Significance
In natural matings, semen delivers spermatozoa and immunoregulatory fluids to the female reproductive tract. Here, a soluble form of CD38 (sCD38) is shown to play an important role in facilitating maternal immune tolerance against the fetus by inducing the development of uterine tolerogenic DCs and forkhead box P3+ (Foxp3+) regulatory T cells. Deficiency of sCD38 in seminal fluid increased the rates of loss of allogeneic fetuses, and this loss was rescued by a direct injection of recombinant sCD38 into the uterus. Thus, seminal sCD38 acts as a pivotal immune suppressor for establishing maternal immune tolerance against the fetus. sCD38 could potentially be used to prevent failed pregnancies.
Keywords: seminal plasma, CD38, regulatory T cells, dendritic cells, fetomaternal tolerance
Abstract
A successful pregnancy depends on a complex process that establishes fetomaternal tolerance. Seminal plasma is known to induce maternal immune tolerance to paternal alloantigens, but the seminal factors that regulate maternal immunity have yet to be characterized. Here, we show that a soluble form of CD38 (sCD38) released from seminal vesicles to the seminal plasma plays a crucial role in inducing tolerogenic dendritic cells and CD4+ forkhead box P3+ (Foxp3+) regulatory T cells (Tregs), thereby enhancing maternal immune tolerance and protecting the semiallogeneic fetus from resorption. The abortion rate in BALB/c females mated with C57BL/6 Cd38−/− males was high compared with that in females mated with Cd38+/+ males, and this was associated with a reduced proportion of Tregs within the CD4+ T-cell pool. Direct intravaginal injection of sCD38 to CBA/J pregnant mice at preimplantation increased Tregs and pregnancy rates in mice under abortive sonic stress from 48 h after mating until euthanasia. Thus, sCD38 released from seminal vesicles to the seminal plasma acts as an immunoregulatory factor to protect semiallogeneic fetuses from maternal immune responses.
Seventy-five percent of pregnancies that are lost represent failure of implantation and are therefore not clinically recognized as pregnancies (1). Recurrent miscarriage (the spontaneous loss of three or more consecutive pregnancies) is a significant health issue for 1–2% of women, with no identifiable biological cause and no effective treatment. During early stages of pregnancy, complex processes help to create a uterine environment that is conducive to a successful pregnancy. These include immunological adaptation to the semiallogeneic fetus. Tolerance to paternal alloantigens is critical for successful reproduction in placental mammals (2, 3). Many studies have proposed that regulatory T cells (Tregs) play an essential role in the development of fetomaternal tolerance in mice and humans (4–7). Seminal plasma contains potent immunoregulatory molecules that contribute to the induction of tolerogenic DCs (tDCs) and ultimately Treg expansion, which is necessary to establish maternal tolerance against paternal antigens (8–10). However, the specific molecules in semen that are responsible for expansion of Tregs and establishment of maternal tolerance remain undefined.
CD38, a mammalian prototype of ADP ribosyl cyclases (ADPRCs), is a type II transmembrane (TM) glycoprotein expressed in many cell types and seminal fluid (11–16). CD38 produces calcium-mobilizing second messengers, cyclic ADP ribose, and nicotinic acid adenine dinucleotide phosphate (11, 12). We previously showed that intact CD38 in prostasomes assists progesterone-induced sperm Ca2+ signaling (13). In addition to its enzymatic role for Ca2+ signaling, CD38 may also have a nonenzymatic role through its interaction with CD31 (17, 18). CD31, a type I TM homophilic or heterophilic receptor, is expressed in endothelial cells and a variety of immune cells (19) and is involved in attenuating the inflammatory response in a variety of inflammatory diseases (20–23). For example, recombinant CD38 inhibits LPS-induced inflammatory signals in mouse macrophages and human DCs through an interaction with CD31 (24, 25).
In this study, we found that CD38 is truncated and released into the seminal plasma from seminal vesicles (SVs) as a soluble form (sCD38) in humans and mice. This finding prompted us to examine whether CD38 plays a role in maternal immune tolerance during pregnancy. We found that sCD38 present in seminal plasma was crucial for the induction of uterine tDC and Tregs, which are responsible for the development of the fetomaternal tolerance.
Results
CD38 Is Truncated and Released from SVs to the Plasma as a Soluble Form.
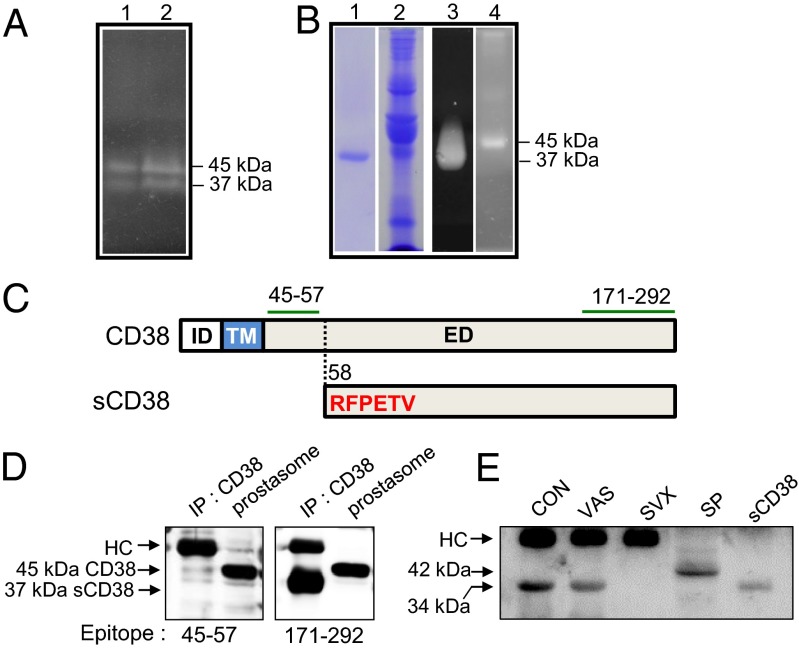
ADPRC activity was detected in human seminal fluid in two proteins with molecular weights of 45 kDa and 37 kDa as identified in in-gel assays (Fig. 1A). Notably, the 37 kDa protein bound to a CD38 immunoaffinity column (Fig. 1B, lanes 1 and 3), whereas the 45 kDa protein did not (Fig. 1B, lanes 2 and 4). This suggests that the 37 kDa protein was a truncated form of CD38, whereas the 45 kDa protein was full-length CD38, which is encapsulated within prostasomes (13), making the CD38 (45 kDa) physically inaccessible for binding to the affinity column. Aminoterminal sequencing of the 37 kDa protein identified its cleavage site as Arg58 close to the TM domain of CD38 (Fig. 1C). Western blot analysis with two different antibodies against CD38 peptides (CD3845–57 and CD38171–292) confirmed that this was the case (Fig. 1D). The soluble form, 37 kDa CD38 (sCD38), was detected in all 19 seminal plasma samples tested (range, 0.5–10.6 μg/mL), including one from a vasectomised individual (7.8 μg/mL).
Fig. 1.
Identification of sCD38 in seminal fluid by in-gel ADPRC assay. (A) Visualization of human seminal fluid proteins with ADPRC activity (lanes 1 and 2 for two healthy volunteers). (B) Coomassie blue staining of proteins eluted from a CD38 immunoaffinity column (lane 1) and proteins that passed through the column (lane 2). In-gel ADPRC activity assay for the proteins eluted from the column (lane 3) and passed through the column (lane 4). (C) Upper diagram shows the intracellular domain (ID), TM domain, and extracellular domain (ED) of CD38, and the lower diagram shows the N-terminal amino acid (aa) sequence (highlighted in red) of sCD38. The segments (aa 45–57 and aa 171–292) denoted by green lines represent epitopes of the antibodies used for immunoblotting. The dotted line indicates the cleavage site within CD38. (D) Proteins immunoprecipitated from seminal fluid and prostasome lysates using anti-CD38 antibodies were blotted with antibodies specific for two different epitopes: amino acids 45–57 and 171–292. HC, heavy chain of Ig. (E) Mouse seminal plasma was collected from B6 females by uterine lavage within 1 h of mating with B6 intact (CON), vasectomised (VAS), and SVX males immunoprecipitated with anti-CD38, and the immunoprecipitates were analyzed for CD38 by Western blotting. Mouse splenocyte lysate (SP) and recombinant mouse sCD38 (sCD38) were included in the experiment as size references of full length and truncated sCD38.
We next used a mouse model to examine the role of seminal CD38 in more detail. We began by asking whether sCD38 was present in mouse seminal fluid. Due to technical difficulties in obtaining mouse semen, we collected uterine lavage fluid from unmated estrous females and mouse SV fluid. sCD38 was not detected in uterine lavage fluid from unmated estrous females, whereas sCD38 was present in mouse SV fluid (Fig. S1). To identify the organ that secreted the sCD38, we collected uterine lavage fluid from the female reproductive tract 1 h after mating. Western analysis demonstrated that the uterine lavage fluid collected from female mice mated with normal or vasectomised mice contained sCD38 (34 kDa). Mouse splenocytes expressed only the full-length CD38 (42 kDa) (Fig. 1E) (26). sCD38 comigrated with recombinant sCD38 lacking the cytoplasmic and TM domains on SDS/PAGE gels (Fig. 1E). By contrast, uterine lavage fluid collected from females mated with SV-deficient (SVX) males did not contain sCD38. These findings indicate that sCD38 in seminal fluid originates from SV and that the truncation of CD38 in SV is common to both humans and mice.
Seminal sCD38 Is Crucial for Fetomaternal Tolerance During Pregnancy.
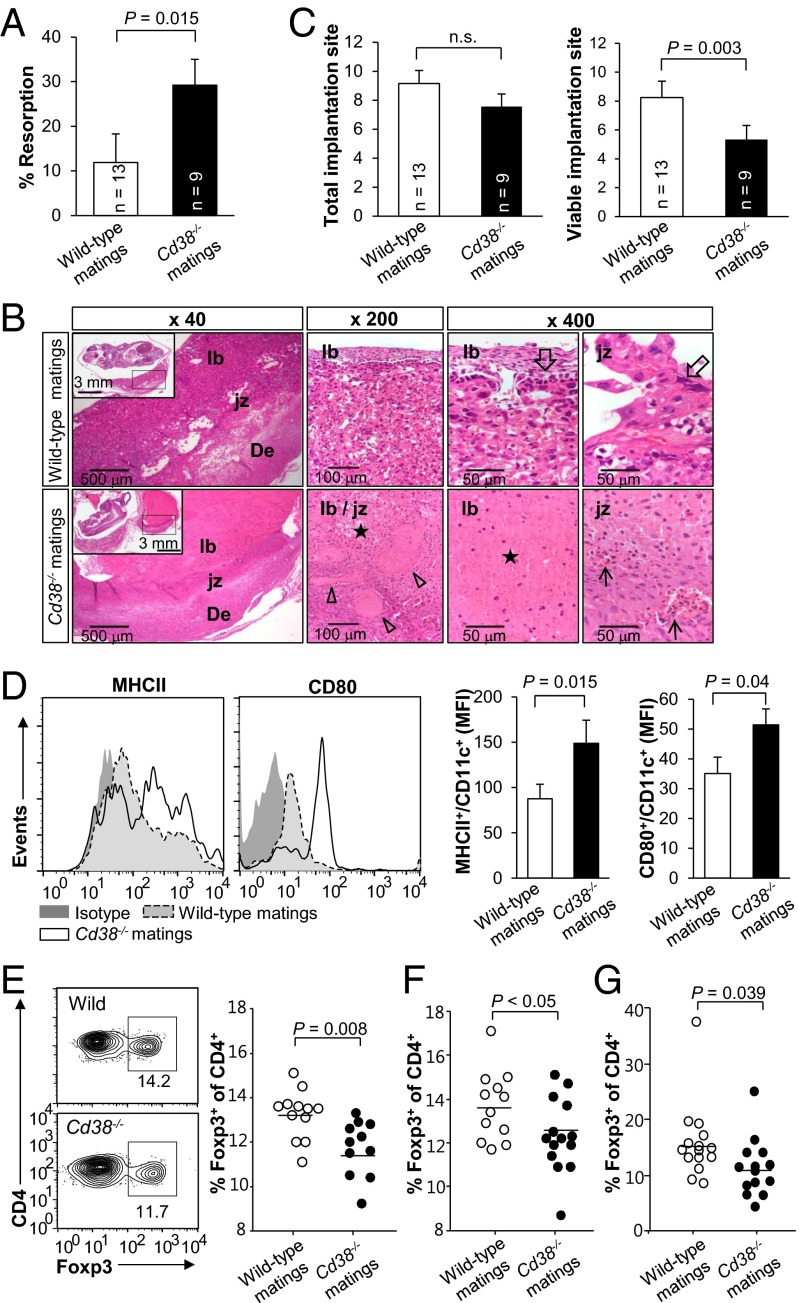
As sCD38 was present in mouse seminal fluid, we next investigated whether seminal sCD38 affected pregnancy, particularly with respect to fetomaternal immune tolerance. We generated an allogeneic pregnancy model based on BALB/c (H-2d) female mice mated with Cd38−/− or wild-type C57BL/6 (H-2b) male mice. Mice were examined at 12.5 d postcoitum (dpc), at which time both implantation and resorption would be visible. We found that female mice mated with Cd38−/− males (Cd38−/− matings) showed higher rates of fetal resorption at 12.5 dpc than females mated with Cd38+/+ males (wild-type matings) (Fig. 2A). In agreement with this finding, histochemical analysis revealed visible signs of inflammation, including thrombosis and massive immune cell infiltration into the placentas of Cd38−/− matings, none of which were present in wild-type matings (Fig. 2B). These findings indicate that a lack of CD38 in seminal fluid induces embryo resorption, presumably due to the impairment of fetomaternal tolerance (3–6). The number of implantations in Cd38−/− matings was similar to that in wild-type matings, whereas the number of surviving fetuses in Cd38−/− matings was reduced compared with that in wild-type matings (Fig. 2C). To exclude the possibility that mouse pregnancy failures can be ovarian-based due to progesterone insufficiency, we compared maternal plasma progesterone levels at 12.5 dpc, but found no significant difference between the two groups (Fig. S2). This suggests that sCD38 reduces the maternal immune response toward the fetus after implantation, but does not affect the implantation itself.
Fig. 2.
Pregnancy impairment in female mice mated with Cd38−/− male mice. Wild-type or Cd38−/− C57BL/6 males were mated with BALB/c females, and plugged mice were killed at 12 dpc. (A) Resorption rates. (B) Histological evaluation of placental tissue. In wild-type mating, the placenta and the junctional zone (jz) area of the placenta located between the labyrinth (lb) and decidual tissues (De) are intact, and all of the cellular components, including trophoblasts (empty arrows), are viable. In Cd38−/− mating, the placenta is necrotic (stars), and the junctional zone area shows vascular congestion (empty arrow heads), hemorrhage, and inflammatory infiltration (arrows). Insets show low-magnification images of the embryos. (C) The number of total and viable implantation sites in wild-type and Cd38−/− matings. Bars, mean ± SEM. (D) Phenotypic analysis of CD11c+ uterine DCs from wild-type or Cd38−/− mating mice at 3.5 dpc. MHC-II and CD80 expression was examined in gated CD11c+ uterus cells. Mean fluorescent intensities (MFIs) of MHC-II and CD80 were plotted as bar graphs (Right panels). Bars, mean ± SEM; number of mice tested for each experiment, 8–9. (E, Left panels) Representative flow cytometric analysis of CD4+Foxp3+ T cells in the PALN in wild-type and Cd38−/− matings at 3.5 dpc. Percentage of CD4+Foxp3+ T cells in the PALN in wild-type and Cd38−/− matings was plotted for statistical evaluation at 3.5 (E, Right panel) and 6.5 dpc (F). (G) Percentage of CD4+Foxp3+ T cells in the uterus in wild-type and Cd38−/− matings at 3.5 dpc.
Previous studies suggest that seminal factors promote the generation of tDCs (9, 10). Therefore, we next examined whether seminal sCD38 inhibited the insemination-induced maturation of uterine DCs by comparing phenotypic maturation markers on uterine DCs in wild-type and Cd38−/− matings. In agreement with the high resorption rates in Cd38−/− matings, the uterine CD11c+ cells from Cd38−/− mating showed higher levels of MHC-II and CD80 expression at 3.5 dpc than those from wild-type matings (Fig. 2D). Because tDCs promote the generation of Tregs (27), we next asked whether sCD38 was necessary for Tregs expansion. Expression of the transcription factor, Foxp3, is a hallmark of mature Tregs expansion (28). We found that para-aortic lymph nodes (PALNs) in Cd38−/− matings were significantly larger (198 ± 60.4%) and contained more CD4+ cells than those in wild-type matings at 3.5 dpc. The numbers of Foxp3+ T cells in PALNs in Cd38−/− and wild-type matings were not different at 3.5 dpc and 6.5 dpc (Fig. S3). However, the percentage of CD4+Foxp3+ T cells in the PALNs from Cd38−/− matings was significantly smaller than that from wild-type matings at both 3.5 (Fig. 2E) and 6.5 dpc (Fig. 2F), indicating that sCD38 induced Foxp3+ Treg-mediated T-cell suppression. This is consistent with previous reports (29, 30) that Tregs are able to suppress effector T cells and thereby cause an expansion of CD4+ cells in the presence of a decreased proportion of Tregs. Likewise, the percentage of CD4+Foxp3+ T cells in the uterus in Cd38−/− matings was also significantly less than that from wild-type matings at 3.5 dpc (Fig. 2G). By comparison, syngeneic matings of C57BL/6 females with wild-type and Cd38−/− males showed no differences in resorption rates, total number of implantation sites, viable implantation sites, and CD4+Foxp3+ T-cell population (Fig. S4). In addition, Cd38−/− females mated with C57BL/6 wild-type and Cd38−/− males showed no difference in fetal loss, indicating that a CD38 deficiency in maternal and/or placental tissue does not affect fetal development, at least in syngeneic pregnancy (Fig. S4). Thus, by inducing Foxp3+ Tregs in females, seminal sCD38 contributes to a successful pregnancy by supporting fetomaternal tolerance.
sCD38 Induces Fetomaternal Tolerance Through Expression of tDCs and Foxp3+ Tregs.
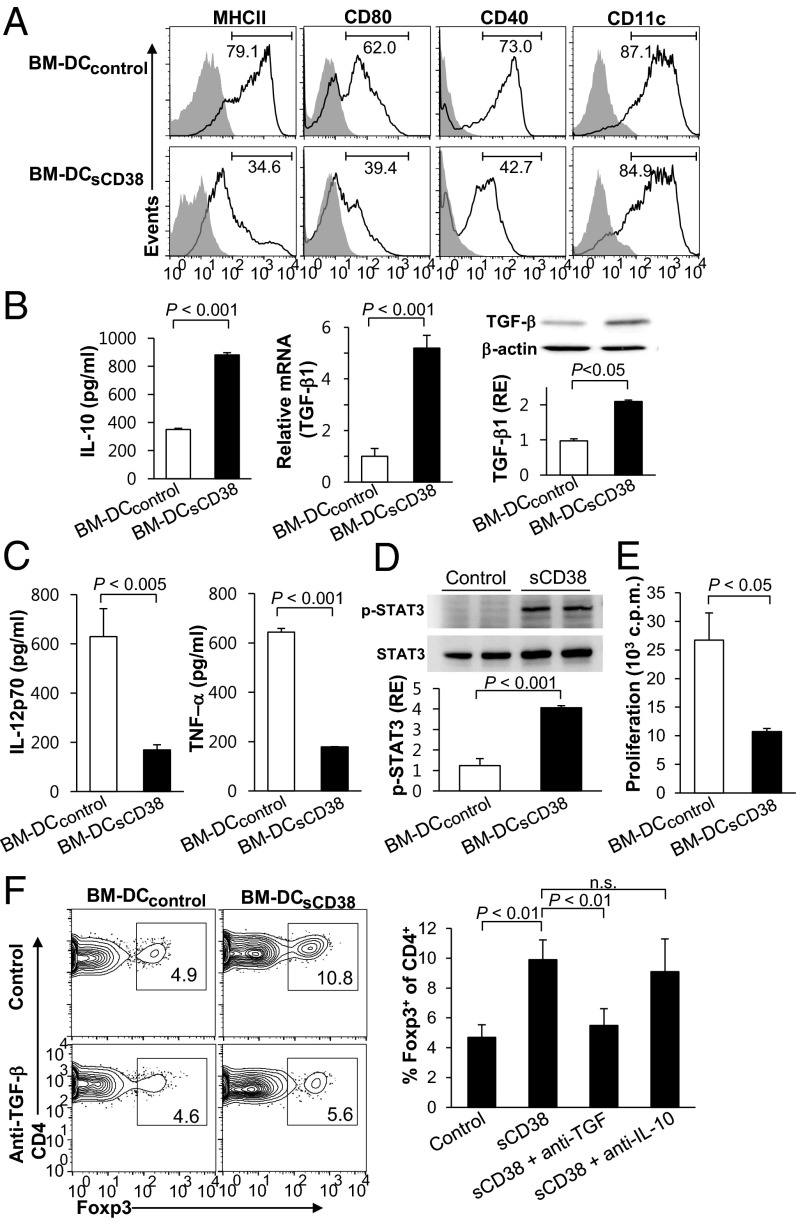
Because the properties of uterine DC in pregnant female mice are markedly influenced by seminal sCD38, we examined whether sCD38 induced tDC, which is characterized by expression of immature DC surface phenotype. To this end, we generated DCs by culturing bone marrow (BM) cells in vitro with GM-CSF in the absence (BM-DCcontrol) or presence (BM-DCsCD38) of recombinant sCD38 for 6 d. Upon LPS stimulation, BM-DCcontrol displayed a typical mature phenotype, including increased expression of MHC-II, CD80, and CD40; however, this was not the case for BM-DCsCD38 (Fig. 3A). Moreover, proteinase K-treated recombinant sCD38 was incapable of suppressing DC maturation (Fig. S5). These data suggest that sCD38 directly suppresses the maturation, causing DCs to maintain an immature phenotype. We also found that upon exposure to LPS, BM-DCsCD38 secreted a higher level of IL-10 (TH2 cytokine) and expressed higher levels of TGF-β mRNA and protein than did BM-DCcontrol (Fig. 3B). Conversely, BM-DCsCD38 inhibited the production of both IL-12p70 and TNF-α (TH1 cytokines) (Fig. 3C), indicating that sCD38 skewed DCs toward an anti-inflammatory phenotype. However, no distinct expression levels of indoleamine 2,3-dioxygenase (IDO) and BM-DCsCD38 and BM-DCcontrol were observed (Fig. S6). Previously, CD31 ligation has been reported to inhibit NF-κB signaling (24, 31). However, sCD38 did not affect NF-κB, Erk, and p38 signaling pathways (Fig. S7). Instead, sCD38 triggered the phosphorylation of a signal transducer and an activator of transcription 3 (STAT3) (Fig. 3D), a transcription factor involved in regulating tDCs (32, 33), indicating that sCD38 induced the differentiation of tDCs via STAT3-dependent pathways. Consistent with this mechanism, BM-DCsCD38 showed impaired activity in a mixed leukocyte reaction containing allogeneic CD4+ T cells compared with BM-DCcontrol (Fig. 3E).
Fig. 3.
sCD38-mediated induction of tDC. (A) DCs were generated from mouse BM cells by culture with GM-CSF for 6 d in the absence (BM-DCcontrol, line histogram) or presence (BM-DCsCD38, filled histogram) of 200 ng/mL sCD38 and then activated with 1 μg/mL LPS for 24 h to induce DC maturation; gray histogram, isotype-matched control. Data are representative of nine experiments. (B and C) The levels of cytokines of LPS-stimulated BM-DCs were measured in IL-10, IL12p70, and TNF-α ELISAs. TGF-β1 mRNA and protein were measured by real-time PCR and immunoblotting, respectively. (Right Bottom) Band intensity of TGF-β1 relative to that of actin. RE, relative expression. (D) Western blot analysis of phosphorylated and total STAT3 in BM-DCcontrol and BM-DCsCD38. (Bottom Panel) Relative band intensity (RE). Data of TGF-β and STAT3 represent the mean ± SD of three experiments. (E) LPS-stimulated BM-DCcontrol and BM-DCsCD38 (2 × 104) were cocultured with allogeneic CD4+ T cells (2 × 105), and proliferation was determined by [3H] thymidine incorporation. Data represent the mean ± SD of three independent experiments. (F) Representative flow cytometric analysis showing the CD4+Foxp3+ T cells in the mixed leukocyte reaction. OVA 323–339 peptide-stimulated BM-DCcontrol or BM-DCsCD38 were cocultured with OT-II CD4+CD25− T cells in the presence or absence of anti–IL-10 or anti–TGF-β mAbs for 5 d. Bar graph represents mean percentage of Foxp3+ among gated CD4+ cells. Bars, mean ± SD of three experiments.
Next, we investigated whether tDCs induced by sCD38 have the potential to induce Foxp3+ Tregs. We observed a substantial reduction in the percentage of Foxp3+ in CD4+ cells when sorted OT-II naïve T cells were cocultured with BM-DCcontrol (4.7 ± 0.8%) versus with BM-DCsCD38 (9.9 ± 1.3%) (Fig. 3F). Furthermore, the degree of induction of Foxp3+ Tregs by BM-DCsCD38 was lower in the presence of anti–TGF-β than that in the absence of anti–TGF-β or anti–IL-10 mAbs. When anti–IL-10 was present instead of TGF-β, the induction of Foxp3+ Tregs by BM-DCsCD38 was not altered. These results indicate that TGF-β released by BM-DCsCD38 was primarily responsible for the induction of Foxp3+ Tregs. Thus, BM-DCsCD38 show features of tDCs, as BM-DCsCD38 are able to induce the differentiation of Tregs, which are crucial for fetomaternal tolerance.
sCD38 Induces the Differentiation of tDCs Through a CD31 Independent Pathway.
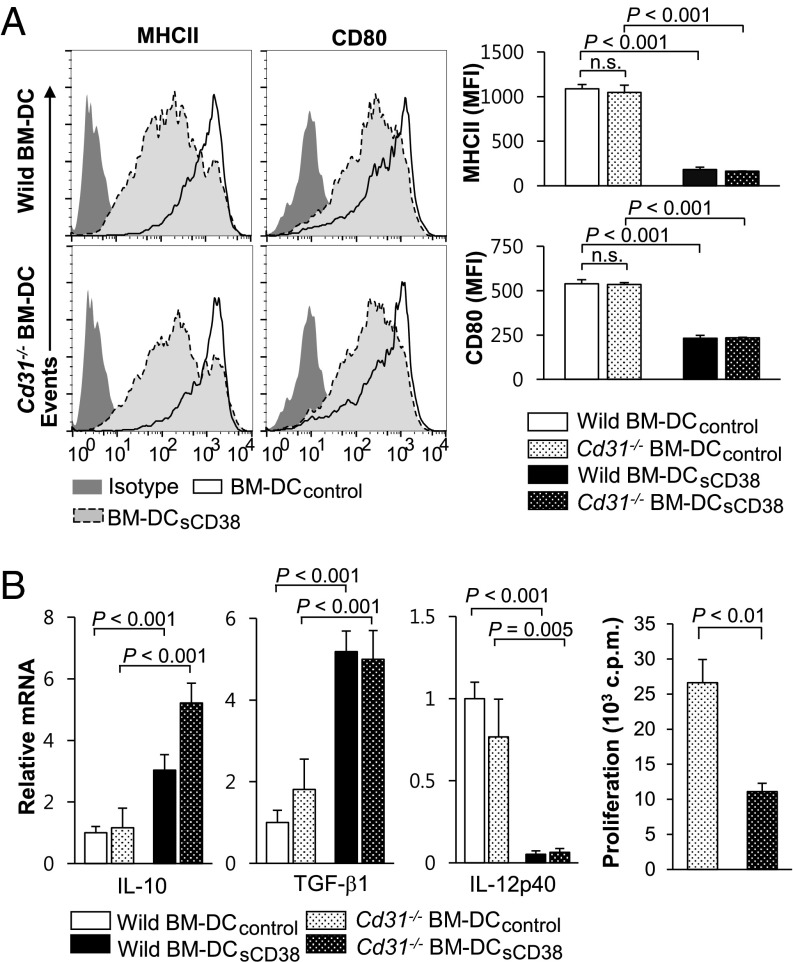
CD31 has been reported to be a counterreceptor for CD38, and CD38-mediated negative regulation of TLR4 signaling was undertaken by CD31 (24, 25). Thus, we evaluated the possibility of CD31 involvement in the sCD38 effects on sustaining immature phenotypes of BM-DC as seen in Fig. 3A. Although a recent study suggested that DCs lacking CD31 favor immunogenic potential (31), our data showed that LPS-stimulated wild-type BM-DCsCD38 and Cd31−/− BM-DCsCD38 expressed similar levels of MHC-II and CD80 (Fig. 4A). Similarly, upon exposure to LPS even without CD31 expression, mRNA levels of IL-10 and TGF-β were higher, whereas the mRNA level of IL-12p40 was lower in sCD38-treated BM-DCsCD38 than those in BM-DCcontrol (Fig. 4B). Regardless of CD31 expression, BM-DCsCD38 maintained weaker allogeneic T-cell proliferative responses than BM-DCcontrol (Fig. 4B). Collectively, these data show that CD31 is not involved in sCD38-driven differentiation of tDCs.
Fig. 4.
CD31 is not involved in sCD38-mediated induction of tDC. (A) BM-DCs were produced from BM cells of wild-type and Cd31−/− mice by culture in the medium containing GM-CSF for 6 d in the presence (BM-DCsCD38) or absence of sCD38 (BM-DCcontrol) and stimulated with 1 μg/mL LPS for 24 h. Expression of MHC-II and CD80 on BM-DCcontrol and BM-DCsCD38 derived from wild-type and Cd31−/− mice was plotted as line histogram (Left panels). MFIs of MHC-II and CD80 were plotted as bar graphs (Right panels). Data represent the mean ± SD of 15 experiments. (B) The mRNA levels of IL-10, TGF-β1, and IL-12p40 in LPS-stimulated BM-DCcontrol and BM-DCsCD38 derived from Cd31−/− mice. Allogeneic T-cell proliferative responses were measured by [3H]thymidine incorporation after coculture with LPS-stimulated BM-DCcontrol and BM-DCsCD38 derived from Cd31−/− mice. Data are expressed as the mean ± SD of four experiments.
sCD38 Reduces Stress-Challenged Fetal Resorption by Expansion of Foxp3+ Tregs.
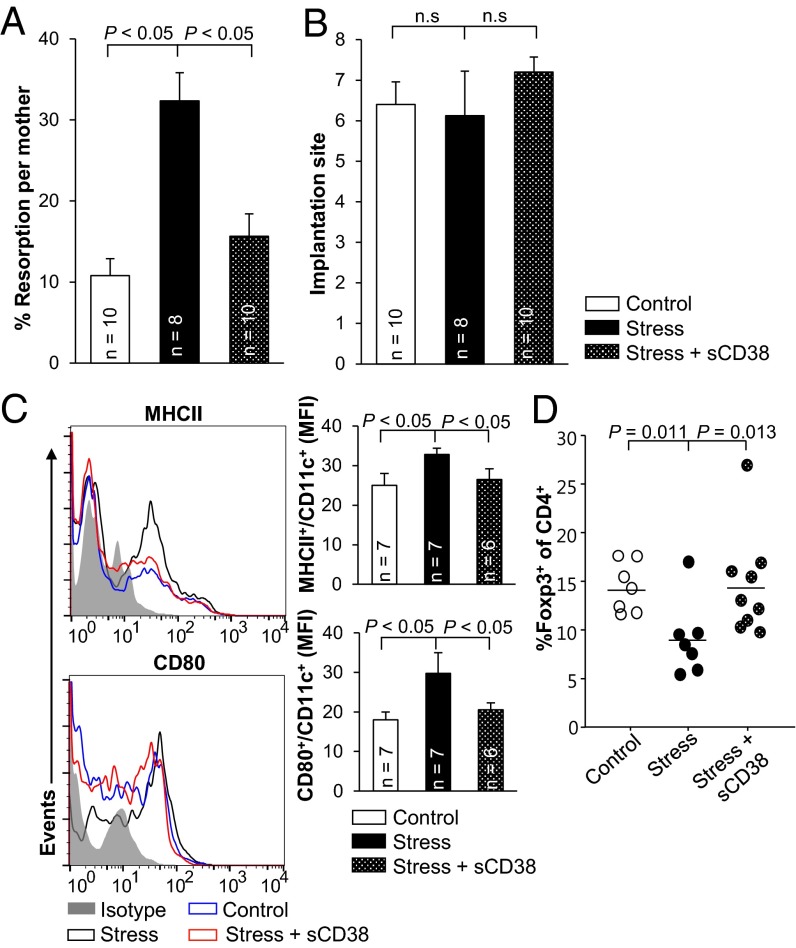
Because sCD38 induces tDCs and then Foxp3+ Tregs, we examined whether intravaginal injection of sCD38 maintained pregnancy in abortion-prone mice under sonic stress (34). Direct injection of sCD38 to the vagina of stress-challenged CBA/J female mice mated with DBA/2J male mice greatly reduced the incidence of fetal resorption (Fig. 5A), although the number of fetal implantations was unchanged (Fig. 5B). To examine the inhibitory effects of sCD38 on the aberrant maturation of uterine CD11c+ cells induced by sonic stress, we characterized the phenotype of CD11c+ cells. Sonic stress induced the maturation of uterine CD11c+ cells with increased levels of MHC-II and CD80 in pregnant CBA/J females mated with DBA/2J males, compared with those in control mice. Administration of sCD38 decreased the number of phenotypically matured uterine CD11c+ cells in stress-challenged mice (Fig. 5C). In addition, the PALNs of female mice injected with sCD38 contained a higher proportion of Foxp3+ cells at 6.5. dpc than Foxp3+ cells of control females (Fig. 5D). Taken together, the results of the present study show that sCD38 causes induction of tDCs and Foxp3+ cells and thereby protects embryos from maternal immune rejection.
Fig. 5.
Direct injection of sCD38 into the vagina protects fetuses from abortion. sCD38 or PBS was injected to the pregnant CBA/J females mated with DBA/2J males intravaginally at 1.5 and 3.5 dpc. Sonic stress was applied from 2.5 dpc until sacrifice. (A) Resorption rates and (B) total number of implantation sites were measured at 12.5 dpc in the mated female mice exposed to sound stress with or without sCD38. Bars, mean ± SEM. (C) Phenotypic analysis of CD11c+ uterine DCs from nonstressed mating mice injected with PBS (Control), stressed mating mice injected with PBS (Stress), and stressed mating mice injected with sCD38 (Stress + sCD38) at 6.5 dpc. Mean fluorescent intensities (MFIs) of MHC-II and CD80 were plotted as bar graphs (Right panels). Bars, mean ± SD. (D) Percentages of CD4+Foxp3+ T cells in the PALNs of Control, Stress, and Stress + sCD38 were calculated by flow cytometry analysis at 6.5 dpc.
Discussion
The role of Tregs in fetomaternal tolerance is essential for normal pregnancy. Studies have shown that the adoptive transfer of Tregs prevented fetal loss in the embryo resorption model (DBA/2J-mated CBA/J female), and the depletion of Tregs led to high levels of embryo resorption in the allogeneic pregnancy model (35, 36). Foxp3+ Tregs are generated in the thymus and periphery from naïve CD4+ T cells (5). Interestingly, recent studies reported that extrathymic Foxp3+ Tregs play a pivotal role in fetomaternal tolerance and that those memorized fetal-specific Tregs rapidly expanded to induce tolerance during the subsequent pregnancy (5, 6). Even in the absence of fertilization, exposure of the female genital tract to seminal plasma induced tolerance to the paternal alloantigen via expansion of Foxp3+ Tregs (8, 37). Seminal plasma confers DCs with immunoregulatory potential, and these DCs are crucial for embryo implantation and the generation of Tregs (9, 10, 34, 38). Seminal fluid contains high levels of TGF-β (39, 40) and prostaglandins (41), both of which have been proposed to contribute to the induction of Foxp3+ Tregs and/or the differentiation of tDCs (9, 42). However, it has been suggested that other additional molecules are involved in the induction of tDCs (9). In the present study, we demonstrated that sCD38-treated BM-DCs transform into tDCs, which in turn promoted the generation of Foxp3+ Tregs. A deficiency of sCD38 in semen led to the immunogenic maturation of uterine DCs and the diminished proportion of Foxp3+ Tregs, resulting in fetal loss (Fig. 2). In addition, the administration of sCD38 restored tolerance through the expansion of Foxp3+ Tregs and prevented fetal loss.
Regarding fetomaternal tolerance induced by sCD38-mediated Foxp3+ Treg expansion, it is likely to be developed via numerous immunomodulatory mechanisms. The expression of MHC-II and costimulatory molecules, which are essential for tolerogenic ability of DC, was low in DCsCD38. In addition, DCsCD38 secrete IL-10, which inhibits DC maturation and reduces their capacity to stimulate CD4+ T-cell–mediated immunity. DCsCD38 produce TGF-β, which induces the differentiation of naïve T cells to Foxp3+ Tregs; DCsCD38 inhibit the production of TH1 cytokines, such as IL-12p70 and TNF-α (Fig. 4).
Although the proportion of Foxp3+ Tregs in the CD4+ T cell was reduced in Cd38−/− matings (Fig. 2 E and F), the number of Foxp3+ Tregs was not reduced (Fig. S2). The explanation for this finding would be that increased mature DCs in Cd38−/− matings (Fig. 2D) induce naïve T cells to produce IL-2, a paracrine Tregs growth factor. Thus, tDCsCD38 may positively regulate the Treg-effector T-cell balance in favor of Tregs through inhibition of T-cell–mediated immunity and induction of Foxp3+ Treg differentiation, suggesting that the percentage of Foxp3+ Tregs may be more important than the sheer number of Foxp3+ Tregs in fetomaternal tolerance. Consistent with reports (36) that the contribution of Tregs at the time of early pregnancy impacts fetal survival later on in gestation, our results showed that the Foxp3+ Tregs proportion was higher in wild-type matings than in Cd38−/− matings at 3.5 dpc, and this difference persisted through 6.5 dpc. These findings suggest that a consistently high proportion of Foxp3+ Tregs, induced by sCD38 during the early phase of pregnancy, may play an important role in fetomaternal tolerance.
Previous studies have reported that maternal inflammation decreased Foxp3+ Treg accumulation, which caused fetal loss, and that the decidua of the surviving fetus exhibited abnormal spiral artery modification (5). Abnormal maternal inflammation restricted the growth of surviving fetuses. Abnormal spiral artery modification in the decidua was associated with complications of pregnancy, including preeclampsia, placental abruption, preterm, and fetal loss (43, 44). In addition, immunosuppressive function of maternal Tregs in pregnant mice was fetal antigen-specific, and depletion of Tregs led to a reduction in body weight of the surviving embryos (6, 45). In our histological analysis of surviving fetuses from Cd38−/− matings, although there was some variability of individual fetuses, hemorrhage and immune cell infiltration could be observed within the decidua. In some cases, spiral arteries clustering in decidua were also observed (Fig. S8A). Moreover, the number of Foxp3+ cells within all layers of decidua from Cd38−/− matings was reduced compared with that from wild-type matings (1.57 ± 0.64 vs. 3.36 ± 1.2) (Fig. S8B). Consistent with histological evaluations, the mean weight of fetuses was reduced more in Cd38−/− matings than in wild-type matings (Fig. S8C). These observations indicate that Cd38−/− matings may show other complications of pregnancy, including low-birth-weight babies, besides fetal loss.
Seminal plasma interacts not only with epithelial cells of the vagina and cervix but also with immune cells such as DCs and causes the recruitment of DCs and macrophages into the endometrium (46, 47). The seminal antigen is processed and displayed on class II MHC cells and transported to draining lymph nodes, activating CD4+ T cells and promoting the acquisition of a regulatory phenotype. Although the present study shows that allogeneic mating with sCD38-deficient mice causes maturation of uterine DCs and results in fetal resorption, this may be due to multiple mechanisms exerted by sCD38, for instance, on other immune cells such as macrophages. Therefore, understanding the mechanisms by which sCD38 affects immune responses is of utmost importance.
Clement et al. (31) recently showed that CD31 is a coinhibitory receptor in the development of tDCs, thus supporting their previous finding that the lack of T-cell CD31 signaling increases autoimmune responses (21–23). The disruption of CD31 signaling favored immunogenic maturation. Also, ligation of CD31 using a homotypic CD31 peptide reduced the expression of costimulatory molecules and proinflammatory cytokines and increased the expression of anti-inflammatory cytokines (31). The role that CD31 plays in inhibitory signaling in effector-adaptive immune cells was explained through the action of Src homology 2 domain tyrosine phosphatases SHP-1/SHP-2, which are recruited by its cytoplasmic immunoreceptor tyrosine-based inhibitory motif (19). The CD31 peptide can also elicit the signaling pathway (48). Contrary to this report, our data showed that the extent of maturation of Cd31−/− and wild-type BM-DCs upon stimulation with LPS was similar (Fig. 4A). Moreover, an inhibitory signaling on DC maturation by a homotypic CD31 peptide was observed in Cd31−/− BM-DCs (Fig. S9). These results suggest that the action of sCD38 in driving the differentiation of tDCs is through an as-yet-unidentified target molecule(s) other than CD31.
sCD38 was first found in the amniotic fluid, suggesting that sCD38 levels likely increase under certain conditions (49). sCD38 is present at extraordinarily high concentrations in seminal plasma, but its levels were below the detection limit of our ELISA (250 pg/mL) in normal serum. The immunoregulatory role of sCD38 may provide crucial insights into how insemination regulates maternal immunity to allow successful pregnancies. Furthermore, this knowledge of the immunoregulatory role of sCD38 is important for understanding the function of sCD38 under pathogenic conditions. Our finding that sCD38 is an important immune regulator for stimulating Treg cells may inform studies to develop novel treatments for recurrent miscarriage with an immune etiology.
Materials and Methods
The Institutional Review Board approved the collection of semen from normal and vasectomised volunteers. sCD38 was detected by Western blotting, an in-gel activity assay, CD38 affinity chromatography, and ELISA. Pregnancy in females mated with wild-type or Cd38−/− male mice was determined by checking the copulation plug (at 0.5 dpc). Embryo tissue sections from pregnant mice were stained with hematoxylin and eosin. Uterine DCs and Foxp3+ Tregs from pregnant mice were analyzed at 3.5 or 6.5 dpc. To assess whether sCD38 induced tDC, BM cells were cultured in differentiation medium in the presence or absence of sCD38. A mouse model of stress-induced fetal abortion was used to evaluate the protective effects of sCD38. Details on materials, assays, and experimental protocol are available in SI Materials and Methods.
All data were analyzed using the Student’s t test or ANOVA as appropriate. A P value < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Young June Kim, Dr. Donghee Kim, and Mr. Chansu Park for critical reading of the manuscript. This study was supported by National Research Foundation Grant 2012R1A3A2026453, funded by the Korean government (Ministry of Science, ICT & Future Planning) (to U.-H.K.), and a BK21 grant recipient at Chonbuk National University (to B.-J.K.)
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413493112/-/DCSupplemental.
References
- 1.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345(19):1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 2.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med. 2013;19(5):548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 3.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol. 2013;13(1):23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 4.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 5.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150(1):29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490(7418):102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito S, Sasaki Y, Sakai M. CD4(+)CD25 high regulatory T cells in human pregnancy. J Reprod Immunol. 2005;65(2):111–120. doi: 10.1016/j.jri.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Robertson SA, et al. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80(5):1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remes Lenicov F, et al. Semen promotes the differentiation of tolerogenic dendritic cells. J Immunol. 2012;189(10):4777–4786. doi: 10.4049/jimmunol.1202089. [DOI] [PubMed] [Google Scholar]
- 10.Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. Am J Reprod Immunol. 2013;69(4):315–330. doi: 10.1111/aji.12107. [DOI] [PubMed] [Google Scholar]
- 11.Lee HC. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J Biol Chem. 2012;287(38):31633–31640. doi: 10.1074/jbc.R112.349464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song EK, et al. NAADP mediates insulin-stimulated glucose uptake and insulin sensitization by PPARγ in adipocytes. Cell Reports. 2012;2(6):1607–1619. doi: 10.1016/j.celrep.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Park KH, et al. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci Signal. 2011;4(173):ra31. doi: 10.1126/scisignal.2001595. [DOI] [PubMed] [Google Scholar]
- 14.Mallone R, et al. Characterization of a CD38-like 78-kilodalton soluble protein released from B cell lines derived from patients with X-linked agammaglobulinemia. J Clin Invest. 1998;101(12):2821–2830. doi: 10.1172/JCI1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zielinska W, Barata H, Chini EN. Metabolism of cyclic ADP-ribose: Zinc is an endogenous modulator of the cyclase/NAD glycohydrolase ratio of a CD38-like enzyme from human seminal fluid. Life Sci. 2004;74(14):1781–1790. doi: 10.1016/j.lfs.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Kramer G, et al. High expression of a CD38-like molecule in normal prostatic epithelium and its differential loss in benign and malignant disease. J Urol. 1995;154(5):1636–1641. [PubMed] [Google Scholar]
- 17.Horenstein AL, Stockinger H, Imhof BA, Malavasi F. CD38 binding to human myeloid cells is mediated by mouse and human CD31. Biochem J. 1998;330(Pt 3):1129–1135. doi: 10.1042/bj3301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deaglio S, et al. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. 1998;160(1):395–402. [PubMed] [Google Scholar]
- 19.Privratsky JR, Newman DK, Newman PJ. PECAM-1: Conflicts of interest in inflammation. Life Sci. 2010;87(3-4):69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, et al. Ig gene-like molecule CD31 plays a nonredundant role in the regulation of T-cell immunity and tolerance. Proc Natl Acad Sci USA. 2010;107(45):19461–19466. doi: 10.1073/pnas.1011748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tada Y, et al. Acceleration of the onset of collagen-induced arthritis by a deficiency of platelet endothelial cell adhesion molecule 1. Arthritis Rheum. 2003;48(11):3280–3290. doi: 10.1002/art.11268. [DOI] [PubMed] [Google Scholar]
- 22.Graesser D, et al. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109(3):383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maas M, et al. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288(1):H159–H164. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- 24.Rui Y, et al. PECAM-1 ligation negatively regulates TLR4 signaling in macrophages. J Immunol. 2007;179(11):7344–7351. doi: 10.4049/jimmunol.179.11.7344. [DOI] [PubMed] [Google Scholar]
- 25.Fedele G, et al. CD38 is expressed on human mature monocyte-derived dendritic cells and is functionally involved in CD83 expression and IL-12 induction. Eur J Immunol. 2004;34(5):1342–1350. doi: 10.1002/eji.200324728. [DOI] [PubMed] [Google Scholar]
- 26.Lund FE, et al. Signaling through murine CD38 is impaired in antigen receptor-unresponsive B cells. Eur J Immunol. 1995;25(5):1338–1345. doi: 10.1002/eji.1830250531. [DOI] [PubMed] [Google Scholar]
- 27.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7(8):610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 28.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of Treg-mediated Tcell suppression. Front Immunol. 2012;3(51):1–20. doi: 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Clement M, et al. CD31 is a key coinhibitory receptor in the development of immunogenic dendritic cells. Proc Natl Acad Sci USA. 2014;111(12):E1101–E1110. doi: 10.1073/pnas.1314505111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilarregui JM, et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10(9):981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 34.Blois SM, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13(12):1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 35.Zenclussen AC, et al. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol. 2006;36(1):82–94. doi: 10.1002/eji.200535428. [DOI] [PubMed] [Google Scholar]
- 36.Shima T, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85(2):121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD. Activating T regulatory cells for tolerance in early pregnancy—The contribution of seminal fluid. J Reprod Immunol. 2009;83(1-2):109–116. doi: 10.1016/j.jri.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Plaks V, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118(12):3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loras B, et al. Seminal transforming growth factor-beta in normal and infertile men. Hum Reprod. 1999;14(6):1534–1539. doi: 10.1093/humrep/14.6.1534. [DOI] [PubMed] [Google Scholar]
- 40.Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007;22(11):2928–2935. doi: 10.1093/humrep/dem281. [DOI] [PubMed] [Google Scholar]
- 41.Templeton AA, Cooper I, Kelly RW. Prostaglandin concentrations in the semen of fertile men. J Reprod Fertil. 1978;52(1):147–150. doi: 10.1530/jrf.0.0520147. [DOI] [PubMed] [Google Scholar]
- 42.Kaliński P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159(1):28–35. [PubMed] [Google Scholar]
- 43.Renaud SJ, et al. Spontaneous pregnancy loss mediated by abnormal maternal inflammation in rats is linked to deficient uteroplacental perfusion. J Immunol. 2011;186(3):1799–1808. doi: 10.4049/jimmunol.1002679. [DOI] [PubMed] [Google Scholar]
- 44.Avagliano L, Bulfamante GP, Morabito A, Marconi AM. Abnormal spiral artery remodelling in the decidual segment during pregnancy: From histology to clinical correlation. J Clin Pathol. 2011;64(12):1064–1068. doi: 10.1136/jclinpath-2011-200092. [DOI] [PubMed] [Google Scholar]
- 45.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci USA. 2010;107(20):9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 47.Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322(1):43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- 48.Fornasa G, et al. TCR stimulation drives cleavage and shedding of the ITIM receptor CD31. J Immunol. 2010;184(10):5485–5492. doi: 10.4049/jimmunol.0902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Funaro A, et al. Identification and characterization of an active soluble form of human CD38 in normal and pathological fluids. Int Immunol. 1996;8(11):1643–1650. doi: 10.1093/intimm/8.11.1643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.