Significance
Insect wing movements must be both precise and fast. This requirement is especially challenging in smaller insects whose flapping frequencies exceed 100 Hz, because the nervous system cannot exercise stroke-by-stroke control at such rates. In flies, the hind wings have evolved into halteres, gyroscopic sense organs that oscillate exactly antiphase to wings. We show that wing–wing and wing–haltere coordination at high frequencies is mediated by passive biomechanical linkages in thorax. This system requires a clutch mechanism in the wing hinge to independently engage each wing with the vibrating thorax. Once the wings are engaged, the gearbox modulates the amplitude of each wing. Thus, the force transmission mechanism from thorax to wings in flies bears remarkable similarity to automobile transmission systems.
Keywords: insect thorax, halteres, insect wings, wing hinge, wing clutch
Abstract
The spectacular success and diversification of insects rests critically on two major evolutionary adaptations. First, the evolution of flight, which enhanced the ability of insects to colonize novel ecological habitats, evade predators, or hunt prey; and second, the miniaturization of their body size, which profoundly influenced all aspects of their biology from development to behavior. However, miniaturization imposes steep demands on the flight system because smaller insects must flap their wings at higher frequencies to generate sufficient aerodynamic forces to stay aloft; it also poses challenges to the sensorimotor system because precise control of wing kinematics and body trajectories requires fast sensory feedback. These tradeoffs are best studied in Dipteran flies in which rapid mechanosensory feedback to wing motor system is provided by halteres, reduced hind wings that evolved into gyroscopic sensors. Halteres oscillate at the same frequency as and precisely antiphase to the wings; they detect body rotations during flight, thus providing feedback that is essential for controlling wing motion during aerial maneuvers. Although tight phase synchrony between halteres and wings is essential for providing proper timing cues, the mechanisms underlying this coordination are not well understood. Here, we identify specific mechanical linkages within the thorax that passively mediate both wing–wing and wing–haltere phase synchronization. We demonstrate that the wing hinge must possess a clutch system that enables flies to independently engage or disengage each wing from the mechanically linked thorax. In concert with a previously described gearbox located within the wing hinge, the clutch system enables independent control of each wing. These biomechanical features are essential for flight control in flies.
From giant Atlas moths (wingspan ∼30 cm) to microscopic wasps (wingspan ∼400 μm) (1), flying insects span nearly three orders of magnitude in body size. Smaller insects typically flap their wings at frequencies that often exceed 100 Hz, thereby limiting the ability of their nervous system to exercise stroke-to-stroke nervous control (2). The insect musculoskeletal system has evolved several adaptations that enable high wing-beat frequencies. Key among these adaptations is the evolution of specialized myogenic or asynchronous flight muscles in combination with the indirect flight muscle (IFM) architecture in insects of the order Diptera, Coleoptera, some Hymenoptera, and Hemiptera (Fig. 1A). Asynchronous muscles are stretch activated, which means that they are primarily activated by externally imposed stretches due to thoracic deformation (3), although periodic neural stimulation is required to maintain the calcium levels for muscle contractility; thus, cycle-by-cycle activation of their motor neurons is not necessary in these muscles (4). Contraction of dorsoventral muscles causes extension of the antagonistic dorsolongitudinal muscles and vice versa, thereby setting up resonant oscillations of the thoracic cavity, which are then translated via a complex wing hinge into large-amplitude wing movements (3, 5–8). The subtler alterations in wing kinematics are actuated by separate sets of steering muscles controlled by direct input from motor neurons within the thoracic ganglia (4, 9).
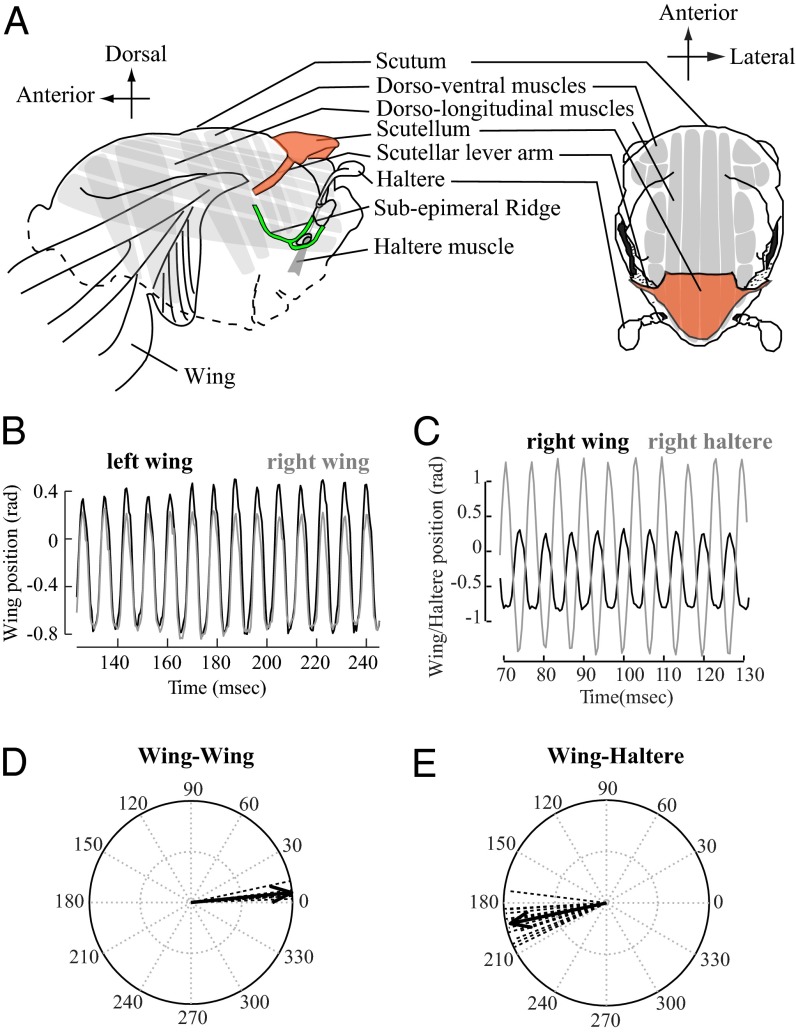
Fig. 1.
Wings and halteres are precisely coordinated at high frequencies ≥100 Hz. (A) Diagram of Dipteran thorax in lateral and dorsal view. (B) The right (gray) and the left (black) wings move in phase with each other. (C) The wing (black) and the ipsilateral haltere (gray) move antiphase to each other. (D) Vector strength representation of control data for wing–wing phase (tethered flies; n = 6; mean phase angle ϕ = 5.63°, vector length r = 0.9965; P < 0.001; nonparametric Moore’s test for uniformity). Dotted lines indicate the mean vector (∼20 wing strokes) for individual insects, and solid line indicates mean for the treatment. (E) Vector strength representation of control data of wing–haltere phase (tethered flies; n = 12; ϕ = 192.14°; r = 0.9572; P < 0.001; right and left side data pooled).
The kinematic changes are mediated via a complex wing hinge (4), but the function and composition of the hinge remains quite unclear, especially in its ability to mediate rapid wing movement. Moreover, faster wing movements require rapid sensory motor integration by the insect nervous system. The hind wings of Diptera have evolved into a pair of mechanosensory halteres that detect gyroscopic forces during flight (10–13). The rapid feedback from halteres is essential for flies to sense and control self-rotations during complex aerobatic maneuvers (13–15). In the majority of flies, the bilateral wings move in-phase, whereas halteres move antiphase relative to the wings. This relative coordination between wings and halteres is extremely precise even at frequencies far exceeding 100 Hz. How do wings and halteres maintain precise coordination at such rapid frequencies? There are two principal hypotheses to address this question. First, as suggested by Pringle (13) in his pioneering studies on halteres, the wings and halteres, although driven by independent set of myogenic muscles, may be mechanically coupled. Second, because haltere sensory feedback influences wing motor neurons (14), it may also be required to drive the precise coordination of wing and haltere motion. To address these questions, it is necessary to understand the contribution of thoracic mechanics and its role in modulating wing kinematics through the wing hinge. Here, we show that the biomechanics of the thorax and wing hinge is essential for wing–wing and wing–haltere coordination, as well as in mediating the independent control of each wing.
Results
To address these questions, it is first necessary to clearly visualize the moving halteres in rapidly flapping insect. Hence, we studied these questions in the soldier fly, Hermetia illucens, because their naturally white halteres could be easily visualized during flight (SI Materials and Methods, Fly-Rearing Procedure). Under tethered and untethered conditions, soldier flies synchronously flap their two wings in-phase, whereas their halteres move antiphase to wings (Fig. 1 B and C). We recorded the wing–wing (Fig. 1 B and D; Materials and Methods; and SI Materials and Methods, Wing–Wing and Wing–Haltere Coordination Experiments) and wing–haltere (Fig. 1 C and E and Movies S1 and S2) motion in tethered flies and used vector strength method (16) to quantify the phase relationships. Here, the phase difference between the wing–wing or wing–haltere pair is converted into a vector such that its angle equals the mean phase difference, and its length is a measure of variability. Thus, vector length values of ∼1 indicate a low variability and significant directionality at the mean angle, whereas values of ∼0 indicate a high variability and absence of directionality with no mean angle. We compared the subsequent experimental data to these baseline controls.
Wings and Halteres Are Passively Coordinated by Mechanical Linkages.
In a first set of experiments on postmortem flies, we tested if the nervous system was actively involved in maintaining the phase relationship between the flapping wing–wing and wing–haltere pairs. When a wing of a dead fly was actuated on one side of the thorax, the contralateral wing moved in-phase, whereas both halteres moved antiphase to the wing pair (Movie S3 and SI Materials and Methods, Experiments on Postmortem Soldier Flies). Because these experiments were performed on postmortem insects, wings and haltere coordination was not mediated by the nervous system. Instead, these experiments show that wings and halteres are coordinated by mechanical linkages embedded within the thorax (13).
Wing–Wing Coordination Is Mediated via Linkages Within the Scutellum.
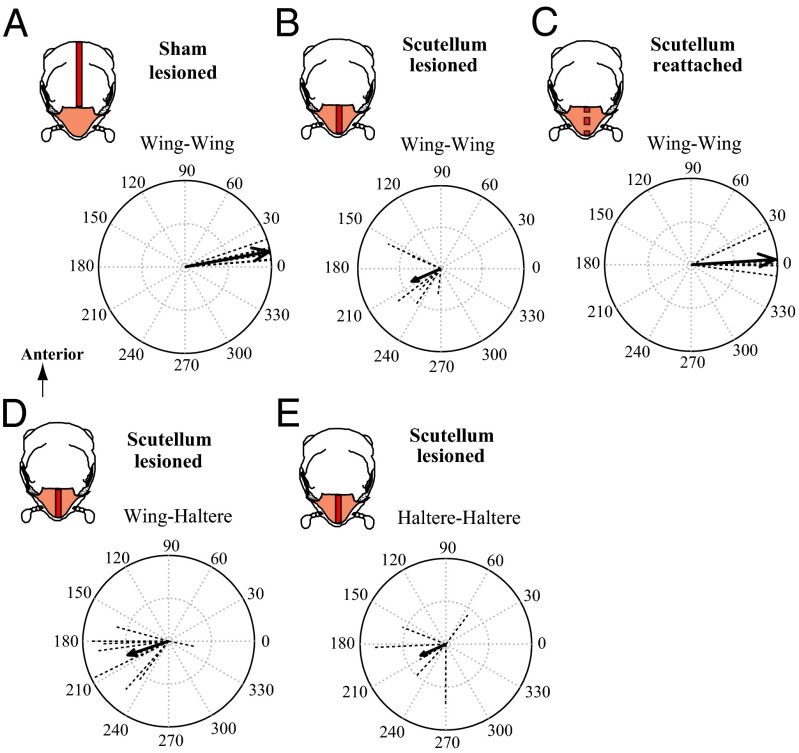
The Dipteran thorax is subdivided into two parts: a large anterior scutum, followed by a posterior small scutellum, which is the reduced hind thorax (Fig. 1A). To identify the linkages within the thorax, we made systematic lesions on the dorsal surface of the scutum or scutellum in live, tethered flies to disrupt strain transfer between wings, and filmed the wing and haltere motion (SI Materials and Methods, Wing–Wing and Wing–Haltere Coordination Experiments). The wing–wing coordination data from these experiments were compared with the control group in which there is near-exact phase synchrony of the flapping wings (Fig. 1C). In a scutum-lesioned group (Materials and Methods), we made systematic surgical cuts along the scutum while keeping the scutellum intact (Fig. S1). If the linkage system is located within the scutum, the coordination between wings should be disrupted. However, the wings flapped in-phase similar to controls, suggesting that the linkage was not in the scutum (Fig. 2A). In contrast, wing–wing coordination in the scutellum-lesioned group of flies was significantly disrupted (Fig. 2B and Movies S4 and S5) (17) but was restored in scutellum-reattached flies, in which the slit scutellum was glued by an adhesive (Fig. 2C). Thus, the bilateral wings in flies are mechanically linked via the scutellum. Although the wing–wing coordination in scutellum-lesioned flies was severely impaired, their halteres maintained antiphase coordination to ipsilateral wings (Fig. 2D) similar to control flies (Fig. 1E), but haltere–haltere coordination was disrupted (Fig. 2E). Thus, wing–haltere coordination is driven by a previously undescribed link, physically independent from the wing–wing scutellar linkage.
Fig. 2.
Wing–wing coordination is mediated by a passive mechanical linkage within the scutellum. Vector strength data for the phase between wing–wing (A–C), wing–ipsilateral haltere (D), and haltere–haltere (E) pairs. (Insets) Treatment type as a dorsal view of thorax with scutum (white) and scutellum (brown), and a line on thorax (red) indicating the surgical lesion. (A) Similar to controls (Fig. 1C), scutum-lesioned flies show well-coordinated wing movements (ϕ = 10.35°, r = 0.9932, n = 6, P < 0.001). (B) Scutellum-lesioned flies have disrupted wing–wing coordination and randomly distributed phase angles (ϕ = 203.63°, r = 0.3822, n = 6, P > 0.01). (C) In flies with reattached scutellum, wing coordination is restored (ϕ = 3.60°, r = 0.9769, n = 6, P < 0.001). (D) In scutellum-lesioned flies, halteres move antiphase to ipsilateral wings (ϕ = 198.79°, r = 0.5033, n = 12, P < 0.001; left and right data pooled) similar to control (Fig. 1E). (E) Haltere–haltere coordination is disrupted in scutellum-lesioned flies (ϕ = 203.89, r = 0.3217, n = 6, P > 0.1).
Each Wing–Haltere Pair Is Coordinated by a Separate Mechanical Linkage.
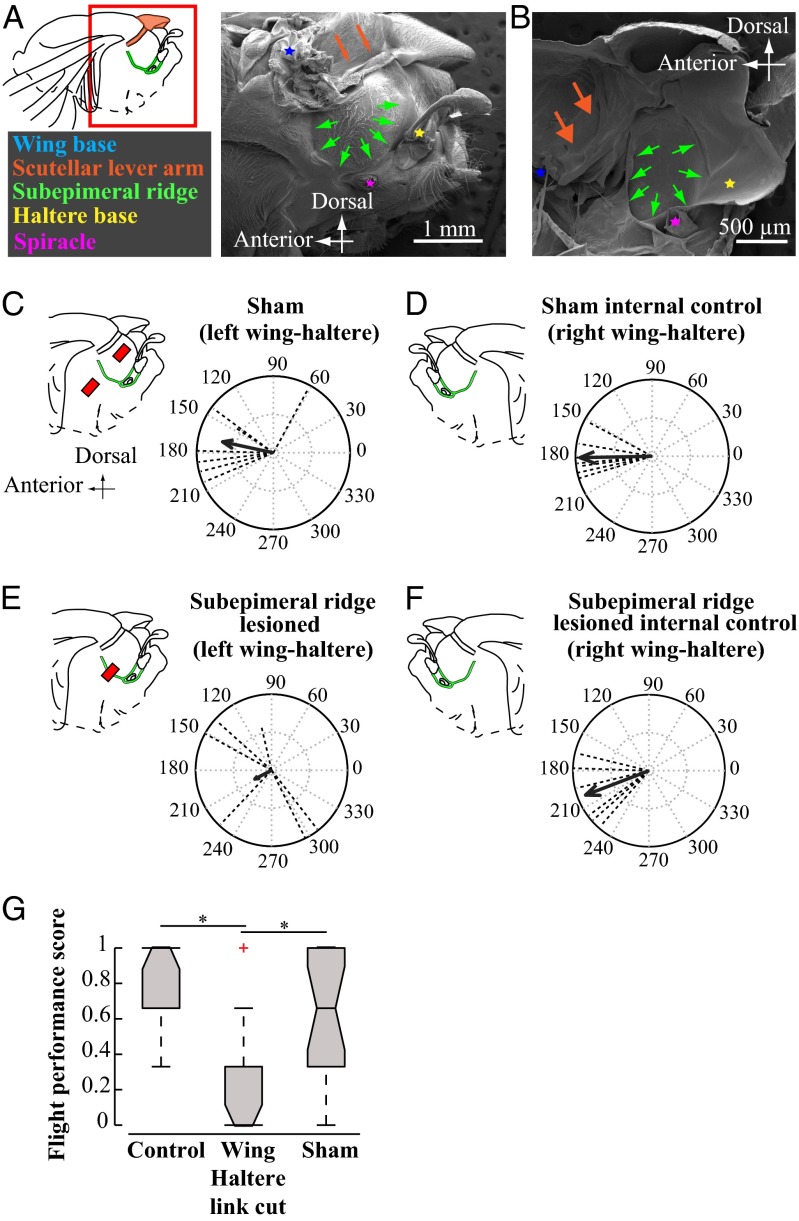
SEM images of fly thoraces revealed a thick piece of cuticle, which we termed the subepimeral ridge, spanning the base of wings and halteres below the epimeron abutting the metathoracic spiracle (Fig. 3 A and B; SI Materials and Methods, SEM of Insect Thorax; and Fig. S2). To test how subepimeral ridge influences wing–haltere coordination, we divided the flies into two groups. In a sham-treated group, we perforated the cuticle above and below the intact subepimeral ridge on only the left side of thorax, allowing the right side to be used as an internal control. The wing–haltere coordination on the left and right (control) side in the sham-treated group remained unaffected (Fig. 3 C and D; Materials and Methods; and Fig. S3), whereas the wings and halteres of the subepimeral ridge-lesioned group could not maintain their antiphase relationship on the lesioned left side (Fig. 3E and Movie S6). Thus, the subepimeral ridge mediates the wing–haltere phase coordination. Severing the subepimeral ridge on the left side did not influence the phase between the right wing–haltere pair, suggesting that they were independently linked (Fig. 3F and Movie S7). Unlike control and sham-treated groups, flies with bilaterally lesioned subepimeral ridge could not initiate or sustain flight, as measured by their ability to recover from free fall in the behavioral drop test assays [Fig. 3G and SI Materials and Methods, Free Flight (Drop Test) Assay for Flies with Severed Subepimeral Ridge]. Thus, the integrity of subepimeral ridge and antiphase coordination between wings and halteres is essential for flight.
Fig. 3.
Each wing–haltere pair is coordinated via a separate mechanical linkage running through the subepimeral ridge. (A, Inset) Red box around the fly wing base highlights the approximate region of the thorax imaged using a SEM. (A and B) SEM images of H. illucens thorax with wing (blue asterisk) and haltere (yellow asterisk) base, scutellar lever arm (brown arrows), epimeral ridge (green arrows), and spiracle (purple asterisk) in lateral (A) and hemisectional (B) views. (C–F) Phase relationships between ipsilateral wing–haltere pair. (C–F, Left) Lateral thorax with red bar indicating lesioned area relative to the supepimeral ridge (green). All treatments were performed on the left side, leaving the right intact as an internal control. (C) In sham-treated flies, the left wing–haltere pair, which is perforated above and below the ridge, continues to move antiphase to each other (ϕ = 167.84°; r = 0.6780; n = 7; P < 0.01; Moore’s test), similar to D, the right side untreated wing–haltere pair that acts as internal control (ϕ = 181.14°; r = 0.9420; P < 0.001, n = 7). (E) Lesioning the subepimeral ridge disrupts antiphase coordination in left wing–haltere pair (ϕ = 207.72°, r = 0.2485, n = 6, P > 0.5), whereas (F) the unlesioned wing–haltere pair on the right side, which acts as internal control, maintains antiphase coordination (ϕ = 201.05°; r = 0.8938, P < 0.005; n = 6). (G) Lesioning the subepimeral ridge and disrupting wing–haltere coordination on both sides impairs flight. We measured flight performance (shown as notched box plots) using the drop test [SI Materials and Methods, Free Flight (Drop Test) Assay for Flies with Severed Subepimeral Ridge; n = 20 per treatment], which measured the ability of each fly to recover from free fall in a vertical cylinder in three separate trials. Flies scored 0 if did not recover flight in all three trials, or 1/3, 2/3, or 1 if it recovered flight, respectively, in one, two, or three of three trials. Significant differences in groups (P < 0.001, asterisk) were identified using nonparametric Kruskal–Wallis ANOVA, followed by the Tukey–Kramer post hoc multicomparison analysis.
Wings and Halteres Act as Coupled Oscillators.
Because the wings and halteres are mechanically linked, the haltere frequency, although independently governed by a single asynchronous haltere muscle (13), should be constrained to follow the wing frequency. We tested this idea by increasing wing frequency by sequentially clipping wing length (Fig. 4A and Materials and Methods). Because stroke frequency increased with decreasing wing length, haltere frequency also concomitantly increased. For wing length above ∼0.6, the haltere motion remained exactly synchronized with wing motion. However, beyond a ∼1.5-fold increase in wing frequency, halteres recovered their original frequency (Fig. 4A and Figs. S4 and S5). In the transition region at wing length of ∼0.6, the halteres continuously shifted from oscillating either at their natural frequency or at the altered wing beat frequency (Fig. S4). Under these circumstances, the right and left halteres could operate at different frequencies, again underscoring the conclusion that the motion of the two halteres was governed by an independent set of linkages. Based on these observations, the wing–haltere system may be modeled as a physical system in which two independently driven oscillators of approximately the same phase and frequency in the unlinked state achieve exact synchronization due to the passive mechanical linkage of finite strength (18). Thus, the mechanical linkage system ensures that the wing and haltere motion remains exactly phase and frequency locked at all times, despite being independently driven by the indirect flight muscles and the haltere muscles, respectively. These linkages are thus essential for the robustness of the wing–haltere coordination even when the frequency of wings may be slightly perturbed e.g., due to wing damage.
Fig. 4.
Passive mechanisms for wing–wing and wing–haltere coordination in flies. (A) Wing (blue) and right haltere (green) frequency as a function of wing length for a representative fly (for more data, see Fig. S5). Individual box plots show distribution of ∼20 wing (and haltere) stroke frequency. (B) SEM image of the wing hinge in P. dux in lateral view showing radial stop (RS), pleural wing process (PWP), PteraleC (PtC), and wing (W). (C–F) Simultaneous visualization and quantification of 3D trajectories of the wing tip and wing hinge configurations. (C–E) As shown for a representative tethered P. dux, during flight onset, the wing beat amplitude as a function of time increases from small (black traces, corresponding to mode 0 gearbox configuration) to large (blue corresponding to mode 1, 2, or 3) in a single stroke (magenta, gearbox in transition), and at flight offset the drop in amplitude reverses from high (blue; mode 1, 2, or 3) to low (black; mode 0) amplitude also occurs in one wing stroke (pink; gearbox in transition). The corresponding trajectories for the whole sequence are shown in D (gray silhouette represents fly body and black points are wing base, which were also digitized) and wing hinge dynamics and corresponding wing amplitudes for several flies is shown in E. Here, stroke amplitudes (mean ± SD, n = 6; five animals) at various hinge configurations were compared using Friedman ANOVA with Tukey’s LSD post hoc test (*P < 0.001). (F) Wing hinge configurations during flight initiation. (Inset) Approximate region around the fly thorax that was filmed at macro magnification. (i and ii) Video stills show the wing hinge configuration (traced as in Movie S8; radial stop, red; pleural wing process, yellow; PteraleC, light blue) for the (i) wing transitioning from mode 0 to higher modes and (ii) engaged wing in mode 2 where the wing is flapping and RS touches the second grove of the PWP. (G) Schematic summary of the Dipteran thoracic mechanics. The A-IFM’s (gray box) drive the wings, which are synchronized via the scutellum (brown spring). Each wing is, however, independently and actively engaged and disengaged by the clutch (black switch) and its amplitude modulated by the gearbox (yellow and red). The halteres are driven by their own asynchronous muscles (gray box) and are mechanically linked to the ipsilateral wing by the wing haltere linkage, the subepimeral ridge (green spring). (G, Insets) The clutch in a disengaged state (a) and engaged state (b). (a) Disengaged state: when the clutch is disengaged, only mode 0 is possible. RS sits posteriorly to the PWP. (b) Engaged state: when the clutch is engaged, modes 1, 2, and 3 are possible. RS either contacts a groove in PWP (modes 1 and 2) or moves in front and around the PWP (mode 3).
A Clutch Mechanism Couples or Decouples Each Wing from the Thoracic Linkages.
Although advantageous for wing–haltere coordination at rapid timescales, mechanical linkages impose constraints on the independent kinematic control of each wing. However, some Diptera can flap a single wing even while the other remains immobile (19). This behavior is also observed in natural circumstances, e.g., during fruit fly courtship behavior when male Drosophila vibrates a single wing to attract females (20). How might wings be unilaterally actuated despite the linkage constraints? To explain this, we proposed that there exists a wing clutch at the base of each wing that is used by flies to actively engage with or isolate their wings from thoracic oscillations. According to this hypothesis, the wings can exist in only two states—either engaged or disengaged, with no intermediates. Engagement of the clutch would allow for thoracic strains to be transmitted and translated into wing motion; this is akin to the action of a mechanical clutch in automobiles, which when engaged allows transmission of energy from the rotating motor to the wheels.
In addition to this putative clutch, previous studies have shown that the Dipteran wing base consists of a separate gearbox that actively controls their wing amplitude (21–23). The basic structure of this gearbox is conserved across Diptera with minor variations (24). Part of the wing base, the radial stop (RS), directly contacts a multigrooved structure on the thorax, the pleural wing process (PWP), in four discrete configurations previously designated as modes 0, 1, 2, and 3 (Fig. 4B). In mode 0, the RS rests posteriorly to PWP and corresponding to this state, the wing remains in resting position. As the wing starts moving, the RS moves from this position to higher modes. During downstroke, in mode 1 and 2, RS briefly contacts grooves 1 and 2 of PWP, respectively, causing distinct changes in wing motion. In mode 3, however, RS moves anterior to and around the PWP but it does not contact any of the PWP grooves. The corresponding amplitude of the wing movement is maximum, indicating that the contact with modes 1 and 2 likely restricts the wing from moving through its maximum amplitude, and hence the PWP acts as a stopper. In all three modes, the wing contacts another putative mechanosensory structure, the PteraleC (PtC), during its downstroke (25). Whereas modes 1–3 correspond to discrete changes in wing kinematics, an earlier study deemed mode 0 to be unphysiological because it only occurred preinitiation or postcessation of flight (21).
The clutch hypothesis predicts that, at the onset and offset of flight, the wings should transition from small to large amplitude motion in discrete rather than gradual steps. We tested this prediction by imaging the 3D wing kinematics using two high-speed cameras at 3,000 frames per second while a synchronized third camera simultaneously recorded the corresponding changes in the gearbox configuration in high magnification (SI Materials and Methods, High-Speed Videography of Wing Hinge). These studies were conducted in a larger sarcophagid flesh fly, Parasarcophaga dux (Thomson), rather than Hermetia illucens, because the gearbox is more clearly visible in the former case. Although these two species of Diptera are phylogenetically distinct, the results of the studies described here are general to Diptera, and the clutch and wing hinge system likely exists in insects of other orders as well (26). As shown in the raw plot of the wing tip kinematics, during flight onset and offset, the wing undergoes sudden discrete changes in wing amplitude that occur within the duration of a single wing stroke (Fig. 4 C and D). The discrete change in wing amplitude is thus consistent with our hypothesis of an underlying clutch that mediates the switch between two discrete states for the wing—a disengaged and an engaged state.
Once their wings are engaged, how do flies modulate wing amplitudes during aerial maneuvers? To understand this, we analyzed and correlated the hinge configuration of tethered insects with the simultaneously captured 3D trajectory of the wing (Materials and Methods). These videos showed that during flight initiation, the wing is in resting position and the gearbox is in mode 0 configuration, which corresponds to small wing beat amplitudes of ∼50° (Fig. 4 C–E, mode 0, black, and Movie S8). However, as the wing hinge transitions to higher modes (mode 1–3), there is a step increase in the wing beat amplitude to about 130° (Fig. 4 C–E, mode 1–3, blue). This increase in wing beat amplitude occurs in about a single wing stoke (Fig. 4 C–E, during engagement, magenta). During flight cessation again, there is a step decrease in amplitude as the wing hinge transitions back to mode 0 (during disengagement shown in pink and mode 0 shown in black). The immobility of the wing during mode 0 indicates that rather than being unphysiological, this mode corresponds to the wing in a disengaged state. The higher modes (modes 1–3) correspond to an engaged wing, and during these the radial stop contacts the grooves in the pleural wing process (Fig. 4F), with discrete correlated changes in wing motion (21, 27).
Are the clutch and gearbox actions separate or part of the same underlying mechanism? Note that in both mode 0 and mode 3, the RS did not contact the PWP, and yet these two states were fundamentally different. Mode 0 corresponded to a wing in resting position, whereas the mode 3 represented the wing flapping with maximum amplitude. Thus, although the gearbox was in a similar state for both modes 0 and 3, the wing was in two different states, i.e., disengaged in the former, but engaged in the latter, which suggests the clutch mechanism is separate from the gearbox.
Moreover, it was also possible in a dead insect to engage the wing by gently pressing the wing vein in the axial direction against the thorax. Once engaged, moving one wing caused the synchronous movement of the contralateral wing, suggesting that the wing was now connected to the mechanical linkage system in the thorax. Because the nervous system is inactive in dead insects, the above observation suggests that the clutch is ultimately a mechanical structure that mediates engagement and disengagement of wings and is under neural control such that it allows flies to unilaterally control their wings if required.
The results described above are summarized in a schematic diagram of the insect thorax, which accounts for all observed frequency and phase relationships between wings and halteres (Fig. 4G). According to this schematic, the indirect asynchronous flight muscles vibrate the scutellum, which mechanically links and coordinates both wings. The scutellar strain transmitted via the subepimeral ridge on each side mediates the antiphase motion of halteres relative to wings. Once engaged, the two wings are constrained to flap with exactly the same phase and frequency due to the scutellar linkage, and similarly the halteres are constrained to flap antiphase to the wings due to the subepimeral ridge linkage. Thus, phase and frequency of the wing and haltere motion are constrained by linkages, although engagement and disengagement of wing are actively driven by the clutch system (Fig. 4G, Inset). Consistent with the gearbox hypothesis, once engaged, the radial stop contacts the grooves in the pleural wing process, with correlated changes in wing motion during higher modes.
Discussion
How the IFM-driven thoracic oscillations translate into wing movements has been a long-standing question in insect flight research. Earlier investigations with CCl4-anesthetized flies showed that their wings assumed either extreme dorsal or extreme ventral positions (17), suggesting a bistability in their wing position. This so-called “click model” was challenged by later studies, which argued that the two wing states were probably artifacts of CCl4 anesthesia. In tethered flight, fly wings could also assume intermediate states (23, 24, 28), which were likely mediated by axillary sclerites in the wing hinge. It was also suggested that the lateral deformations of scutum move the scutellar lever arm, causing wing motion (29). Together, these findings led to a modification of the click mechanism to include a gear change mechanism involving the radial stop and the pleural wing process in wing amplitude modulation (21, 30). Although the precise mechanisms for wing motion were different in each of the above models, they all implicated the scutellar lever arm in the actuation of each wing. These results are corroborated by our study, which explicitly demonstrates that the sclerotized scutellar linkage is necessary for coordinating the phase of both wings. In addition to the above, we show that a pair of previously undescribed linkages, the subepimeral ridges, independently coordinates the phase and frequency of wings and halteres. These linkages ensure that the wings and halteres always beat with exactly the same frequency and relative phase even in case the frequency of the wing changes, e.g., due to damage to the wings (Fig. 4A).
The mechanism by which the subepimeral ridge sets the phase difference between wings and halteres at 180° is presently unclear. Nevertheless, our free-flight studies indicate that the integrity of the subepimeral ridges and hence the maintenance of the precise 180° phase difference between wings and halteres is essential for proper flight. Mechanical interlinking of the thoracic elements in concert with the action of asynchronous muscles ensures that the wing beat frequency in Dipteran insects can be greatly enhanced without compromising the precise coordination of wings and halteres; this in turn ensures that halteres provide accurate, rapid mechanosensory feedback to the wing motor system during flight (14).
We also demonstrate that there must exist a wing clutch that can functionally engage or isolate the wing from thoracic oscillations, thereby enabling independent bilateral control of wings despite the physical constraints due to linkages. Because of the complexity of wing hinge morphology, we were unable to directly image the actual structure of the clutch, but its existence appears to be unequivocal. Indeed, there are several indications that the wing clutch inferred here is also likely present in other insects. For example, in endothermic insects, which elevate their thoracic temperatures by oscillating flight muscles, some (e.g., hawk moths) show visible shivering of their wings during warm-up, whereas in others (e.g., bees) the wings remain relatively immobile during warm-up, suggesting that the wings in the case of latter insects, such as bees, are disengaged during warm-up.
The thoracic architectural features described in this study are thus critical for rapid wing–wing and wing–haltere coordination in flies, and similar features may also exist in other insects that need to coordinate various limbs during fast locomotion (31–34). In addition to the evolutionary implications of the mechanisms described here, this study also provides important design principles for engineers in their efforts to develop microrobotic insects.
Materials and Methods
We reared soldier flies, H. illucens, on an artificial diet, and flesh flies, P. dux, on goat meat in the laboratory (rearing procedures described in SI Materials and Methods, Fly-Rearing Procedure).
Wing–Wing and Wing–Haltere Coordination Experiments.
The 1- to 4-d-old soldier flies were cold anesthetized for 5 min in an icebox. We performed the surgical treatments before flies recovered from the cold anesthesia.
Surgical procedures for the contralateral wing–wing coordination experiment.
In the control flies, we handled the flies for a few minutes similar to experimental flies, but performed no surgeries. In the scutum-lesioned flies, we cut the scutum longitudinally with a scalpel blade (no. 11 blade; Fine Science Tools Inc.) and left the scutellum intact. In the scutellum-lesioned flies, we cut the scutellum but left the scutum intact. In the reattached scutellum flies, we first cut the scutellum, tethered the fly and then glued the slit using cyanoacrylate glue and sodium bicarbonate. After filming, we visually confirmed that the scutellum was reattached in these flies after they died. Additionally, one wing of the dead fly was actuated using forceps to confirm that the contralateral wing also moved. We then carefully removed the layer of glue on the scutellum and reactuated the wing to ensure the absence of contralateral wing motion. These procedures were performed for each wing. Thus, we confirmed that the scutellum had indeed been reattached after being lesioned. Data from flies that did not meet the above criteria in our postmortem examination were discarded.
Surgical procedures for the ipsilateral wing–haltere coordination.
All of the surgeries were performed on the left side of the thorax leaving the right side intact as an internal control. In the sham treatment, we perforated the cuticle above and below the subepimeral ridge, but left the subepimeral ridge intact. In the subepimeral ridge lesion treatment, we used an Ultra-Fine Micro Knife (Fine Science Tools Inc.) to lesion the subepimeral ridge between left wing and haltere at a location anterior to the posterior thoracic spiracle. We removed a small part of the subepimeral ridge to ensure that the strain transfer across the subepimeral ridge, which is an internal invagination in the thoracic cuticle, was disrupted. Because the haltere muscles lay close to the ridge and posterioventral to the spiracle, we lesioned the ridge anterior to the spiracle away from the muscles to prevent damage to the haltere muscles. The amplitudes of oscillations of the left (treated) haltere were similar to the right (control) haltere, suggesting that the haltere muscles were left undamaged.
Details of tethering, filming procedure, and analysis are provided in SI Materials and Methods, Wing–Wing and Wing–Haltere Coordination Experiments.
Wing-Beat Frequency Manipulation by Changing Wing Length.
Using the same tethering procedure and filming conditions as described in SI Materials and Methods, Wing–Wing and Wing–Haltere Coordination Experiments, we initially filmed flies flapping with intact wings. We then turned off the lights to inhibit flight, and clipped the wings were to an appropriate length. We serially shortened the wings using a pair of scissors (Fine Science Tools Inc.) under a dissection microscope using a light source fitted with a red filter (which cut off all wavelengths below 610 nm). We used the wing vein patterns as landmarks. After each round of wing clipping, we filmed the fly as it flapped, and from these films obtained four data points, which included one intact and three reduced wing lengths. In some cases, we made finer cuts to obtain 5–6 data points, for a clearer resolution of wing and haltere frequency data. In the latter trials, the flies were tethered dorsally for a more ready elicitation of flight, and also the increase the duration of each flight bout. The time period and thus the frequency of a single wing and haltere stroke were calculated from the video by counting the number of frames per wing stroke. We analyzed 20 strokes per flight bout at each wing length.
Analysis of the Wing Hinge Videos.
Based on the position of wing hinge components in the ultramacro view of the wing base in the third camera, we divided the entire flight bout into five different states: (i) the initial disengaged state, before the onset of flight; (ii) during engagement—i.e., the initial engagement of the wing when the radial stop moves over the gearbox; (iii) engaged, during which the radial stop engages with the gearbox and the wing flaps over large amplitudes; (iv) during disengagement, the offset of flight during which the radial stop moves over the gearbox again; and (v) back to disengaged. Using the other two videos, we reconstructed the wing beat amplitude during each of these five phases (for details, see SI Materials and Methods, High Speed Videography of Wing Hinge).
Supplementary Material
Acknowledgments
We thank Anand Krishnan, Umesh Mohan, and Aparna Nair for assisting the study, and L. Kundanati and N. Gundiah from the Indian Institute of Science, Bangalore, for help with SEM. This study was funded by the Air Force Office of Scientific Research, Asian Office of Aerospace Research and Development (AOARD 114057), International Technology Center–Pacific, and a Ramanujan fellowship from the Department of Science and Technology, Government of India (to S.P.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412279112/-/DCSupplemental.
References
- 1.Polilov AA. The smallest insects evolve anucleate neurons. Arthropod Struct Dev. 2012;41(1):29–34. doi: 10.1016/j.asd.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Dudley R. The Biomechanics of Insect Flight. Princeton Univ Press; Princeton, NJ: 2000. [Google Scholar]
- 3.Josephson RK, Malamud JG, Stokes DR. Asynchronous muscle: A primer. J Exp Biol. 2000;203(18):2713–2722. doi: 10.1242/jeb.203.18.2713. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson MH, Tu MS. The function of dipteran flight muscle. Comp Biochem Physiol A. 1997;116(3):223–238. [Google Scholar]
- 5.Bastian J, Esch H. Nervous control of indirect flight muscles of honey bee. Z Vgl Physiol. 1970;67(3):307. [Google Scholar]
- 6.Roeder KD. Movements of the thorax and potential changes in the thoracic muscles of insects during flight. Biol Bull. 1951;100(2):95–106. doi: 10.2307/1538681. [DOI] [PubMed] [Google Scholar]
- 7.Chapman RF. The Insects. 3rd Ed Harvard Univ Press; Cambridge, MA: 1982. [Google Scholar]
- 8.Pringle JWS. The Croonian Lecture, 1977. Stretch activation of muscle: Function and mechanism. Proc R Soc Lond B Biol Sci. 1978;201(1143):107–130. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- 9.Wisser A, Nachtigall W. Functional-morphological investigations on the flight muscles and their insertion points in the blowfly Calliphora-erythrocephala (insecta, Diptera) Zoomorphology. 1984;104(3):188–195. [Google Scholar]
- 10.Nalbach G. The halteres of the blowfly Calliphora.1. Kinematics and dynamics. J Comp Physiol A. 1993;173(3):293–300. [Google Scholar]
- 11.Nalbach G. Extremely non-orthogonal axes in a sense organ for rotation: Behavioural analysis of the dipteran haltere system. Neuroscience. 1994;61(1):149–163. doi: 10.1016/0306-4522(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 12.Fox JL, Daniel TL. A neural basis for gyroscopic force measurement in the halteres of Holorusia. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194(10):887–897. doi: 10.1007/s00359-008-0361-z. [DOI] [PubMed] [Google Scholar]
- 13.Pringle JWS. The gyroscopic mechanism of the halteres of Diptera. Philos Trans R Soc Lond B Biol Sci. 1948;233(602):347–384. [Google Scholar]
- 14.Fayyazuddin A, Dickinson MH. Convergent mechanosensory input structures the firing phase of a steering motor neuron in the blowfly, Calliphora. J Neurophysiol. 1999;82(4):1916–1926. doi: 10.1152/jn.1999.82.4.1916. [DOI] [PubMed] [Google Scholar]
- 15.Sherman A, Dickinson MH. A comparison of visual and haltere-mediated equilibrium reflexes in the fruit fly Drosophila melanogaster. J Exp Biol. 2003;206(2):295–302. doi: 10.1242/jeb.00075. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: Some physiological mechanisms of sound localization. J Neurophysiol. 1969;32(4):613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- 17.Boettiger EG, Furshpan E. The mechanics of flight movements in Diptera. Biol Bull. 1952;102(3):200–211. [Google Scholar]
- 18.Strogatz SH. Non-linear Dynamics and Chaos. Perseus; New York: 1994. [Google Scholar]
- 19.Nachtigall W, Wilson DM. Neuro-muscular control of dipteran flight. J Exp Biol. 1967;47(1):77–97. doi: 10.1242/jeb.47.1.77. [DOI] [PubMed] [Google Scholar]
- 20.Bennet-Clark HC, Ewing AW. The wing mechanism involved in the courtship of Drosophila. J Exp Biol. 1968;49:117–128. [Google Scholar]
- 21.Nalbach G. The gear change mechanism of the blowfly (Calliphora erythrocephala) in tethered flight. J Comp Physiol A. 1989;165(3):321–331. [Google Scholar]
- 22.Wisser A. Wing beat of Calliphora erythrocephala: Turning axis and gearbox of the wing base (insecta, Diptera) Zoomorphology. 1988;107(6):359–369. [Google Scholar]
- 23.Miyan JA, Ewing AW. Further observations on Dipteran flight: Details of the mechanism. J Exp Biol. 1988;136:229–241. [Google Scholar]
- 24.Miyan JA, Ewing AW. How Diptera move their wings: A re-examination of the wing base articulation and muscle systems concerned with flight. Philos Trans R Soc London Ser B Biol Sci. 1985;311(1150):271–302. [Google Scholar]
- 25.Miyan JA, Ewing AW. A wing synchronous receptor for the Dipteran flight motor. J Insect Physiol. 1984;30(7):567–574. [Google Scholar]
- 26.Nachtigall W, Wisser A, Eisinger D. Flight of the honey bee. VIII. Functional elements and mechanics of the “flight motor” and the wing joint—one of the most complicated gear-mechanisms in the animal kingdom. J Comp Physiol B. 1998;168(5):323–344. [Google Scholar]
- 27.Balint CN, Dickinson MH. The correlation between wing kinematics and steering muscle activity in the blowfly Calliphora vicina. J Exp Biol. 2001;204(24):4213–4226. doi: 10.1242/jeb.204.24.4213. [DOI] [PubMed] [Google Scholar]
- 28.Miyan JA, Ewing AW. Is the click mechanism of Dipteran flight and artifact of CCL4 anesthesia. J Exp Biol. 1985;116:313–322. [Google Scholar]
- 29.Ennos AR. A comparative-study of the flight mechanism of Diptera. J Exp Biol. 1987;127:355–372. [Google Scholar]
- 30.Pfau HK. Critical comments on a ‘novel mechanical model of dipteran flight’. J Exp Biol. 1987;128:463–468. [Google Scholar]
- 31.Burrows M. Energy storage and synchronisation of hind leg movements during jumping in planthopper insects (Hemiptera, Issidae) J Exp Biol. 2010;213(3):469–478. doi: 10.1242/jeb.037861. [DOI] [PubMed] [Google Scholar]
- 32.Leston D, Pringle JWS, White DCS. Muscular activity during preparation for flight in a beetle. J Exp Biol. 1965;42(3):409–414. [Google Scholar]
- 33.Wootton RJ. In: Flying Insects and Robots. Floreano D, Zufferey JC, Srinivasan MV, Ellington C, editors. Springer; Heidelberg: 2010. pp. 207–217. [Google Scholar]
- 34.Patek SN, Dudek DM, Rosario MV. From bouncy legs to poisoned arrows: Elastic movements in invertebrates. J Exp Biol. 2011;214(12):1973–1980. doi: 10.1242/jeb.038596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.