Significance
Increased heart rate predicts all-cause mortality; however, the major causes for elevated basal heart rate values and annual changes in them have not been systematically studied, possibly because of the high individual variability involved. Our study addressed the question, “which physiological and psychological parameters determine basal pulse and its annual changes?,” in a cohort of 17,380 apparently healthy volunteers. This is, to the best of our knowledge, the first statistics-based search for the major interactions between physiological and psychological determinants of basal pulse and annual pulse changes; it indicates, perhaps not surprisingly, that consistent exposure to terror threats ignites fear-induced exacerbation of preexisting neuro-immune risks of all-cause mortality and proposes a set of risk-predicting parameters that may have translational value.
Keywords: pulse, terror, C-reactive protein, acetylcholinesterase, cholinergic status
Abstract
Recent international terror outbreaks notably involve long-term mental health risks to the exposed population, but whether physical health risks are also anticipated has remained unknown. Here, we report fear of terror-induced annual increases in resting heart rate (pulse), a notable risk factor of all-cause mortality. Partial least squares analysis based on 325 measured parameters successfully predicted annual pulse increases, inverse to the expected age-related pulse decline, in approximately 4.1% of a cohort of 17,380 apparently healthy active Israeli adults. Nonbiased hierarchical regression analysis among 27 of those parameters identified pertinent fear of terror combined with the inflammatory biomarker C-reactive protein as prominent coregulators of the observed annual pulse increases. In comparison, basal pulse primarily depended on general physiological parameters and reduced cholinergic control over anxiety and inflammation, together indicating that consistent exposure to terror threats ignites fear-induced exacerbation of preexisting neuro-immune risks of all-cause mortality.
Recent international terror outbreaks involve mass psychological trauma, leading to long-term mental health risks in the exposed population (1, 2). Fear-induced reactions involve cortical and limbic brain regions that together enhance threat-predictive sensory stimuli (3) by interacting with cholinergic signaling pathways (4) in the hippocampus (5), the central amygdala (6), and the prefrontal cortex, especially in adults (7). Imminent fear may even cause immediate cardiac death [e.g., after an earthquake (8)]. However, whether fear exposure elevates cardiac risks of death to otherwise healthy civilians, and if so, what are the causes of such risks, remains unknown.
Pulse is a promising modifiable predictor of cardiovascular and all-cause mortality. Elevated pulse associates with increased systemic inflammation and endothelial dysfunction in older adults (9) and predicts increased risk of death from ischemic heart disease (10). Changes in basal pulse and pulse variability are tightly associated with sudden cardiac death and all-cause mortality, also in asymptomatic men (11). Pulse reflects a complex trait, determined by multiple genetic, environmental, and other endogenous factors that play a substantial role in population variation (12). These include excessive inflammation, shown to associate with pulse increases (13), to be controlled by cholinergic imbalance (decreased vagal tone or increased sympathetic activity) (14), and to increase mortality (15). However, whether specific psychological factors determine the basal pulse and annual pulse changes in active adults is still unknown, perhaps because the intensity of psychological phenomena largely depends on external sources and is highly variable.
Although fear of terror (FOT) is universal, Israel has been exposed to the repeated stress of multiple wars and terror attacks for more than 60 y, with a major impact on the entire society (16). To approach the health risks involved in FOT, we therefore explored the parameters determining resting heart rate (pulse) and its annual change in the Israeli population.
Results
Fear-of-Terror and Inflammation Copredict Pulse Increases.
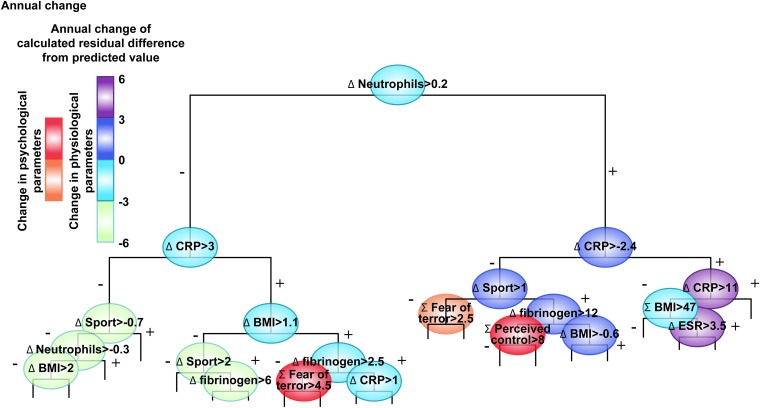
Average maximal pulse values tend to decrease with aging (17), such that pulse increases are the exception. To search for the key parameters involved, we screened a cohort of 17,380 apparently healthy working adult volunteers for predictors of elevated (residual) pulse. A total of 325 routinely and successively measured parameters (16) were included, spanning physiological parameters (including sports activity, smoking), inflammation biomarkers [e.g., C-reactive protein (CRP), fibrinogen], and psychological measures of anxiety, FOT, and perceived control over one’s life. A dynamic regression analysis tree (SI Materials and Methods) that included a validated selection of 27 of these components, each by itself and also as its change from baseline (annual difference), identified inflammatory profile, FOT, and perceived control over one’s life as prominent determinants of annual pulse increases (Fig. 1).
Fig. 1.
FOT-inducible pulse changes: Hierarchical plot of predictors of residual annual pulse change. An analysis regression tree was constructed of the residual score of annual pulse change [adjusted for age, sex, and body mass index (BMI) for 9,946 of the 17,380 cohort members (details in SI Materials and Methods)]. Branches were hierarchically organized, such that the higher is more important and each branch divides the rest of the cohort using the calculated cut-point value noted in the circle (left side = statement in parent circle confirmed). Circles are color-coded by the calculated effect on residual annual pulse change (increase or decrease; scale). Of the physiological, psychological, and medical parameters entered into the model, inflammation parameters, sport activity, and FOT and perceived control emerged as significant predictors. ESR, erythrocyte sedimentation rate.
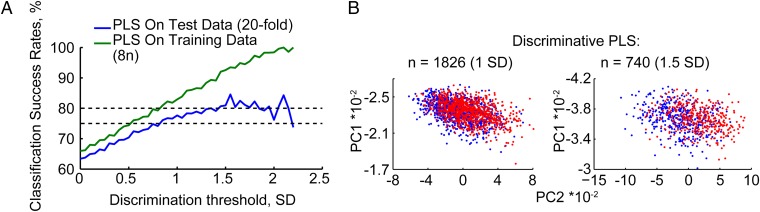
To classify the cohort members by the extent of annual change in their pulse, we performed an independent partial least squares (PLS) analysis on these 325 parameters (18). By reprocessing of the collected multivariate data and removing those variations from the descriptor variables that are not correlated to the property variables, such analyses enable reduced model complexity with preserved prediction ability. Classification success rate calculated for 95% of these volunteers (training data) with a 20-fold cross-validation (test data) predicted annual pulse changes >1.5 SD in approximately 8.2% of the cohort, yielding 81–85% accuracy of separation between individuals predicted to present declined or elevated pulse (Fig. 2A). Principal component separation classified participants with 1.5 SD more effectively than those with 1.0 SD (Fig. 2B).
Fig. 2.
PLS identified pulse changes. (A) PLS classification of pulse changes. Shown are classification success rates as a function of threshold for pulse changes on a training dataset of 95% of the volunteers (green) and a 20-fold cross-validation test dataset (blue) for the noted discrimination SD threshold values. (B) Discriminative classification results. Shown are example classified principal component values for pulse changes (up, red; down, blue) at 1 and 1.5 SD levels for the noted numbers of volunteers.
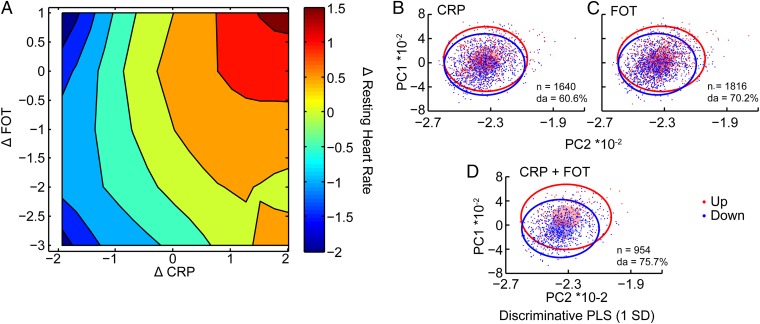
To interrogate potential interactions between the major relevant parameters, we plotted the increases in CRP (19) against FOT levels. Three-dimensional plots including pulse measures showed that participants who presented the largest annual increase of square root-transformed pulse (Fig. 3A, top right corner) showed synergistic association of elevated CRP and FOT scores. An inverse discriminative PLS-based separation of pulse values at 1 SD level classified those volunteers with increases in both CRP and FOT values more successfully than those with either CRP or FOT increases (Fig. 3 B–D), together supporting the notion that CRP and FOT interactions are prominent determinants of the observed pulse change.
Fig. 3.
FOT–CRP interactions affect pulse changes. (A) Coelevated FOT and CRP strongly correlate with pulse increases. Triple association of FOT, inflammation, and annual pulse change presents the differences between the observed and expected value for individuals with the same age, sex, and BMI. Annual pulse changes (color code) were square root transformed. Note the increasing tendency for elevated pulse (red) in those participants with coincreased FOT and CRP. (B–D) CRP and FOT changes coclassify volunteers with pulse variations. Volunteers with elevated or decreased pulse (1 SD) were classified by CRP (B), FOT (C), or CRP and FOT (D). Small translucent ellipsoids represent 0.5 × SD and outer circles 3 × SD for each group. To illustrate the true (eight-dimensional) separation between volunteers we present the discrimination ability (DA) with closer values to their own group mean than to the other group.
Basal Pulse Involves Inflammation and Cholinergic Parameters.
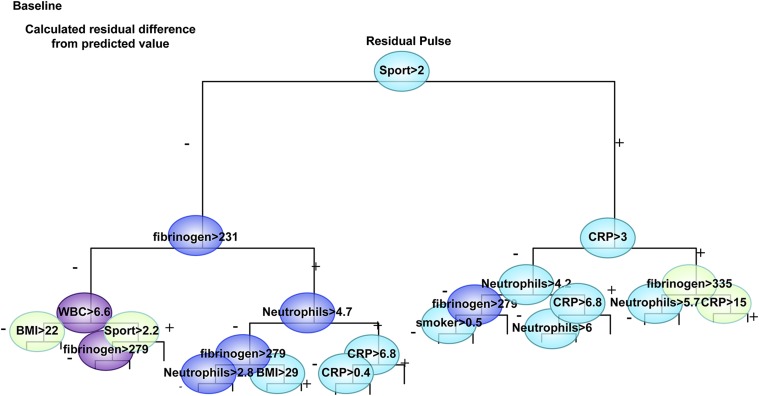
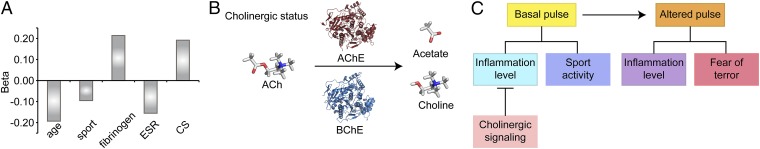
A nonbiased hierarchical regression tree analysis adjusted for age, sex, and BMI showed data-driven cutoff values of restrained inflammation (CRP < 3 mg/L and fibrinogen < 231 mg/dL) and sport activity (> 2 h/wk) as prominent determinants of resting pulse (Fig. 4). Neutrophil and leukocyte counts, smoking, and BMI > 22 kg/m2 represented the next set of predictors for elevated pulse. These discriminating cutoff values are identical to known cutoff values for reducing the risk of heart attack and stroke (20). In contrast, anxiety scores were absent from the regression of basal pulse values.
Fig. 4.
CRP and cholinergic interactions affect basal pulse values: Hierarchical plot of predictors of residual pulse. An analysis regression tree was constructed of the residual score of basal pulse (adjusted for age, sex, and BMI) for 17,380 cohort members, essentially as in Fig. 1. Of the physiological, psychological, and medical parameters entered into the model, inflammation parameters and sport activity but not FOT and perceived control emerged as significant predictors.
Given the impact of inflammation on pulse measurements, and because cholinergic signaling blocks inflammatory effects (14, 21), we have further applied a dedicated test for cholinergic signaling in a subset cohort (398 men and 214 women) of the study’s volunteers. Briefly, we measured serum cholinergic status, the total ability to hydrolyze acetylcholine that reflects cumulative acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities (22), and decreases in which reflect mortality risks after cardiac events (23). In a regression analysis of healthy adults, cholinergic status values corroborated the cholinergic antiinflammatory effect, such that participants with elevated inflammation (CRP > 3 mg/L) showed significantly higher total cholinergic status (P = 0.021 and P = 0.016 for men and women, respectively). Additionally, addition of cholinesterase activity to the model of pulse prediction yields a significant measure in men, but not women (Fig. 5A; R2 change = 0.03, F change = 3.6, P = 0.03), compatible with the sex-related nature of cholinergic status and inflammation values (24).
Fig. 5.
Cholinergic status (CS) and pulse. (A) A multiple linear regression model was constructed with pulse as the dependent variable and age, current smoking, vascular, anxiety, and inflammation risk factors as independent variables. The variables that remained significantly associated with pulse were age, sport, inflammatory markers, and cholinergic status. The addition of cholinergic status was significant (F change 3.56, P = 0.03). (B) Cholinergic status. Shown is a schematic representation of the cholinergic status test, which measures cumulative AChE and BChE capacities to hydrolyze acetylcholine into choline and acetate. (C) General scheme. Basal pulse values are primarily affected by sport and inflammation, which may be controlled by cholinergic signaling. Pulse changes, in comparison, are coaffected by inflammation and FOT.
We found major FOT contributions to annual pulse increases, and effects of inflammation and cholinergic measures on basal pulse values, together supporting the hypothesis that consistent exposure to terror threats potentiates malregulated cardiac physiology, increasing preexisting neuro-immune risks of all-cause mortality. Culturally embedded stress has been shown to effect timing of death (25); however, to the best of our knowledge, this is the first large-scale search for the physiological and psychological parameters that together affect pulse increases. Specifically, we find FOT to contribute to pulse changes that synergistically associate with elevated CRP levels. Figure 5 B and C present this working hypothesis schematically.
Cholinergic status values improved pulse predictions even when controlled for all other risk factors in men but not in women (24), compatible with the antiinflammatory effect of cholinergic signaling (14) as well as with previous reports of protective acetylcholine effects on ischemic myocardium (26), and with the pulse improvement effects of the AChE inhibitor pyridostigmine (27).
Given that information on pulse and its time-related changes is easy to obtain and follow up, our findings may be useful in identifying asymptomatic people at risk who could benefit from primary prevention measures, for example by vagal stimulation, antiinflammatory or anticholinesterase medications, or by physical activity, that limits increases in cardiovascular mortality risk (28). Future studies may focus on stress-inducible epigenetics (29), microRNA-mediated (21), and genetics changes (30). Identifying parameters whose changes may limit the FOT-inducible risk may be especially beneficial for stress-reactive individuals with moderate to high pulse rates.
Materials and Methods
The data used in this study were collected as part of the “TAMCIS: Tel Aviv Medical Center Inflammation Survey.” Study participants (N = 10,972 men and 6,408 women) were all apparently healthy employees, attending a center for periodic health examinations, for a routine health examination during the years 2002–2013.
Participants were recruited individually by an interviewer while waiting their turn for the clinical examination. They represent 91.5% of the examinees during this period. We systematically checked for nonresponse bias and found that nonparticipants did not differ from participants on any of the socio-demographic or the biomedical variables (details in SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank the study participants for volunteering these data. This study was supported by the Israel I-Core center of excellence for Mass Trauma (H.S.) and the Israel Science Foundation (S.T.). S.S.-T. was supported by Eshkol and The Edmond and Lily Safra Center of Brain Science (ELSC) postdoctoral fellowships; and N.Y. and N.W. were supported by ELSC and Einstein predoctoral fellowships, respectively.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 1248.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418264112/-/DCSupplemental.
References
- 1.Whalley MG, Brewin CR. Mental health following terrorist attacks. Br J Psychiatry. 2007;190:94–96. doi: 10.1192/bjp.bp.106.026427. [DOI] [PubMed] [Google Scholar]
- 2.Ghuman SJ, Brackbill RM, Stellman SD, Farfel MR, Cone JE. Unmet mental health care need 10-11 years after the 9/11 terrorist attacks: 2011-2012 results from the World Trade Center Health Registry. BMC Public Health. 2014;14:491. doi: 10.1186/1471-2458-14-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kass MD, Rosenthal MC, Pottackal J, McGann JP. Fear learning enhances neural responses to threat-predictive sensory stimuli. Science. 2013;342(6164):1389–1392. doi: 10.1126/science.1244916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovett-Barron M, et al. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343(6173):857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viviani D, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333(6038):104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS, Morrison JH. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413–419. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- 9.Nanchen D, et al. PROSPER Group Resting heart rate and incident heart failure and cardiovascular mortality in older adults: Role of inflammation and endothelial dysfunction: The PROSPER study. Eur J Heart Fail. 2013;15(5):581–588. doi: 10.1093/eurjhf/hfs195. [DOI] [PubMed] [Google Scholar]
- 10.Fox K, et al. Heart Rate Working Group Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 11.Jouven X, et al. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352(19):1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 12.Eijgelsheim M, et al. Genome-wide association analysis identifies multiple loci related to resting heart rate. Hum Mol Genet. 2010;19(19):3885–3894. doi: 10.1093/hmg/ddq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sajadieh A, et al. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25(5):363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Tracey KJ. Understanding immunity requires more than immunology. Nat Immunol. 2010;11(7):561–564. doi: 10.1038/ni0710-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brook RD, Julius S. Autonomic imbalance, hypertension, and cardiovascular risk. Am J Hypertens. 2000;13(6 Pt 2):112S–122S. doi: 10.1016/s0895-7061(00)00228-4. [DOI] [PubMed] [Google Scholar]
- 16.Melamed S, Shirom A, Toker S, Berliner S, Shapira I. Association of fear of terror with low-grade inflammation among apparently healthy employed adults. Psychosom Med. 2004;66(4):484–491. doi: 10.1097/01.psy.0000130963.52755.b9. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson L, et al. Using chemometrics for navigating in the large data sets of genomics, proteomics, and metabonomics (gpm) Anal Bioanal Chem. 2004;380(3):419–429. doi: 10.1007/s00216-004-2783-y. [DOI] [PubMed] [Google Scholar]
- 19.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 21.Shaked I, et al. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity. 2009;31(6):965–973. doi: 10.1016/j.immuni.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Loewenstein-Lichtenstein Y, et al. Genetic predisposition to adverse consequences of anti-cholinesterases in ‘atypical’ BCHE carriers. Nat Med. 1995;1(10):1082–1085. doi: 10.1038/nm1095-1082. [DOI] [PubMed] [Google Scholar]
- 23.Arbel Y, et al. Decline in serum cholinesterase activities predicts 2-year major adverse cardiac events. Mol Med. 2014;20(1):38–45. doi: 10.2119/molmed.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sklan EH, et al. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in Health, Risk Factors, Exercise Training, and Genetics study. Proc Natl Acad Sci USA. 2004;101(15):5512–5517. doi: 10.1073/pnas.0307659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franzke CW, et al. Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMs. EMBO J. 2002;21(19):5026–5035. doi: 10.1093/emboj/cdf532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ando M, et al. Efferent vagal nerve stimulation protects heart against ischemia-induced arrhythmias by preserving connexin43 protein. Circulation. 2005;112(2):164–170. doi: 10.1161/CIRCULATIONAHA.104.525493. [DOI] [PubMed] [Google Scholar]
- 27.Dewland TA, Androne AS, Lee FA, Lampert RJ, Katz SD. Effect of acetylcholinesterase inhibition with pyridostigmine on cardiac parasympathetic function in sedentary adults and trained athletes. Am J Physiol Heart Circ Physiol. 2007;293(1):H86–H92. doi: 10.1152/ajpheart.01339.2006. [DOI] [PubMed] [Google Scholar]
- 28.Nauman J, Janszky I, Vatten LJ, Wisløff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA. 2011;306(23):2579–2587. doi: 10.1001/jama.2011.1826. [DOI] [PubMed] [Google Scholar]
- 29.Sailaja BS, Cohen-Carmon D, Zimmerman G, Soreq H, Meshorer E. Stress-induced epigenetic transcriptional memory of acetylcholinesterase by HDAC4. Proc Natl Acad Sci USA. 2012;109(52):E3687–E3695. doi: 10.1073/pnas.1209990110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanin G, et al. Competing targets of microRNA-608 affect anxiety and hypertension. Hum Mol Genet. 2014;23(17):4569–4580. doi: 10.1093/hmg/ddu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.