Significance
Antibodies produced by B cells provide a protective barrier to our organism against the penetration and dissemination of microorganisms. Each antibody recognizes a specific antigen through variable (V) region domains of pairs of immunoglobulin (Ig) heavy (H) and light (L) chains. In mammals, VDJ recombination generates a highly diversified preimmune pool of VH and VL domains. Acquisition of a functional VH rearrangement is thought to prevent further VDJ recombination at the IgH locus. Instead, mice cloned from a terminally differentiated B cell unravel the ability of VDJ recombination to revise a functionally rearranged VH gene through VH replacement. We show that up to 20% of the antibody V gene repertoire of mature B-lymphocytes can be generated through VH replacement.
Keywords: B cells, VH replacement, antibody repertoire, IgA, nuclear cloning
Abstract
In mammals, VDJ recombination is responsible for the establishment of a highly diversified preimmune antibody repertoire. Acquisition of a functional Ig heavy (H) chain variable (V) gene rearrangement is thought to prevent further recombination at the IgH locus. Here, we describe VHQ52NT; Vκgr32NT Ig monoclonal mice reprogrammed from the nucleus of an intestinal IgA+ plasma cell. In VHQ52NT mice, IgA replaced IgM to drive early B-cell development and peripheral B-cell maturation. In VHQ52NT animals, over 20% of mature B cells disrupted the single productive, nonautoimmune IgH rearrangement through VH replacement and exchanged it with a highly diversified pool of IgH specificities. VH replacement occurred in early pro-B cells, was independent of pre–B-cell receptor signaling, and involved predominantly one adjacent VH germ-line gene. VH replacement was also identified in 5% of peripheral B cells of mice inheriting a different productive VH rearrangement expressed in the form of an IgM H chain. In summary, editing of a productive IgH rearrangement through VH replacement can account for up to 20% of the IgH repertoire expressed by mature B cells.
A functional immune system relies on the ability of B-lymphocytes to recognize foreign antigens in a highly specific fashion through the Ig receptor (also called B-cell receptor, or BCR). Each BCR consists of two identical Ig heavy (H) and light (L) chains, which are expressed on the surface of the B cell together with an Igα/Igβ heterodimer to form a signaling unit. In most vertebrates, the ability of the immune system to generate a highly diversified repertoire of antibody specificities relies on the stochastic assembly of variable (V), diversity (D), and joining (J) gene segments that encode for the antigen-binding domain of IgH and IgL chains of the BCR. This process, called V(D)J recombination, is mediated by the ordered recruitment at Ig loci of the RAG-1/2 endonucleases, followed by nonhomologous end-joining factors that catalyze the joining of the cleaved DNA segments (1). The latter processes are often accompanied by trimming and insertion of n-nucleotides at junctional ends. All together these mechanisms contribute to generate a highly diversified pool of Ig specificities.
Ig receptor editing provides B cells with the opportunity to exchange BCR specificity through secondary VDJ recombination. Whereas IgL chain editing was shown to play a central role in the neutralization of autoimmune BCR specificities expressed by newly generated immature B cells (2), the contribution of IgH receptor editing to antibody repertoire diversification has remained largely unappreciated. Two mechanisms promote secondary IgH rearrangements. In VH-to-JH direct joining, RAG proteins cleave at Recombination Signal Sequences (RSSs) of VH and JH elements lying, respectively, upstream and downstream of the original VH rearrangement, followed by microhomology-driven joining of the Ig gene segments. This mechanism has been mainly observed in IgH knock-in mice carrying nonproductive IgH rearrangements (3, 4). VH replacement relies, instead, on evolutionary conserved cryptic RSSs embedded within the framework region 3 of most VH germ-line genes (5). During VH replacement, cryptic recombination signal sequences (cRSS) within the V gene of a preexisting VH rearrangement are engaged in a RAG-mediated recombination reaction together with RSSs of an upstream VH germ-line gene, in accordance with the 12/23 rule (reviewed in ref. 6). VH replacement generates a novel VH rearrangement that carries a different V gene and shares with the original one part of its Complementary Determining Region 3 (CDR3). Studies in B-lymphoma cell lines were the first to identify VH replacement as a mechanism to edit both in-frame (IF) and out-of-frame (OF) IgH rearrangements (7, 8). Later analyses with IgH knock-in mice confirmed in vitro studies, unraveling the potential of VH replacement to rescue progenitor B cells carrying nonproductive VH rearrangements (4, 9). VH replacement has also been proposed to diversify the preimmune repertoire of productive IgH specificities in both human and mice (10–12). Bioinformatic analyses of IgH V gene repertoires obtained through next-generation sequencing have shown limitations to identify VH replacements (13). Studies on IgH transgenic mice have in part overcome such limitations (9, 11, 14–17). However, the targeting strategy to generate most IgH knock-in mice may severely limit the interpretation of VH replacement data obtained from such models. Indeed, in most IgH knock-in mice, prerearranged VH genes replace the four JH segments of the IgH locus. This atypical chromosomal configuration may affect the rate and nature of secondary IgH rearrangements. Intergenic Control Region 1 (IGCR1), which is crucial for ordered and lineage-specific VDJ recombination (18), represents one example of a cis regulatory element that is excised from the IgH locus during physiologic V-to-DJ recombination, but is retained in most IgH knock-in animals.
Recent advancements in ES gene targeting strategies have allowed the establishment of next-generation IgH knock-in mice where the insertion of a particular VH rearrangement into the JH locus is coupled to Cre recombinase-assisted deletion of the intervening region between DH-proximal VH genes and the JH locus (4). This elegant approach relies on multiple targeting steps that are time consuming and may preclude germ-line transmission of targeted ES cells. Instead, somatic cell nuclear transfer (SCNT) technology applied to B-lymphocytes allows the rapid generation of IgH monoclonal mice carrying VH rearrangements placed in their physiologic location (19).
Here, we applied SCNT to establish a novel mouse strain (VHQ52NT; Vκgr32NT) starting from the nucleus of a terminally differentiated IgA+ intestinal plasma cell (PC). Cloned mice allowed the investigation of B-cell development and IgH repertoire diversification under conditions where a single, productive IgH rearrangement consisting of a DH-proximal Q52 VH gene was expressed from an IgA class-switched IgH locus. We could show that a BCR specificity selected in the lamina propria (LP) of the small intestine and expressed in the form of IgA could effectively drive early B-cell development and instruct peripheral B-cell subset differentiation. VHQ52NT mice allowed the study of the contribution of VH replacement to the diversification of the IgH antibody repertoire in mice starting with a single productive nonautoimmune IgH specificity. Surprisingly, our results indicate that up to 20% of IgH specificities expressed in the pool of mature B cells can be generated through VH replacement.
Results
Nuclear Reprogramming of Intestinal PCs.
We applied SCNT to reprogram terminally differentiated IgA+ PCs isolated from the LP of the small intestine of mice housed under specific pathogen-free conditions. Nuclear transferred ES (ntES) cell lines were established from independent IgA cloned embryos. Derivation of ntES lines from IgA PCs was confirmed by genomic PCR amplification of Ig H and L chain V gene rearrangements. Chimeric mice were obtained through blastocyst injection of one representative IgA ntES cell line. Southern blotting analysis and PCR amplification of tail-tip genomic DNA of chimeric offspring confirmed germ-line transmission of cloned Ig V gene rearrangements (Fig. 1A and Fig. S1 A and B). These data indicate that PCs can undergo nuclear reprogramming to become pluripotent stem cells.
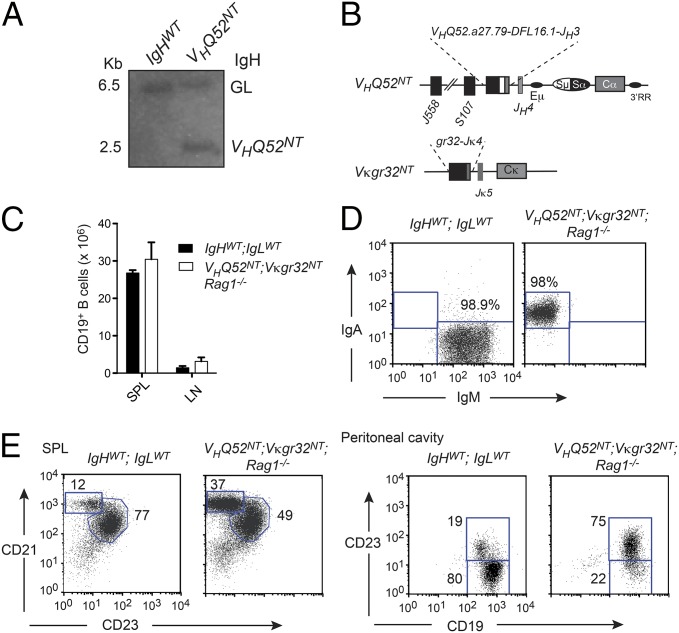
Fig. 1.
B-cell development in VHQ52NT; Vκgr32NT monoclonal mice. (A) IgH Southern blotting analysis of wild-type and VHQ52NT HT mice. Bands corresponding to IgH germ line (GL) and VHQ52NT alleles are indicated. (B) Structure of rearranged IgH and Igκ loci in IgA transnuclear mice. (C) Numbers of CD19+ B cells in SP and inguinal LNs of control (IgHWT;IgLWT) and IgA monoclonal mice (VHQ52NT; Vκgr32NT; Rag1−/−), determined by flow cytometric analysis. (D) Representative flow cytometric analysis of splenic CD19+-gated B cells in control (n = 2) and IgA monoclonal mice (n = 2). (E) Representative flow cytometric analysis of splenic CD19+ B cells in controls and IgA monoclonal mice (n = 2). Peritoneal cavity B cells were analyzed after gating, respectively, on IgM+ (IgHWT;IgLWT) or IgA+ (VHQ52NT; Vκgr32NT; Rag1−/−) cells (n = 2). Numbers indicate percentage of boxed B-cell subsets.
IgA Can Replace IgM to Drive B-Cell Development.
IgA transnuclear mice allowed us to test whether an IgA BCR selected by an intestinal PC could replace IgM to drive B-cell development. IgA monoclonal mice inherited a productive, unmutated, VH rearrangement consisting of the DH-proximal VHQ52.a27.79 gene joined to DFL16.1 and JH3 segments. The VL gene rearrangement consisted of Vκgr32 joined to Jκ4 (Fig. 1B and Fig. S1B). Mice inheriting prerearranged VH and VL genes were called, respectively, VHQ52NT and Vκgr32NT.
VHQ52NT;Vκgr32NT heterozygous (HT) mice were analyzed on the Rag1-deficient genetic background to study B-cell development under conditions of Ig monospecificity. VHQ52NT; Vκgr32NT; Rag1−/− mice showed normal numbers of CD19+ B cells, all expressing surface IgA (sIgA), in spleen (SP) and lymph nodes (LNs) (Fig. 1 C and D). Immunophenotypic analysis revealed the presence in the SP of mature follicular/B-2 (FO; CD19+CD21+CD23+) and marginal zone (MZ; CD19+CD21hiCD23lo) B cells (Fig. 1E). In contrast, B-1 B cells (CD19hiCD23lo) were largely missing in the peritoneal cavity of IgA monoclonal mice (Fig. 1E). These results indicate that a BCR specificity selected by an intestinal PC and expressed in the form of IgA can drive early B-cell development and promote differentiation into mature FO and MZ B cells.
The VHQ52NT; Vκgr32NT BCR Is Neither Autoreactive Nor Signals Autonomously.
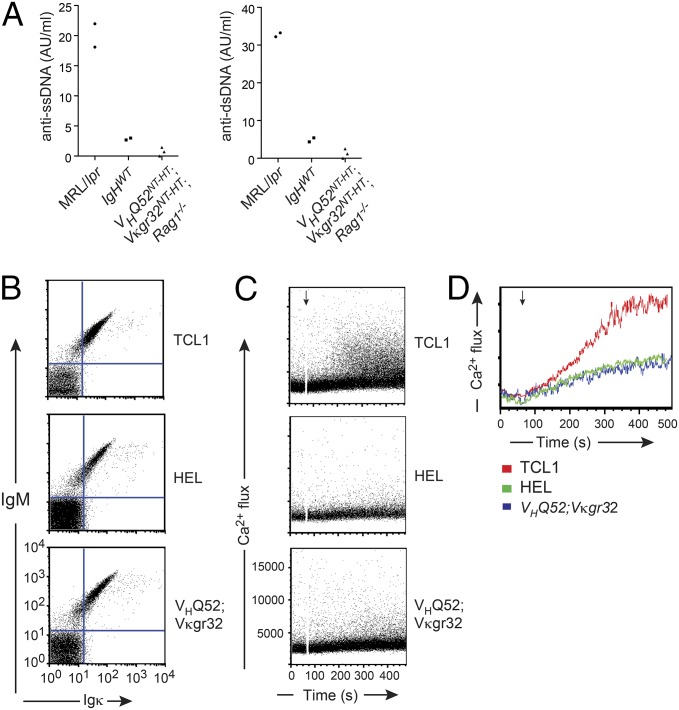
Derivation of transnuclear mice from an intestinal PC predicts that the cloned BCR recognized a gut luminal antigen. Despite multiple attempts, we failed, thus far, to identify the selecting antigen. The polyreactive nature of IgA antibodies may lead to the recognition of self-antigens (20). To determine whether the BCR of VHQ52NT; Vκgr32NT B cells recognized common self-antigens represented by single- and double-stranded DNA, we measured anti-DNA antibody reactivity in the serum of VHQ52NT;Vκgr32NT;Rag1−/− monoclonal mice. ELISAs revealed minimal anti-DNA reactivity in the serum of IgA monoclonal mice, which was comparable to that of wild-type littermate controls and significantly lower than that of autoimmune-prone MRL-lpr/lpr mice (Fig. 2A). We also tested whether the cloned IgA BCR displayed spontaneous (antigen-independent) self-aggregation, possibly resulting from the binding to an internal epitope (21, 22). For this, we measured spontaneous Ca+ mobilization in Rag-2; λ5; SLP65 triple knockout (TKO) pro-B cells that were reconstituted with a BCR (in the form of IgM or IgA) carrying VHQ52NT and Vκgr32NT specificities (Fig. 2B and Fig. S2A). As controls, TKO progenitors were reconstituted with either an autonomously active BCR, cloned from an autoreactive TCL1 transgenic B cell or a nonautonomously active hen egg lysozyme-specific BCR (HEL) (Fig. 2A). Expression of the TCL1-derived BCR induced a robust autonomous intracellular Ca+ flux in TKO progenitors upon tamoxifen-dependent activation of an ERT2–SLP65 fusion protein (21). In sharp contrast, BCR specificities from both IgA transnuclear and HEL-specific B cells failed to trigger spontaneous Ca+ mobilization in response to SLP65 activation (Fig. 2 C and D and Fig. S2 B and C). These results, together with the observations that IgA monoclonal mice (both VHQ52NT-HT and VHQ52NT-HT; Vκgr32NT-HT animals) aged in a comparable fashion to wild-type littermate controls lacked signs of systemic autoimmunity and displayed a normal (or, at most, lower) proportion of sIgλ+ B cells (Fig. S2D), render it unlikely that the IgA BCR expressed by transnuclear B cells is self-reactive.
Fig. 2.
Transnuclear IgA neither recognizes DNA nor signals spontaneously. (A) Serum titers of antibodies reactive against, respectively, single- (ss) and double (ds)-stranded DNA in IgA monoclonal mice (n = 3), age-matched littermate controls (IgHWT; n = 2), and MRL/LPR (n = 2) animals. Each dot represents one animal. (B) Surface expression of IgH (IgM isotype) and IgL chains isolated from IgA monoclonal B cells reconstituted in Rag-2; Slp65; λ5 TKO pro-B cells. Autonomously active TCL1-derived and nonautonomously active HEL-specific BCRs served as controls. (C) Flow cytometric measurement of spontaneous Ca2+ flux upon treatment with 4-OHT (black arrow) of BCR-complemented TKO pro-B cells to induce the activity of an ERT2–SLP65 transgene. (D) Overlay of spontaneous Ca+ fluxes shown in C. Data in B–D are representative of two experiments.
VHQ52NT HT Mice Have a Substantial Number of IgM+ B Cells.
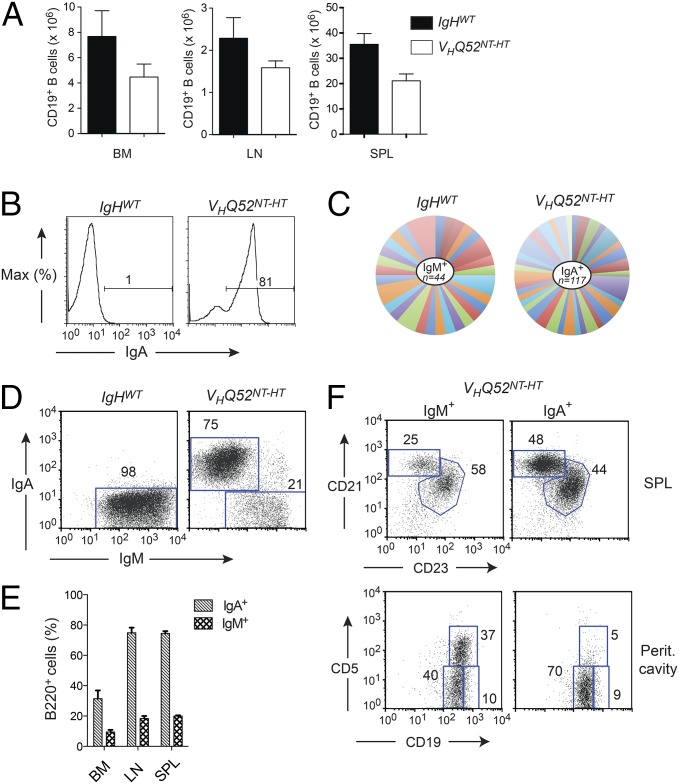
Next, we analyzed B-cell development in VHQ52NT HT mice (VHQ52NT-HT) on a Rag-proficient background. CD19+ B cells in primary and secondary lymphoid organs were only modestly reduced in VHQ52NT-HT mice compared with age-matched littermate controls (Fig. 3A). FACS analysis of SP and LN cell suspensions from VHQ52NT-HT animals revealed that most B cells expressed sIgA (Fig. 3B). Analysis of Vκ gene rearrangements in sorted splenic IgA+ B cells revealed a highly diversified repertoire of IgL chains (Fig. 3C), thus excluding major restrictions in the pairing of the VHQ52NT IgA H chain. Remarkably, around 20% of B cells in the SP and LNs of VHQ52NT-HT mice lacked sIgA and expressed instead IgM (Fig. 3 D and E). A similar fraction of sIgM+ B cells was found in the bone marrow (BM; Fig. 3E). Both IgA+ and IgM+ single-producer B cells matured into FO and MZ B cells (Fig. 3F), with the latter subset enriched among IgA+ B cells (Fig. 3F). In contrast, peritoneal cavity B-1 B cells consisted mainly of IgM+ B cells (Fig. 3F), in agreement with data obtained from VHQ52NT;Vκgr32NT;Rag1−/− mice. VH gene rearrangement analysis revealed a highly diversified IgH repertoire expressed by IgM+ B cells of VHQ52NT-HT mice (Fig. S3A). All together, these results indicate that the antibody repertoire of IgA monoclonal mice undergoes diversification through IgH editing.
Fig. 3.
A substantial number of B cells lose sIgA expression in VHQ52NT mice. (A) Absolute number of CD19+ B cells in BM, inguinal LNs and SP of controls (n = 6), and VHQ52NT HT (n = 7) mice. (B) Frequency of splenic CD19+-gated sIgA+ B cells in representative VHQ52NT-HT HT animals and littermate controls, as determined by flow cytometric analysis. Numbers indicate percentage of sIgA+ B cells. (C) Pie chart representation of Vκ gene rearrangement analysis obtained from splenic IgM+ B cells of controls (n = 2) and IgA+ B cells of VHQ52NT-HT mice (n = 6). Each colored segment represents a unique rearrangement. Segment size defines frequency of individual Vκ rearrangements. (D) Representative flow cytometric analysis of SP B cells in controls (n = 6) and VHQ52NT-HT mice (n = 7) stained for sIgM and sIgA, respectively. Numbers indicate frequencies of CD19+-gated boxed B cells. (E) Frequency of sIgM+ and sIgA+ cells among B220+ B cells in lymphoid organs of VHQ52NT-HT mice (n = 7). (F) Representative flow cytometric analysis of cell suspensions from SP and peritoneal cavity lavages of VHQ52NT-HT mice, gating, respectively, on sIgM+ and sIgA+ B cells. Numbers indicate frequency of boxed B-cell subsets.
VHQ52NT Mice Diversify the IgH Repertoire Through VH Replacement.
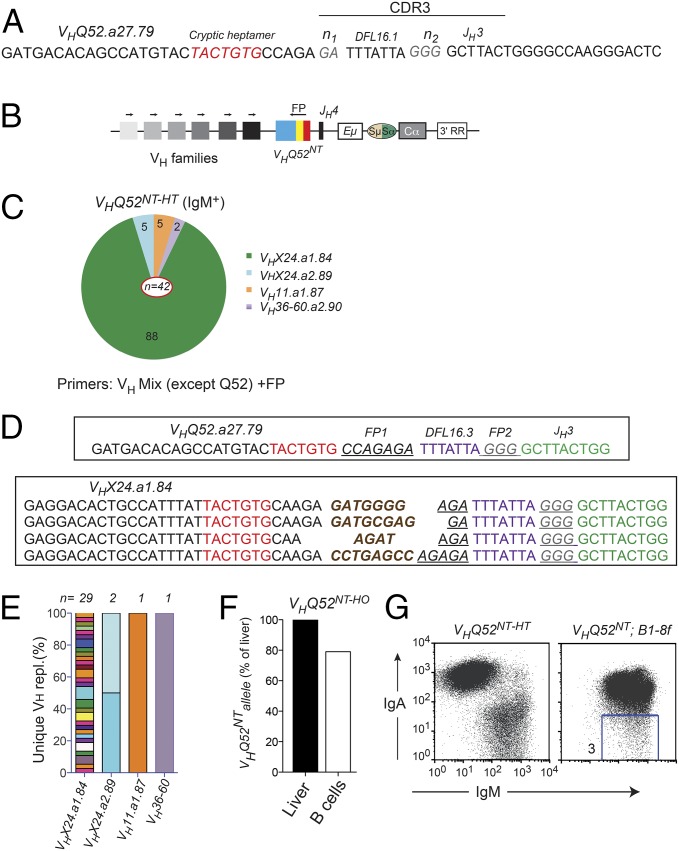
The substantial number of IgM+ B cells found in lymphoid organs of VHQ52NT-HT mice pointed to the silencing/inactivation of the VHQ52NT allele in these cells. Because IgM+ B cells were not identified in VHQ52NT;Vκgr32NT;Rag-1−/− animals, we hypothesized that Rag-dependent VH replacement was responsible for VHQ52NT inactivation. To this end, we performed genomic PCR on sorted IgM+ splenic B cells, using a pool of forward primers complementary to members of the main VH families, except Q52. As a reverse primer, we used an oligonucleotide annealing to a footprint (FP) sequence of the CDR3 of VHQ52NT (Fig. 4 A and B). An RT-PCR approach using cDNA from sorted IgM+ B cells complemented the DNA analysis (Fig. S4A). Both strategies generated PCR products that were subsequently cloned and sequenced. Sequence analyses revealed the consistent replacement of the original VHQ52.a27.79 gene of VHQ52NT with other germ-line VH genes (Fig. 4C, Fig. S4B, and Table S1). All VH replacements shared with the original VHQ52NT rearrangement part of the CDR3 FP sequence and the JH3 segment (Fig. 4D). VH replacements were further diversified by de novo n-nucleotide addition (Fig. 4D and Table S1). Importantly, CDR3 sequence analysis indicated that most VH replacements in VHQ52NT-HT mice were unique, thus underscoring the polyclonal origin of IgM+ B cells (Fig. 4E, Fig. S4C, and Table S1). In accordance with the sIgA-negative phenotype, VH replacements in IgM+ B cells of VHQ52NT-HT mice were predominantly OF (Table S1). This result is compatible with a scenario whereby the occurrence of OF VH replacements caused the inactivation of VHQ52NT, which in turn prompted further VDJ recombination on the second IgH chromosome favoring the generation of IgM+ B cells. To estimate the frequency of secondary VH rearrangements in an unbiased fashion, we performed IgH Southern blotting analysis on genomic DNA from VHQ52NT homozygous splenic B cells. We compared the intensity of the bands corresponding to the VHQ52NT allele between B cells and liver cells that were used as negative control (Fig. S4D). Quantification of the data revealed that around 22% of splenic IgA+ B cells disrupted one VHQ52NT allele (Fig. 4F).
Fig. 4.
VHQ52NT is edited through VH replacement. (A) CDR3 nucleotide sequence of VHQ52NT. Cryptic heptamer and n-nucleotides are indicated, respectively, in red and gray. (B) PCR strategy to identify VH replacements. Arrows indicate PCR primers. Colored blocks symbolize VH families. An oligonucleotide annealing to a unique sequence (FP) within the CDR3 of VHQ52NT was used as a reverse primer. (C) Pie representation of VH gene use in VH replacements cloned from splenic IgM+ B cells of VHQ52NT-HT mice (n = 4). Numbers indicate frequency of rearrangements carrying the indicated VH genes. PCR primers used for the analysis are indicated. (D) CDR3 nucleotide sequence of representative VH replacements cloned from IgM+ B cells of VHQ52NT-HT mice using the VHX24.a1.84 donor VH gene. First line shows CDR3 sequence of the original VHQ52NT rearrangement. Underlined are VHQ52NT FP sequences indicated, respectively, as FP1 and FP2. De novo added n-nucleotides are labeled in brown. cRSSs are indicated in red. (E) Frequency of unique VH replacements cloned from IgM+ splenic B cells of VHQ52NT-HT mice, as assessed through CDR3 sequence analysis. Replacements using the same VH germ-line gene were grouped. (F) Southern blotting quantification of VHQ52NT gene copy number in splenic IgA+ B cells of VHQ52NT homozygous (HO) mice. Data were normalized for DNA input and represented as relative to VHQ52NT copy number in liver cells of transnuclear mice. (G) Representative flow cytometric analysis of splenic CD19+ B cells of VHQ52NT;B1-8f double IgH insertion mice (n = 4). Number within dot plot refers to frequency of IgM-only B cells.
To validate our results, we used VHQ52NT as a reporter allele to monitor VH replacement in B1-8f IgH knock-in mice (23). Flow cytometric analysis of splenocytes of double IgH knock-in mice revealed a major population of B cells expressing concomitantly sIgM and sIgA (Fig. 4G). Importantly, we also detected a distinct subset of IgM-only B cells that ranged between 3% and 5% of splenic B cells (Fig. 4G and Fig. S4E). VH rearrangement analysis in sorted IgM-only B cells identified OF VH replacements that disrupted the VHQ52NT allele (Table S1). We rarely identified sIgA-only B cells, possibly because inactivation of B1-8f through VH replacement is rendered unlikely by its chromosomal location (JH locus) and the lack of a cRSS (23). In summary, using two independent mouse models starting with a highly restricted repertoire of productive IgH specificities, we found that 3–20% of their mature B cells diversified antibody specificity through VH replacement.
VH Replacements Are Already Detected in Pro-B Cells.
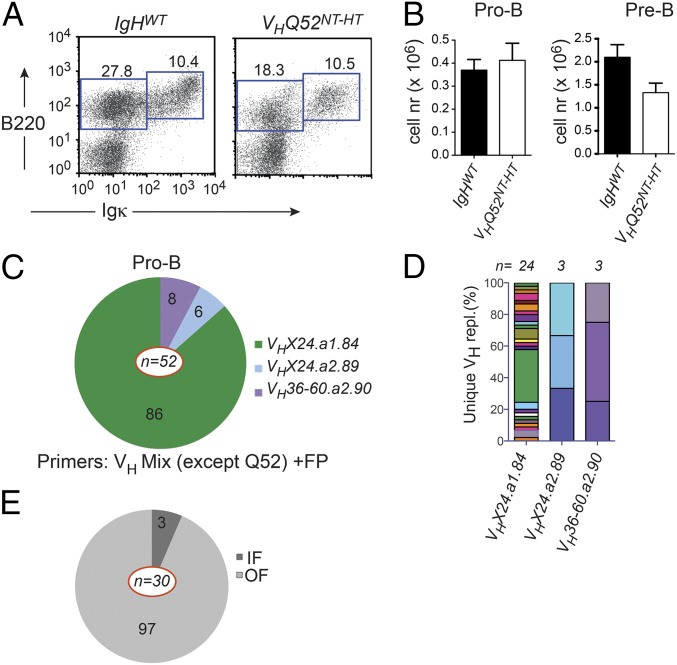
To investigate the temporal onset of VH replacement, we first evaluated early B-cell development in VHQ52NT-HT mice. Flow cytometric analysis revealed reduced numbers of Igκ−B220lo BM B-cell progenitors in VHQ52NT mice in comparison with controls (Fig. 5A). The impairment was mainly caused by fewer CD25+ pre-B cells, whereas CD43+ pro-B cells were largely comparable in number to controls (Fig. 5B). To test whether VH replacement had already occurred in early B-cell progenitors, we sorted B220loIgκ−CD43+ pro-B cells from VHQ52NT-HT mice and performed VH replacement analysis. Sequencing of PCR products revealed a considerable number of unique VH replacements, which were also detected in their CD25+ pre–B-cell progeny (Fig. S5A). V gene use in VH replacements cloned from pro-B cells indicated a similar pattern to that of SP IgM+ B cells (Fig. 5 C and D, Fig. S5 A and C, and Table S1). Ninety-seven percent of unique VH replacements sequenced from pro-B cells were nonproductive (Fig. 5E). In contrast, close to 10% of VH replacements in pre-B cells were IF (Fig. S5B). These results suggest that OF VH replacements block the development of pro-B cells unless rescued by functional secondary IgH rearrangements.
Fig. 5.
VH replacement is detected in pro-B cells. (A) Representative flow cytometric analysis of BM cells in VHQ52NT-HT mice and age-matched littermate controls (IgHWT). Numbers indicate frequencies of gated progenitor (B220+Igκ−) and sIg+ (B220+Igκ+) B cells. (B) Absolute number of BM pro-B (B220+Igκ−CD43+CD25−) and pre-B (B220+Igκ−CD43−CD25+) cells in representative cases of controls (IgHWT) (n = 5) and VHQ52NT-HT mice (n = 4) as assessed by flow cytometric analysis. (C) VH gene use in VH replacements cloned from pro-B cells of VHQ52NT-HT mice. Numbers within pie segments indicate frequency of germ-line VH genes involved in VH replacements (n = 52). (D) Frequency of unique VH replacements cloned from BM pro-B cells of VHQ52NT-HT mice, assessed by CDR3 sequence analysis. VH replacements carrying the same VH gene were grouped. (E) Frequency, respectively, of IF and OF VH replacements cloned from pro-B cells of VHQ52NT-HT mice. Numbers refer to percentage of unique VH replacements (n = 30).
VH Replacement Occurs Independently of Pre-BCR Expression/Signaling.
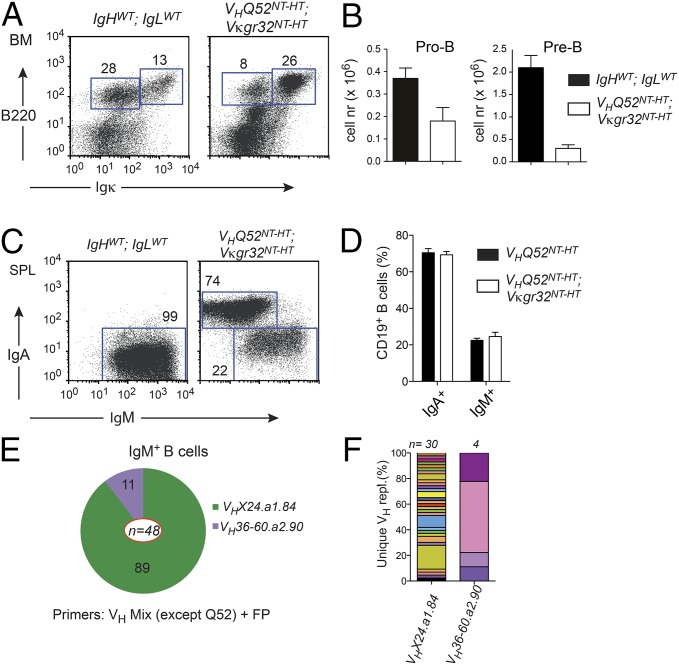
The lower number of pre-B cells in VHQ52NT mice may result from impaired expression and/or signaling of a pre-BCR composed of an IgA H chain (24). Altered pre-BCR function may, in turn, prolong RAG-mediated recombination at the IgH locus, hence facilitating VH replacement. To test this hypothesis, we analyzed VHQ52NT-HT;Vκgr32NT-HT double Ig insertion mice. The latter animals displayed a significant reduction in both pro-B cells and pre-B cells, as a result of the premature expression of pairing IgH/IgL chains (Fig. 6 A and B). Remarkably, flow cytometric analysis of splenic cell suspensions from IgH/L insertion mice revealed a fraction of sIgM+ B cells comparable to that of VHQ52NT-only animals (Fig. 6 C and D). Molecular analysis confirmed the inactivation of VHQ52NT through OF VH replacements in IgM+ B cells of VHQ52NT-HT;Vκgr32NT-HT mice (Fig. 6 E and F and Table S1). These results indicate that pre-BCR expression and/or signaling is not required to initiate VH replacement of a productive IgH rearrangement.
Fig. 6.
VH replacement occurs before pre-BCR expression. (A) Representative flow cytometric analysis of BM cells from VHQ52NT-HT; Vκgr32NT-HT double Ig insertion mice and littermate controls (IgHwt; IgLwt). Numbers indicate frequency of boxed progenitors (B220loIgκ−) and sIg+ B cells. (B) Average number of BM pro-B (B220loIgκ−CD43+CD25−) and pre-B (B220loIgκ−CD43−CD25+) cells in VHQ52NT-HT; Vκgr32NT-HT (n = 4) and control (n = 7) mice. (C) Representative flow cytometric analysis of gated CD19+ B cells in SP of IgHwt; IgLwt controls and VHQ52NT-HT; Vκgr32NT-HT mice (n = 6). Numbers indicate frequency of B cells in boxed gates. (D) Comparison of IgA+ and IgM+ SP B-cell frequencies between VHQ52NT-HT (n = 8) and VHQ52NT-HT; Vκgr32NT-HT double-insertion mice (n = 6). (E) VH gene use in VH replacements (n = 48) cloned from splenic IgM+ B cells of VHQ52NT-HT; Vκgr32NT-HT animals (n = 3). Numbers within pie segments indicate frequency of VH replacements using the indicated germ-line VH genes. (F) Frequency of unique VH replacements (n = 34) cloned from IgM+ B cells of VHQ52NT-HT; Vκgr32NT-HT mice, assessed by CDR3 sequence analysis. VH replacements carrying the same VH germ-line gene were grouped.
VHQ52NT Is Preferentially Replaced by One Neighboring Germ-Line V Gene.
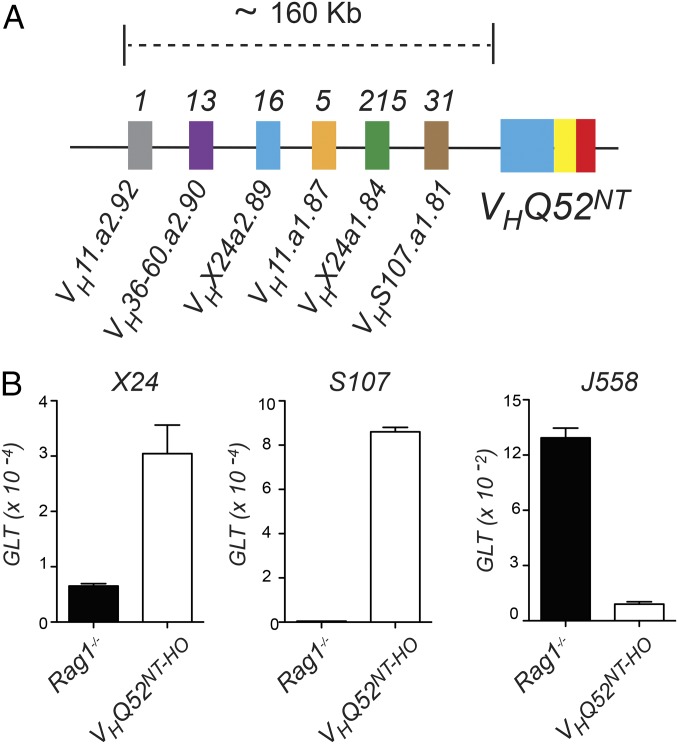
Progenitor B cells inheriting an OF VH rearrangement recruit an unbiased set of donor germ-line VH genes to promote VH replacement (4). In sharp contrast, our data show that pro-B cells carrying a productive IgH rearrangement use a highly restricted set of VH germ-line genes for VH replacement. In particular, VHX24 was identified in over 80% of VH replacements. Alignment to the Igh locus indicated that V genes involved in VH replacement in VHQ52NT mice mapped within a 160-Kb genomic interval proximal to VHQ52NT (Fig. 7A). We tested whether preferential VH gene use correlated with higher expression of their corresponding germ-line transcripts (GLTs). For this, we established IL-7–dependent progenitor B-cell cultures. Quantitative RT-PCR (qRT-PCR) analysis revealed that GLTs for VHX24 and S107 members, which mapped closely to VHQ52NT and were frequently recruited in VH replacement, were substantially increased in VHQ52NT progenitor B cells compared with Rag1 mutant pro-B cells that were used as control. Conversely, GLTs for members of the distal J558 VH family were markedly higher in Rag-1 mutant pro-B cells compared with VHQ52NT progenitors (Fig. 7B). These results indicate that pro-B cells edit a productive VH rearrangement using preferentially neighboring germ-line V genes as recombination substrates.
Fig. 7.
VH replacement of VHQ52NT employs adjacent VH donor genes. (A) Schematic view of the DH-proximal portion of the IgH locus mapping upstream of the VHQ52NT rearrangement. Colored blocks indicate functional germ-line VH genes ordered based on their physical distance from VHQ52NT. Numbers above blocks indicate unique VH replacements using the indicated VH genes. (B) qRT-PCR determination of VH GLTs for the indicated VH families in pro–B-cell cultures established from Rag1-mutant and VHQ52NT-HO mice. Levels of GLTs are relative to those of the housekeeping gene Rplp0.
Partial Impairment of IgH Allelic Exclusion in VHQ52NT Mice.
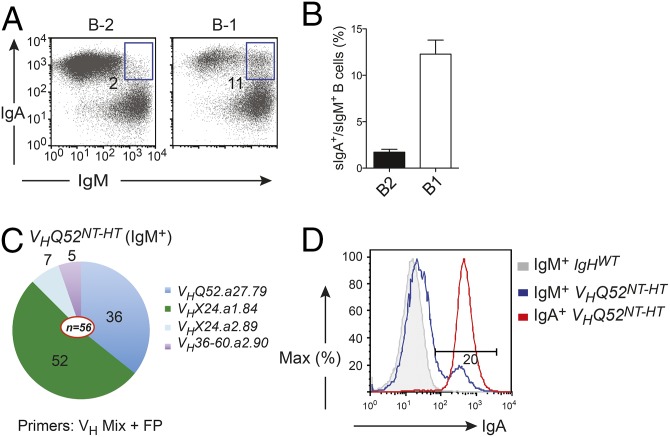
The substantial differences between IgA and IgM cytoplasmic domains may impact the ability of IgA to enforce IgH allelic exclusion. For this reason, we determined the IgH isotype status expressed by peripheral B cells of VHQ52NT-HT mice. Over 80% of transnuclear B cells expressed IgA only on the surface. The remaining fraction consisted of IgM-expressing B cells. Among them, a small, yet consistent, population of sIgA+/sIgM+ double-positive B cells was identified in lymphoid organs of VHQ52NT-HT mice. In particular, whereas B-2 B cells included 2–5% of sIgA+/sIgM+ cells, B-1 B cells consisted of close to 10% of IgH double producers (Fig. 8 A and B). Next, we investigated whether sIgM-only B cells included cells that retained the original VH rearrangement but were unable to express it on the surface in the form of IgA due to a failure to pair with the IgL chain. To address this, we performed VH gene rearrangement analysis in sorted IgM+ B cells including in the PCR primer mixture a VH primer to amplify Q52 family members. Sequencing analysis revealed that a substantial number of PCR products represented the original VHQ52NT rearrangement (Fig. 8C). In support of this result, we found that around 20% of IgM+ B cells in VHQ52NT-HT mice expressed IgA H in the cytoplasm (Fig. 8D). These results indicate that signaling through the cytoplasmic tail of the IgA H chain can enforce IgH allelic exclusion, although with lower efficiency than IgM. The observation that B cells, retaining the VHQ52NT allele, can undergo V-to-DJ recombination on the second IgH chromosome suggests that the latter process can be uncoupled from VH replacement.
Fig. 8.
IgH allelic inclusion is observed in a small fraction of VHQ52NT mature B cells. (A) Representative flow cytometric determination of sIgA/IgM double producers within, respectively, gated B2 (CD19+B220+) and B1 (B220loCD19hi) B cells in the peritoneal cavity of VHQ52NT-HT mice (n = 5). Numbers indicate frequency of boxed sIgA/sIgM double producers. (B) Average frequency of sIgA/sIgM double producers among the indicated B-cell subsets in the peritoneal cavity of VHQ52NT-HT mice (n = 5). (C) VH gene use in purified IgM+ splenic B cells of VHQ52NT-HT mice, assessed by genomic PCR using primers annealing to V genes of the main VH families including Q52. Numbers indicate frequency of IgH rearrangements (n = 56) consisting of the indicated VH genes. (D) Intracellular flow cytometric determination of IgA H expression in the indicated subset of SP B cells in controls (IgHWT) and VHQ52NT-HT mice.
Discussion
Here, we describe the cloning of mice from the nucleus of an IgA-secreting PC. The derivation of pluripotent ES cells through reprogramming of a PC unravels the epigenetic plasticity of terminally differentiated B cells, extending previous work (19).
IgA monoclonal mice were established from a PC residing in the LP of the small intestine, possibly as a result of the recognition of a luminal antigen (25). Although the selecting antigen remains yet undefined, several evidences exclude that the cloned IgA BCR is autoreactive (20). First, serum antibodies in VHQ52NT; Vκgr32NT monoclonal mice lacked reactivity to common self-antigens represented by single- and double-stranded DNA. Second, Rag2; λ5; SLP65 TKO pro-B cells reconstituted with a BCR carrying VHQ52NT; Vκgr32NT specificities lacked autonomous calcium mobilization, hence excluding spontaneous self-aggregation (26). Third, Igκ/λ light chain ratio was comparable between IgA transnuclear and littermate controls. Fourth, IgA cloned mice lacked signs of systemic autoimmunity and aged similarly to littermate controls.
Mice expressing a prerearranged VH rearrangement from an IgA class-switched locus generated close-to-normal numbers of mature B cells, the majority of which expressed IgA. In monoclonal VHQ52NT; Vκgr32NT mice, splenic mature B cells consisted of both FO and MZ B cells. Instead, peritoneal cavity B-1 B cells were largely missing. These results are in accordance with, and extend, previous data (24) indicating that IgA can replace IgM to drive early B-cell development and instruct peripheral B-cell maturation. Whereas VH gene use may influence the FO versus MZ B-cell fate (27), our data reveal that BCR extrinsic factors critically contribute to this decision. Also, the development of FO and MZ B cells at the expense of B-1 B cells may reflect the expression by transnuclear B cells of a BCR specificity enabling selectively the development of a B2/MZ B-cell progenitor (28, 29). Weak tonic BCR signaling may also contribute to the preferential development of FO and MZ B cells in IgA transnuclear mice (30).
We anticipated that transnuclear mice on the Rag-proficient background retained expression of the VHQ52NT specificity among mature B cells. Remarkably, instead, a substantial fraction of SP B cells in VHQ52NT mice lost expression of the original VH rearrangement. In particular, in VHQ52NT HT mice, over 20% of peripheral B cells expressed IgM instead of IgA. An allele-specific PCR strategy revealed that in the latter cells the VHQ52NT allele was consistently inactivated through OF VH replacements. These results are compatible with a scenario whereby an initial OF VH replacement, disrupting VHQ52NT, prompts further VDJ recombination on the second IgH chromosome, allowing the rescue of those B cells that acquire a productive VH rearrangement. IF VH replacements were also identified, hence contributing to the diversification of the IgA+ B-cell pool. CDR3 sequence analysis of VH replacements revealed a highly diversified pool of secondary IgH rearrangements. Within the yet-limited coverage of the IgH repertoire of VHQ52NT mice, these results suggest that transnuclear B cells undergoing VH replacement do not represent a rare population that has undergone substantial clonal expansion. Southern blotting analysis performed on VHQ52NT homozygous B cells revealed that ∼20% of peripheral IgA+ B cells inactivated one VHQ52NT allele. Because VH-to-JH direct joining events were rarely found in VHQ52NT B cells, we conclude that VH replacement represents the preferred mechanism for the diversification of the productive, preimmune, IgH V gene repertoire.
VH replacement was proposed to occur in pro-B as well as in immature B cells, where it may contribute to neutralize BCR autoreactivity (4, 12, 15, 31). In VHQ52NT mice, VH replacements were already found in Ig−B220loCD43+ BM pro-B cells. Because VH replacement was accompanied by de novo addition of n-nucleotides, our data indicate that this process likely occurs in an early pro-B cell that expresses Tdt (32). The high frequency of VH replacements seen in VHQ52NT mice could result from impaired pro-B to pre-B cell transition. However, the analysis of VHQ52NT; Vκgr32NT double Ig insertion mice, largely lacking the progenitor B-cell compartment, showed frequencies of IgM+ B cells that were similar to those of VHQ52NT-only animals. Hence, whereas a contribution of the pre-BCR to the induction of IgH editing cannot be formally excluded, our results, in accordance with previous evidences (4), support a scenario whereby VH replacement of a productive IgH rearrangement starts in an early pro-B cell before pre-BCR expression/signaling.
The loss of a large genomic segment of the CH locus resulting from IgA class switch recombination could contribute to the high rate of VH replacements observed in VHQ52NT mice, through the loss of putative, yet unidentified, negative cis regulatory elements. To address this question, we crossed VHQ52NT mice to B1-8f IgH knock-in mice. In Ig double-insertion mice, VHQ52NT served as a reporter allele to monitor VH replacement in B cells transiting through early B-cell development as a result of B1-8f expression. Whereas most B cells were IgH allelically included, a distinct subset of them ranging between 3% and 5% lost the VHQ52NT allele through VH replacement. The different frequency of B cells undergoing VH replacement in VHQ52NT; B1-8f mice compared with VHQ52NT-only animals renders unlikely the existence of regulatory elements embedded in the CH locus that influence VH replacement. Instead, these results point to differences between IgA and IgM H chains, or levels of their corresponding transcripts (33), in the regulation of IgH locus accessibility to the recombination machinery.
Analysis of V-gene use in VH replacements of VHQ52NT B cells revealed a different scenario from the one described for mice carrying OF VH rearrangements (4). Indeed, IgA transnuclear mice showed a highly restricted set of VH genes engaged in VH replacement. In particular, VHX24.a1.84 contributed to over 80% of all VH replacements. Notably, all VH germ-line genes recruited in VH replacement mapped in close proximity to the VHQ52NT rearrangement. The biased use of VH genes in VH replacement could reflect the limited time to which the productive VHQ52NT rearrangement is exposed to the VDJ recombination machinery. This condition may facilitate the targeting of the RAG proteins to neighboring V genes, as previously suggested (34). The evidence that GLTs for VH genes proximal to VHQ52NT were more abundant in transnuclear pro-B cells compared with controls supports this hypothesis. Higher germ-line transcription of DH-proximal V genes coupled to their preferential recruitment in VH replacement may result from the lack of IGCR1 on the VHQ52NT allele (18). VH replacement may hence represent a mechanism that acquires relevance for the editing of productive VH rearrangements carrying DH-proximal V genes. The failure of VHQ52NT B cells to recruit V genes of distal VH families may depend on VH replacement occurring in a developmental window that precedes IgH locus contraction (35). The identification of a small subset (1–2%) of IgM+ B cells in ovalbumin-specific IgG1 and hematoagglutinin-specific IgG2b transnuclear mice (both using distal J558 VH genes) suggests that secondary IgH rearrangements may also occur in these animals (36, 37).
The IgA H chain cytoplasmic domain is longer and differs substantially in the primary sequence from that of IgM (38). We wondered whether such differences affected the ability of the IgA H chain to signal IgH allelic exclusion. Flow cytometric data revealed that over 80% of B cells in VHQ52NT mice expressed IgA only. The remaining fraction consisted of sIgM+ B cells. Around 20% of the latter cells (accounting for 4% of total peripheral B cells) expressed cytoplasmic IgA H, which was confirmed by successful PCR amplification of the VHQ52NT rearrangement. Moreover, around 2% of B2 B cells and over 10% of peritoneal cavity B-1 B cells expressed both IgA and IgM on the surface. From these results, we conclude that the IgA cytoplasmic tail is able to instruct IgH allelic exclusion, although with lower efficiency than IgM. Similar data were reported for the IgG1 H chain (36).
In summary, this study unravels the contribution of VH replacement to the diversification of the productive preimmune IgH repertoire of mice starting with a restricted pool of antibody specificities. Using two independent Ig transgenic models, we show that 3% or more (reaching a maximum of 20%) of mature B cells edited their initially productive IgH rearrangement through VH replacement. Our results are in agreement and complement data presented in the accompanying paper by Sun et al. (39).
The concomitant presence in HT VHQ52NT mice of diversified pools of, respectively, IgM- and IgA-only B cells offers the unprecedented opportunity to study in a competitive setup the properties of IgA and IgM BCRs to control homeostasis of B cells and their recruitment into T-cell–dependent and –independent immune responses, especially in mucosa-associated lymphoid tissues.
Materials and Methods
Animal Care.
Animals were housed under specific pathogen-free conditions at the IFOM-IEO Campus and maintained according to protocols approved by the IFOM Institutional Animal Care and Use Committee and the Italian Ministry of Health. Rag1−/− mice were purchased from Jackson Laboratories. B1-8f mice were previously described (23). Experiments were performed with 8–16-wk-old animals.
Generation of IgA Transnuclear Mice.
Small intestine LP cells of C57BL/6 X B10D2 F1 mice were prepared as previously described (40) and stained with fluorescent-labeled anti-B220, anti–MHC-II, and anti-IgA antibodies to isolate B220−MHC-II−IgA+ PCs using a MoFlo (Beckman Coulter) cell sorter. SCNT procedures have been previously published (41) and are summarized in SI Materials and Methods.
Flow Cytometry and Cell Sorting.
Single-cell suspensions from lymphoid organs were stained using fluorescent- or biotin-conjugated monoclonal antibodies against mouse CD19 (ID3), CD21 (8D9), CD23 (B3B4), CD45R/B220 (R3A3-6B2), CD25 (7D4), TCRβ (H57-59), CD5 (53-7.3), and IgA (mA-6E1), all from eBioscience. Anti-CD43 (S7) was from BD Biosciences. Anti-IgM (R33.24.12) and anti-Igκ (R33-18-10) antibodies were conjugated in house. For intracellular staining, B cells were fixed, permeabilized in Cytofix/Cytoperm (BD Biosciences), and stained with IgA-specific antibody. Samples were acquired on a FACSCalibur (BD Biosciences) and data analyzed with FlowJo software (Tree Star). Cell sorting was performed on FACSAria (BD Biosciences) after size exclusion of dead cells.
Analysis of Ig V Gene Rearrangements.
Genomic DNA or total RNA was extracted using the All Prep DNA/RNA mini kit according to the manufacturer’s protocol (Qiagen). For VH replacement analysis, genomic DNA was PCR amplified using a mixture of VH family-specific primers (42) in combination with a VHQ52NT FP-specific primer. PCR conditions are listed in SI Materials and Methods. qRT-PCR analysis of VH replacements was performed using cDNA prepared from purified IgM+ B cells using a mixture of VH family-specific primers (42) combined with an oligonucleotide complementary to Cα. VH rearrangements occurring on the second IgH chromosome in IgM+ VHQ52NT-HT B cells were amplified by RT-PCR using VH family-specific primers together with an oligonucleotide annealing to Cµ. Igκ V gene rearrangements were amplified by RT-PCR using a mixture of degenerate forward primers annealing to most Vκ genes (30), in combination with a Cκ reverse primer. PCR products were cloned and subjected to Sanger sequencing. V gene analyses were performed using IGBLAST. Primer sequences are listed in Table S2.
Quantification of VH GLTs.
qRT-PCR was performed in triplicate with SYBR Green-I Master Mix using primer combinations for GLTs listed in Table S2. Values were calculated using the comparative CT method. To normalize for cDNA input, a segment of the housekeeping Rplp0 gene was amplified with primers listed in Table S2.
Southern Blotting Analysis.
Southern blotting was performed on genomic DNA isolated from CD19+ splenic B cells, as previously described (43) and summarized in SI Materials and Methods.
BCR Complementation of Rag2; λ5; SLP65 TKO Pro-B Cells.
Rag2; λ5; SLP65 TKO pro-B cells expressing a tamoxifen-inducible ERT2–SLP65 fusion protein were previously described (26). SLP65 signaling function was restored incubating TKO pro-B cells with 1 µM of 4-Hydroxytamoxifen (4-OHT, Sigma-Aldrich). VHQ52NT and Vκgr32NT specificities were cloned, respectively, into pMIZCC and pMIZYN retroviral vectors, as previously described (26). Retroviral vectors for TCL1-derived IgH and IgL chains were previously described (22). The HEL-specific HyHEL 10 antibody was described in ref. 44. Primers used for cloning of VHQ52NT and Vκgr32NT IgH and IgL chains are listed in Table S2. Retrovirus production and transduction of TKO pro-B cells was performed as previously described (26).
Measurement of Intracellular Calcium Flux.
BCR reconstitution of Rag2; λ5; SLP65 TKO pro-B cells was revealed by staining cells with goat anti-mouse IgM (Jackson ImmunoResearch), anti-mouse Igκ (Southern Biotech), or rat anti-mouse IgA (e-Bioscience) antibodies, respectively. Ca2+ mobilization was measured as described before (26). Briefly, 5 × 106 transduced pro-B cells expressing ERT2–SLP65 were incubated with 5 µg/mL of Indo1 (Molecular probes) and 0.5 μg/mL of Pluronic F-127 (Molecular Probes) in Iscove medium supplemented with 1% serum (Vitromex) at 37 °C for 45 min. After loading, cells were centrifuged, resuspended in Iscove medium with 1% FCS, and stimulated with 1 mM of 4-OHT. Calcium flux was measured on an LSR II flow cytometer (Becton Dickinson).
Enzyme Linked Immunosorbent Assay.
Titration of serum anti-DNA antibodies was performed by ELISA, as previously described (45). Procedures are summarized in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank F. Alberghini for sharing preliminary data and the remaining members of the S.C. group for helpful discussions. We acknowledge N. Hövelmeyer and Ari Waisman for sharing reagents and S. P. Mahrt for suggestions. T. Kurosaki and K. Kometani kindly shared unpublished data. This study was supported by grants from the RIKEN Research Center for Allergy and Immunology through the International Research Collaboration Award (to S.C. and S.F.), the Italian Association for Cancer Research (to S.C.), and the European Research Council through the Italian Ministry of Health (Progetto IDEAS-Fondo per gli Investimenti Ricerca di Base). S.C. was supported by the Italian Foundation for Cancer Research Foundation and the Giovanni Armenise-Harvard Foundation. R.K. was supported by an European Molecular Biology Organization long-term fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417988112/-/DCSupplemental.
References
- 1.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109(Suppl):S45–S55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 2.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6(10):728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 3.Koralov SB, Novobrantseva TI, Hochedlinger K, Jaenisch R, Rajewsky K. Direct in vivo VH to JH rearrangement violating the 12/23 rule. J Exp Med. 2005;201(3):341–348. doi: 10.1084/jem.20041577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koralov SB, Novobrantseva TI, Königsmann J, Ehlich A, Rajewsky K. Antibody repertoires generated by VH replacement and direct VH to JH joining. Immunity. 2006;25(1):43–53. doi: 10.1016/j.immuni.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Radic MZ, Zouali M. Receptor editing, immune diversification, and self-tolerance. Immunity. 1996;5(6):505–511. doi: 10.1016/s1074-7613(00)80266-6. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Burrows PD, Cooper MD. The molecular basis and biological significance of VH replacement. Immunol Rev. 2004;197:231–242. doi: 10.1111/j.0105-2896.2004.0107.x. [DOI] [PubMed] [Google Scholar]
- 7.Kleinfield R, et al. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. Nature. 1986;322(6082):843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- 8.Reth M, Gehrmann P, Petrac E, Wiese P. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. Nature. 1986;322(6082):840–842. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- 9.Lutz J, Müller W, Jäck HM. VH replacement rescues progenitor B cells with two nonproductive VDJ alleles. J Immunol. 2006;177(10):7007–7014. doi: 10.4049/jimmunol.177.10.7007. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, et al. Contribution of Vh gene replacement to the primary B cell repertoire. Immunity. 2003;19(1):21–31. doi: 10.1016/s1074-7613(03)00170-5. [DOI] [PubMed] [Google Scholar]
- 11.Cascalho M, Wong J, Wabl M. VH gene replacement in hyperselected B cells of the quasimonoclonal mouse. J Immunol. 1997;159(12):5795–5801. [PubMed] [Google Scholar]
- 12.Davila M, et al. Multiple, conserved cryptic recombination signals in VH gene segments: Detection of cleavage products only in pro B cells. J Exp Med. 2007;204(13):3195–3208. doi: 10.1084/jem.20071224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng W, et al. Trials and tribulations with VH replacement. Front Immunol. 2014;5:10. doi: 10.3389/fimmu.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taki S, Meiering M, Rajewsky K. Targeted insertion of a variable region gene into the immunoglobulin heavy chain locus. Science. 1993;262(5137):1268–1271. doi: 10.1126/science.8235657. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Nagy Z, Prak EL, Weigert M. Immunoglobulin heavy chain gene replacement: A mechanism of receptor editing. Immunity. 1995;3(6):747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, et al. The site and stage of anti-DNA B-cell deletion. Nature. 1995;373(6511):252–255. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Geissal ED, Li W, Stollar BD. Repertoire diversification in mice with an IgH-locus-targeted transgene for the rearranged VH domain of a physiologically selected anti-ssDNA antibody. Mol Immunol. 2005;42(12):1475–1484. doi: 10.1016/j.molimm.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Guo C, et al. CTCF-binding elements mediate control of V(D)J recombination. Nature. 2011;477(7365):424–430. doi: 10.1038/nature10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochedlinger K, Jaenisch R. Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature. 2002;415(6875):1035–1038. doi: 10.1038/nature718. [DOI] [PubMed] [Google Scholar]
- 20.Shimoda M, Inoue Y, Azuma N, Kanno C. Natural polyreactive immunoglobulin A antibodies produced in mouse Peyer’s patches. Immunology. 1999;97(1):9–17. doi: 10.1046/j.1365-2567.1999.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meixlsperger S, et al. Conventional light chains inhibit the autonomous signaling capacity of the B cell receptor. Immunity. 2007;26(3):323–333. doi: 10.1016/j.immuni.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Dühren-von Minden M, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489(7415):309–312. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]
- 23.Lam KP, Kühn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 24.Duchez S, et al. Premature replacement of mu with alpha immunoglobulin chains impairs lymphopoiesis and mucosal homing but promotes plasma cell maturation. Proc Natl Acad Sci USA. 2010;107(7):3064–3069. doi: 10.1073/pnas.0912393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benckert J, et al. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J Clin Invest. 2011;121(5):1946–1955. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Köhler F, et al. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity. 2008;29(6):912–921. doi: 10.1016/j.immuni.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Kaplinsky J, et al. Antibody repertoire deep sequencing reveals antigen-independent selection in maturing B cells. Proc Natl Acad Sci USA. 2014;111(25):E2622–E2629. doi: 10.1073/pnas.1403278111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7(3):293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 29.Tung JW, Mrazek MD, Yang Y, Herzenberg LA, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc Natl Acad Sci USA. 2006;103(16):6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casola S, et al. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5(3):317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, et al. Regulation of VH replacement by B cell receptor-mediated signaling in human immature B cells. J Immunol. 2013;190(11):5559–5566. doi: 10.4049/jimmunol.1102503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasserman R, Li YS, Hardy RR. Down-regulation of terminal deoxynucleotidyl transferase by Ig heavy chain in B lineage cells. J Immunol. 1997;158(3):1133–1138. [PubMed] [Google Scholar]
- 33.Lutz J, et al. Pro-B cells sense productive immunoglobulin heavy chain rearrangement irrespective of polypeptide production. Proc Natl Acad Sci USA. 2011;108(26):10644–10649. doi: 10.1073/pnas.1019224108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yancopoulos GD, Alt FW. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]
- 35.Kosak ST, et al. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296(5565):158–162. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- 36.Dougan SK, et al. IgG1+ ovalbumin-specific B-cell transnuclear mice show class switch recombination in rare allelically included B cells. Proc Natl Acad Sci USA. 2012;109(34):13739–13744. doi: 10.1073/pnas.1210273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dougan SK, et al. Antigen-specific B-cell receptor sensitizes B cells to infection by influenza virus. Nature. 2013;503(7476):406–409. doi: 10.1038/nature12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Word CJ, Mushinski JF, Tucker PW. The murine immunoglobulin alpha gene expresses multiple transcripts from a unique membrane exon. EMBO J. 1983;2(6):887–898. doi: 10.1002/j.1460-2075.1983.tb01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun A, et al. VH replacement in primary immunoglobulin repertoire diversification. Proc Natl Acad Sci USA. 2015;112:E458–E466. doi: 10.1073/pnas.1418001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413(6856):639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 41.Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394(6691):369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 42.Ehlich A, Martin V, Müller W, Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr Biol. 1994;4(7):573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 43.Casola S. Conditional gene mutagenesis in B-lineage cells. Methods Mol Biol. 2004;271:91–109. doi: 10.1385/1-59259-796-3:091. [DOI] [PubMed] [Google Scholar]
- 44.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 45.Hao Z, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29(4):615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.