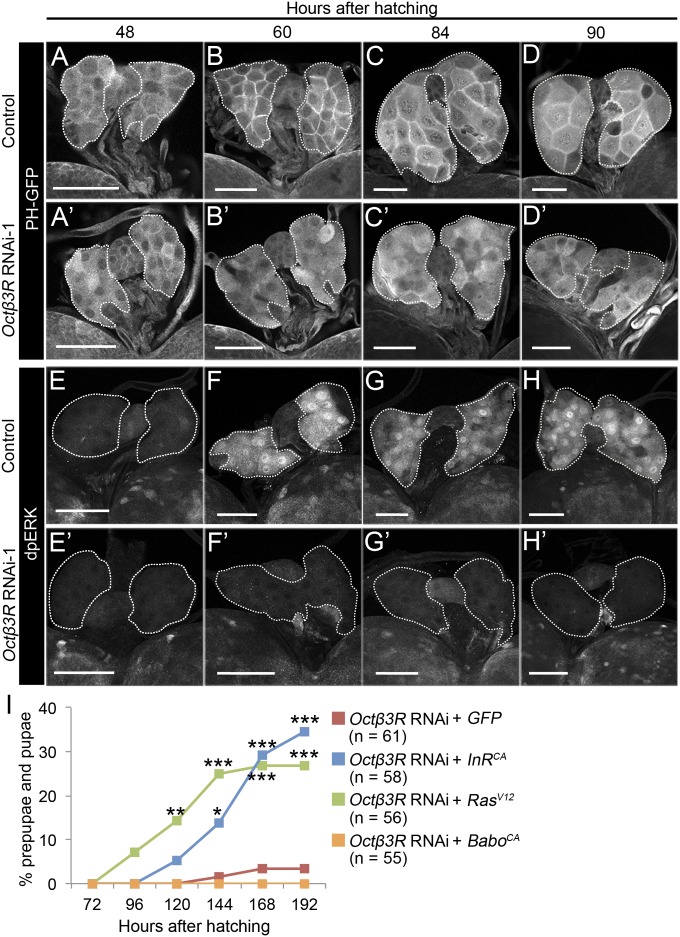
Because ecdysone biosynthesis in the PG is under the control of Ilps and PTTH signaling (2–4), we next examined whether Octβ3R function is required to activate these signaling pathways. To detect Ilps signaling activity, we used a pleckstrin-homology domain fused to GFP (PH-GFP), which is recruited to the plasma membrane when insulin signaling is activated (25). In the PG cells of control larvae, PH-GFP was only weakly localized to the plasma membrane at 48 hAH, whereas its membrane localization became increasingly evident at 60, 84, and 90 hAH (Fig. 4 A–D). By contrast, in PG cells of phm>Octβ3RRNAi-1 larvae, the tight localization of PH-GFP to the plasma membrane was no longer detectable (Fig. 4 A′–D′), indicating that activation of Ilps signaling had been disrupted. Moreover, overexpression of a constitutively active form of the Ilps receptor InR (InRCA) was able to rescue the larval arrest in phm>Octβ3RRNAi-1 animals (Fig. 4I). Next, we performed immunostaining of the diphosphorylated form of ERK (dpERK), a downstream signaling component of the PTTH pathway. We found that dpERK expression was very weak at 48 hAH, but was activated in the PG of control larvae at 60, 84, and 90 hAH (Fig. 4 E–H); by contrast, this activation was reduced in the PG of phm>Octβ3RRNAi-1 larvae (Fig. 4 E′–H′). Expression of a constitutively active form of another downstream PTTH signaling component, Ras (RasV12), rescued the larval–prepupal transition defect in phm>Octβ3RRNAi-1 animals (Fig. 4I). These results show that Octβ3R function is required to activate Ilps and PTTH signaling in the PG and that these signaling pathways execute the larval–prepupal transition. Although activation of both the Ilps and PTTH signaling pathways requires Activin/TGFβ signaling in the PG (12), expression of a constitutively active form of the Activin/TGFβ receptor Baboon (BaboCA) failed to rescue the larval–prepupal transition defect in phm>Octβ3RRNAi-1 animals (Fig. 4I). This observation suggests that Octβ3R acts downstream or independent of Activin/TGFβ signaling to regulate Ilps and PTTH signaling in the PG.
Fig. 4.
The Ilps and PTTH signaling pathways are disrupted by Octβ3R knockdown in the PG. (A–H and A′–H′) Expression of PH-GFP (A–D and A′–D′) and dpERK (E–H and E′–H′) in the PG of control [phm-22-Gal4+PH-GFP (A–D), phm-22-Gal4 (E–H)] and PG-specific Octβ3R knockdown [phm > Octβ3RRNAi-1+PH-GFP (A′–D′), phm > Octβ3RRNAi-1 (E′–H′)] larvae at 48, 60, 84, and 90 hAH. Immunostaining was performed using antibodies against GFP (A–D and A′–D′) and dpERK (E–H and E′–H′). PGs are outlined by dashed lines. (Scale bars, 50 μm.) (I) Percentages of larvae that developed into prepupae/pupae at representative stages. PG-specific Octβ3R knockdown larvae expressing GFP [phm > Octβ3RRNAi-1+GFP (red)], InRCA [phm > Octβ3RRNAi-1+InRCA (blue)], RasV12 [phm > Octβ3RRNAi-1+RasV12 (green)], and BaboCA [phm > Octβ3RRNAi-1+BaboCA (yellow)] were cultured from 48 hAH, and the number of prepupae/pupae was counted at each stage. n, the number of animals examined. Significance was calculated using the χ2 test (*P < 0.05; **P < 0.01; ***P < 0.001).
Octβ3R Signaling Is Required at Around 60 hAH in PG for Execution of the Larval–Prepupal Transition.
The observations described above demonstrate that phm>Octβ3RRNAi affects Ilps and PTTH signaling in the PG as early as 60 hAH, raising the question of when Octβ3R function is required in the PG for execution of the larval–prepupal transition. To address this issue, we used the Gal80ts and Gal4/UAS system (26), which restricts expression of Octβ3R dsRNA in the PG at 18 °C, but allows its expression at 28 °C. The results of temperature upshift and downshift experiments revealed that the larval–prepupal transition was impaired only when Octβ3R dsRNA was expressed in the PG at around 60 hAH (Fig. S5). Notably, 60 hAH is the critical period during which larvae attain CW under nutrient-rich conditions (2, 13, 14). As noted above, when larvae are starved before attainment of CW, they are unable to transit into the prepupal stage (2, 13, 14). By contrast, starved larvae can successfully transit to prepupal/pupal stage without developmental delay once they have attained CW by growing beyond the critical period (∼56 hAH) under nutrient-rich conditions in standard Drosophila medium (13, 14). Thus, we hypothesized that Octβ3R signaling acts downstream of the body-size checkpoint, or attainment of CW, to allow the larval–prepupal transition.
Several lines of evidence support our hypothesis. First, Octβ3R function is required for activation of Ilps and PTTH signaling detected in the PG at 60 hAH. By contrast, at 48 hAH, before the attainment of CW, neither signaling pathway is active in the PG (Fig. 4). Second, Ilps and PTTH signaling was not activated in the PG when the larvae were starved from 48 hAH onward (early starvation), whereas these signaling pathways were active when the larvae were starved after 60 hAH (late starvation) (Fig. S6). Finally, a ligand for Octβ3R, tyramine, was detectable in the PG at 60 hAH, but decreases after this stage under a nutrient-rich condition (Fig. S7 A and B). This decrease in tyramine was abrogated by early starvation but not by late starvation (Fig. S7 C–E). Assuming that this decrease in tyramine in the PG is due to its secretion from PG cells, it is reasonable to propose that attainment of CW causes tyramine secretion from the PG at around 60 hAH, which in turn activates Octβ3R to regulate the Ilps and PTTH pathways, leading to the larval–prepupal transition (Fig. S8).
This study demonstrates, for the first time to our knowledge, that monoaminergic regulation plays a pivotal role in ecdysone biosynthesis to induce metamorphosis and that Octβ3R acts as an upstream regulator essential for the Ilps and PTTH signaling. In addition, our data indicate that Octβ3R ligands are produced in the PG to stimulate ecdysone biosynthesis in an autocrine manner. Autocrine signaling has been proposed to mediate the community effect, in which identical neighboring cells are coordinated in their stimulation and maintenance of cell type-specific gene expression and their differentiation, as observed in muscle development of amphibian embryos (27, 28). Thus, we propose that monoaminergic autocrine signaling among PG cells acts to increase their responsiveness to Ilps and PTTH, thereby allowing coordinated expression of ecdysone biosynthetic genes within a time window following exposure to neuropeptides.
Our findings raise the larger question of whether monoamine acts as part of an evolutionarily conserved mechanism of steroid hormone production. In vertebrates, there is limited evidence of monoaminergic regulation of steroid hormone biosynthesis. For example, in cultured adrenal glands, catecholamine stimulates the biosynthesis of the steroid hormone cortisol in a paracrine manner to elicit a stress reaction (29). Another example is the Leydig cells of the mammalian testes, in which the steroid hormone testosterone is produced mainly in response to pituitary gonadotropin. However, catecholamine signaling through β-adrenergic receptors, the orthologs of Octβ3R, also promotes the production of testosterone from cultured fetal Leydig cells (30, 31), which may be the site of catecholamine synthesis in the fetal and mature human testes (32). Thus, monoamines may play a conserved role in modulating and/or stimulating steroid hormone production during physiological and developmental transitions.
Materials and Methods
Drosophila Stocks.
Flies were maintained on a standard Drosophila medium at 25 °C. phm-22-Gal4 (a gift from Michael B. O’Conner, University of Minnesota, Minneapolis) was used to express the following UAS constructs: UAS-Octβ3RRNAi-1 (31348R-1) and UAS-Tdc2RNAi-1 (10687R-1) from National Institute of Genetics, Japan; UAS-dicer2 (v60008), UAS-Octβ3RRNAi-2 (v106519), and UAS-TbhRNAi-1 (v107070) from the Vienna Drosophila RNAi Center, Austria; UAS-Tdc1RNAi (25801), UAS-Tdc2RNAi-2 (25871), UAS-TbhRNAi-2 (27667), UAS-mCD8::GFP (5130), UAS-RasV12 (4847), UAS-InRCA (8263), PT-GFP (8164), and tubulin (tub)-Gal80ts (7017) from Bloomington Drosophila Stock Center in the United STates. UAS-BaboCA is a gift from Michael B. O’Conner. See SI Materials and Methods and Table S1 for detailed genotypes of the flies used in this study.
Concentration Measurement of 20E and 20E Feeding.
Ten larvae were rinsed with distilled water and collected in a 1.5-mL microcentrifuge tube. Methanol (100 μL) was added, and the larvae were homogenized with a plastic pestle at room temperature. Next, 400 μL of methanol was added, and the tubes were vortexed. The samples were then centrifuged at 130,000 × g for 15 min at 4 °C, and 350 μL of supernatant was dried in a centrifugal vacuum evaporator. 20E was quantitated by ELISA using 20E EIA antiserum, 20E AchE tracer, and Ellman’s reagent (Cayman Chemical) as previously described (33).
For 20E feeding, 48-hAH larvae were transferred to freshly made cornmeal medium with or without 1 mg/mL 20E (Sigma). Larvae were cultured at 25 °C under a 12-h light/dark cycle. The number of prepupae/pupae was scored at 24-h intervals.
mRNA and Protein Expression Analyses.
See SI Materials and Methods and Tables S2 and S3 for details about quantitative RT-PCR, in situ hybridization, antibody preparation, and immunostaining methods.
Supplementary Material
Acknowledgments
We thank Dr. Michael B. O’Connor for providing fly strains and antibodies and Dr. N. Yamanaka for critical reading of the manuscript. We also thank the Drosophila Genetic Resource Centers (Japan) and the Bloomington and Vienna Drosophila Stock Centers for providing us with fly strains. This work was supported in part by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (JSPS), the Ministry of Education, Culture, Sport, Science and Technology of Japan, PRESTO/JST, the Inamori Foundation, and the Naito Foundation. Y.S.-N. was a recipient of JSPS fellowships.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414966112/-/DCSupplemental.
References
- 1.Roa J, et al. Metabolic control of puberty onset: New players, new mechanisms. Mol Cell Endocrinol. 2010;324(1-2):87–94. doi: 10.1016/j.mce.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Rewitz KF, Yamanaka N, O’Connor MB. Developmental checkpoints and feedback circuits time insect maturation. Curr Top Dev Biol. 2013;103:1–33. doi: 10.1016/B978-0-12-385979-2.00001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niwa YS, Niwa R. Neural control of steroid hormone biosynthesis during development in the fruit fly Drosophila melanogaster. Genes Genet Syst. 2014;89(1):27–34. doi: 10.1266/ggs.89.27. [DOI] [PubMed] [Google Scholar]
- 4.Tennessen JM, Thummel CS. Coordinating growth and maturation - insights from Drosophila. Curr Biol. 2011;21(18):R750–R757. doi: 10.1016/j.cub.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem Soc Trans. 2006;34(Pt 6):1256–1260. doi: 10.1042/BST0341256. [DOI] [PubMed] [Google Scholar]
- 6.McBrayer Z, et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell. 2007;13(6):857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rewitz KF, Yamanaka N, Gilbert LI, O’Connor MB. The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science. 2009;326(5958):1403–1405. doi: 10.1126/science.1176450. [DOI] [PubMed] [Google Scholar]
- 8.Walkiewicz MA, Stern M. Increased insulin/insulin growth factor signaling advances the onset of metamorphosis in Drosophila. PLoS ONE. 2009;4(4):e5072. doi: 10.1371/journal.pone.0005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldwell PE, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol. 2005;15(20):1785–1795. doi: 10.1016/j.cub.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Mirth C, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol. 2005;15(20):1796–1807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Colombani J, et al. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310(5748):667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- 12.Gibbens YY, Warren JT, Gilbert LI, O’Connor MB. Neuroendocrine regulation of Drosophila metamorphosis requires TGFbeta/Activin signaling. Development. 2011;138(13):2693–2703. doi: 10.1242/dev.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirth CK, Shingleton AW. Integrating body and organ size in Drosophila: Recent advances and outstanding problems. Front Endocrinol (Lausanne) 2012;3:49. doi: 10.3389/fendo.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callier V, Nijhout HF. Body size determination in insects: A review and synthesis of size- and brain-dependent and independent mechanisms. Biol Rev Camb Philos Soc. 2013;88(4):944–954. doi: 10.1111/brv.12033. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. 2002;47:883–916. doi: 10.1146/annurev.ento.47.091201.145302. [DOI] [PubMed] [Google Scholar]
- 16.Rybczynski R, Gilbert LI. Protein kinase C modulates ecdysteroidogenesis in the prothoracic gland of the tobacco hornworm, Manduca sexta. Mol Cell Endocrinol. 2006;251(1-2):78–87. doi: 10.1016/j.mce.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Ohhara Y, Kayashima Y, Hayashi Y, Kobayashi S, Yamakawa-Kobayashi K. Expression of β-adrenergic-like octopamine receptors during Drosophila development. Zoolog Sci. 2012;29(2):83–89. doi: 10.2108/zsj.29.83. [DOI] [PubMed] [Google Scholar]
- 18.Yoshiyama T, Namiki T, Mita K, Kataoka H, Niwa R. Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development. 2006;133(13):2565–2574. doi: 10.1242/dev.02428. [DOI] [PubMed] [Google Scholar]
- 19.Niwa R, et al. Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the ‘Black Box’ of the ecdysteroid biosynthesis pathway. Development. 2010;137(12):1991–1999. doi: 10.1242/dev.045641. [DOI] [PubMed] [Google Scholar]
- 20.Ou Q, Magico A, King-Jones K. Nuclear receptor DHR4 controls the timing of steroid hormone pulses during Drosophila development. PLoS Biol. 2011;9(9):e1001160. doi: 10.1371/journal.pbio.1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophila β-adrenergic-like octopamine G-protein coupled receptors. J Neurochem. 2005;94(2):547–560. doi: 10.1111/j.1471-4159.2005.03251.x. [DOI] [PubMed] [Google Scholar]
- 22.Roeder T. Tyramine and octopamine: Ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 23.Cole SH, et al. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: Distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280(15):14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 24.Monastirioti M, Linn CE, Jr, White K. Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16(12):3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell. 2002;2(2):239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- 26.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302(5651):1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 27.Gurdon JB, Lemaire P, Kato K. Community effects and related phenomena in development. Cell. 1993;75(5):831–834. doi: 10.1016/0092-8674(93)90526-v. [DOI] [PubMed] [Google Scholar]
- 28.Standley HJ, Zorn AM, Gurdon JB. eFGF and its mode of action in the community effect during Xenopus myogenesis. Development. 2001;128(8):1347–1357. doi: 10.1242/dev.128.8.1347. [DOI] [PubMed] [Google Scholar]
- 29.Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Morphological and functional studies of the paracrine interaction between cortex and medulla in the adrenal gland. Microsc Res Tech. 1997;36(6):520–533. doi: 10.1002/(SICI)1097-0029(19970315)36:6<520::AID-JEMT9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Pointis G, Latreille MT. Catecholamine-induced stimulation of testosterone production by Leydig cells from fetal mouse testis. J Reprod Fertil. 1987;80(1):321–326. doi: 10.1530/jrf.0.0800321. [DOI] [PubMed] [Google Scholar]
- 31.Stojkov NJ, et al. Repeated immobilization stress disturbed steroidogenic machinery and stimulated the expression of cAMP signaling elements and adrenergic receptors in Leydig cells. Am J Physiol Endocrinol Metab. 2012;302(10):E1239–E1251. doi: 10.1152/ajpendo.00554.2011. [DOI] [PubMed] [Google Scholar]
- 32.Davidoff MS, et al. Catecholamine-synthesizing enzymes in the adult and prenatal human testis. Histochem Cell Biol. 2005;124(3-4):313–323. doi: 10.1007/s00418-005-0024-x. [DOI] [PubMed] [Google Scholar]
- 33.Rewitz KF, Yamanaka N, O’Connor MB. Steroid hormone inactivation is required during the juvenile-adult transition in Drosophila. Dev Cell. 2010;19(6):895–902. doi: 10.1016/j.devcel.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.