Significance
Mammalian hibernators can reduce their metabolic rate by 95% and body temperature to 2 °C. However, their central and peripheral nervous systems retain activity even in cold, through unknown mechanisms. We report here that neurons from hibernating squirrels express uncoupling protein 1 (UCP1), a protein known as a heat generator in brown adipose tissue. We show that squirrel UCP1 acts as the typical thermogenic protein and is up-regulated during torpor, suggesting its thermogenic capability is important during hibernation. Accordingly, we found that the temperature of squirrel brain during the deep torpor associated with hibernation is warmer than the surrounding tissues. We hypothesize that neuronal UCP1 allows squirrels to withstand the long hibernation season and tolerate temperatures prohibitively low for survival and neuronal function in nonhibernating species.
Keywords: thirteen-lined ground squirrel, uncoupling protein, hibernation, thermogenesis, UCP1
Abstract
Hibernating mammals possess a unique ability to reduce their body temperature to ambient levels, which can be as low as −2.9 °C, by active down-regulation of metabolism. Despite such a depressed physiologic phenotype, hibernators still maintain activity in their nervous systems, as evidenced by their continued sensitivity to auditory, tactile, and thermal stimulation. The molecular mechanisms that underlie this adaptation remain unknown. We report, using differential transcriptomics alongside immunohistologic and biochemical analyses, that neurons from thirteen-lined ground squirrels (Ictidomys tridecemlineatus) express mitochondrial uncoupling protein 1 (UCP1). The expression changes seasonally, with higher expression during hibernation compared with the summer active state. Functional and pharmacologic analyses show that squirrel UCP1 acts as the typical thermogenic protein in vitro. Accordingly, we found that mitochondria isolated from torpid squirrel brain show a high level of palmitate-induced uncoupling. Furthermore, torpid squirrels during the hibernation season keep their brain temperature significantly elevated above ambient temperature and that of the rest of the body, including brown adipose tissue. Together, our findings suggest that UCP1 contributes to local thermogenesis in the squirrel brain, and thus supports nervous tissue function at low body temperature during hibernation.
Thirteen-lined ground squirrels (Ictidomys tridecemlineatus) are obligatory hibernating mammals. They are found across a wide range of latitudes, from southern Canada to the Gulf of Mexico. In northern habitats with long winters and limited food resources, ground squirrels breed only once a year, in late spring, and then hibernate in underground burrows from October to April. Hibernation consists of cycling between bouts of torpor and brief interbout arousal periods, each usually lasting less than 24 h (1, 2).
During the long hibernation season, torpid animals undergo dramatic perturbations, including reduction in heart, respiratory, and overall metabolic rates, as well as decreases in core body temperature from 37 °C to just above ambient temperature (often as low as 1–5 °C) (2). Despite such a depressed physiologic phenotype, torpid animals remain sensitive to their environment (3). For example, during extreme winters, when ambient burrow temperatures reach the freezing point of water, most hibernating mammals increase metabolic activity and warm up as a safety precaution to prevent freezing (4). Similarly, arousal can be triggered by sound, touch, or a sudden increase in ambient temperature (3, 5). Thus, hibernating animals maintain activity in their peripheral and central nervous systems. Indeed, physiologic experiments conducted on nerve fibers isolated from torpid hamsters and squirrels demonstrated the ability to generate action potentials at temperatures prohibitively low for their nonhibernating relatives (i.e., rats, mice) (6–8).
Deeply hibernating animals can keep their brain temperatures elevated above ambient by several degrees (9). This may reflect a potential mechanism to support the functionality and integrity of the nervous system in the cold. In the search for a molecular basis for this process, we investigated the expression of uncoupling protein 1 (UCP1). UCP1 is known to be expressed in the inner mitochondrial membrane of brown adipose tissue (BAT) (10, 11), where it generates heat by dissipating the proton gradient set up by the electron-transport chain (12, 13). In hibernating mammals, as well as in human infants and small rodents, UCP1-mediated nonshivering thermogenesis plays a crucial role in the maintenance of core body temperature (14–16). Although UCP1 was originally thought to be restricted to the BAT of placental mammals, recent studies have challenged phylogenetic and tissue distributions, with it being found in marsupials, monotremes, and nonmammals (17–20). Intriguingly, UCP1 mRNA up-regulation was observed in the nervous tissue of cold-exposed common carp (Cyprinus carpio) (19). It was suggested that this temperature-induced UCP1 expression in carp may support local cranial endothermy necessary to survive winter dormancy. Trace amounts of UCP1 mRNA have been detected in mouse cortex (21, 22), but no expression was observed in peripheral nervous tissues (23).
Here, using differential transcriptomics alongside immunohistologic, biochemical, and functional analyses, we show that squirrel neurons from central and peripheral nervous system express a functional ortholog of UCP1. UCP1 protein is localized in neuronal mitochondria and is up-regulated during torpor compared with the summer active state. Functional analysis showed that squirrel UCP1 is capable of decoupling the electron transporting chain, suggesting a role in neuronal thermogenesis. Finally, we show that squirrel brain is warmer than the surrounding tissues, including BAT, in torpid hibernating animals. Our findings suggest a previously unexplored role for UCP1 in maintaining functionality of the nervous system in mammals during hibernation.
Results
Squirrel Nervous Tissue Expresses UCP1.
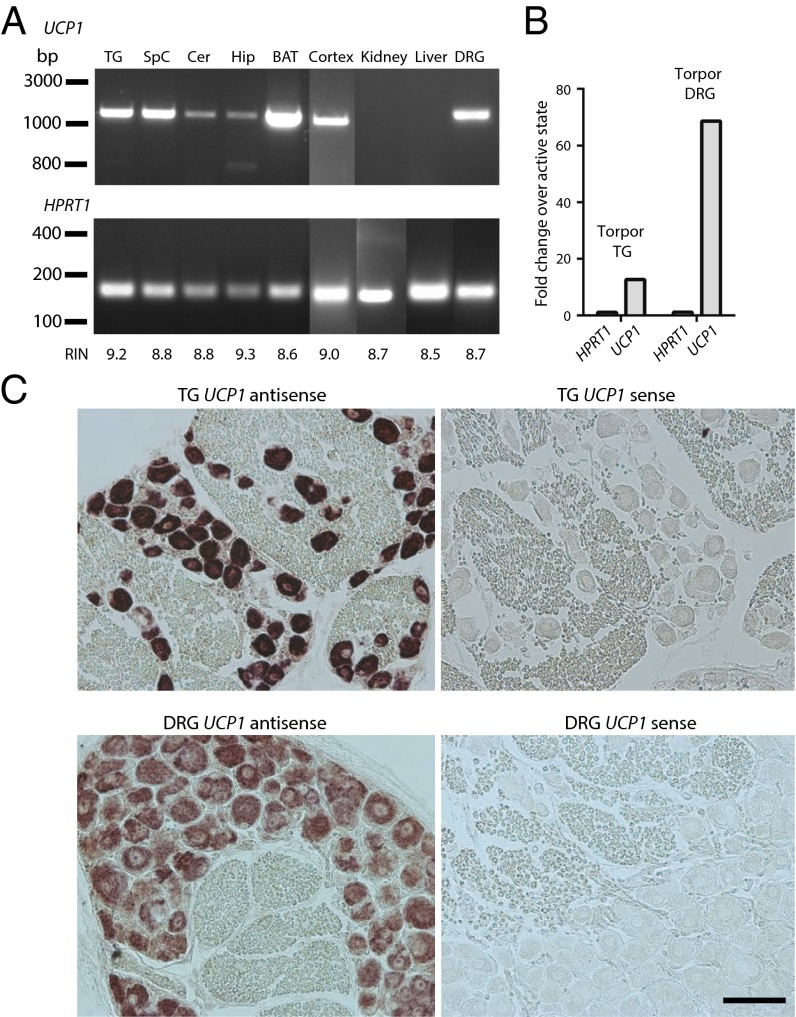
In hibernating squirrels, we detected UCP1 transcripts not only in BAT but also in central (cortex, cerebellum, hippocampus, spinal cord) and peripheral (trigeminal and dorsal root ganglia) nervous tissues of torpid animals (Fig. 1A). We confirmed UCP1 identity by direct sequencing of PCR products from each sample. In contrast, we did not detect UCP1 in kidney and liver, even though we amplified transcripts for the housekeeping gene hypoxanthine phosphoribosyltransferase 1 (HPRT1) (24) from all tissues (Fig. 1A).
Fig. 1.
UCP1 is expressed in neurons of torpid squirrels. (A) PCR amplification of UCP1 and housekeeping gene HPRT1 transcripts from cDNA isolated from various tissues of torpid squirrels. Transcripts were amplified by 35-cycle PCR reactions. The RNA integrity number (RIN) was calculated to assess RNA quality in each sample. TG, trigeminal ganglia; SpC, spinal cord; Cer, cerebellum; Hip, hippocampus; BAT, brown adipose tissue; DRG, dorsal root ganglia; HPRT1, hypoxanthine-guanine phosphoribosyltransferase. (B) Differential transcriptome analysis of TG and DRG from torpid and active squirrels shows up-regulation of UCP1 expression during torpor; the control HPRT1 gene remained unchanged (n = 3 animals for torpor state; n = 2 animals for summer active state). (C) RNA in situ hybridization in TG and DRG from torpid squirrels, showing neuron-specific UCP1 staining (n = 32 sections for TG; n = 40 sections for DRG; n ≥ 2 animals). (Scale bar, 100 µm.)
Transcriptome analysis of trigeminal ganglia (TG) and dorsal root ganglia (DRG) from summer active and hibernating torpid squirrels revealed a dramatic up-regulation of UCP1 during torpor (13- and 68-fold increase in TG and DRG, respectively) (Fig. 1B). The expression of the control HPRT1 transcript remained stable in all conditions (1.1-fold increase in torpor vs. active state for TG and DRG). Notably, we did not detect molecular markers that are normally present in BAT and involved in the regulation of UCP1 expression and activation pathways (15, 25–27) (Table S1). Examination of cDNAs encoding UCP1 using both conventional cloning and de novo transcript assembly did not reveal the presence of isoforms in central and peripheral nervous tissues. To explore UCP1 expression at the cellular level, we examined the distribution of UCP1 transcripts by RNA in situ hybridization. UCP1 was consistently detected in somatosensory neurons of torpid squirrels, but not in the surrounding tissue (Fig. 1C). Taken together, these observations demonstrate that UCP1 transcripts are present in the neurons of hibernating squirrels.
UCP1 Protein Is Present in Neuronal Mitochondria of Torpid Squirrels.
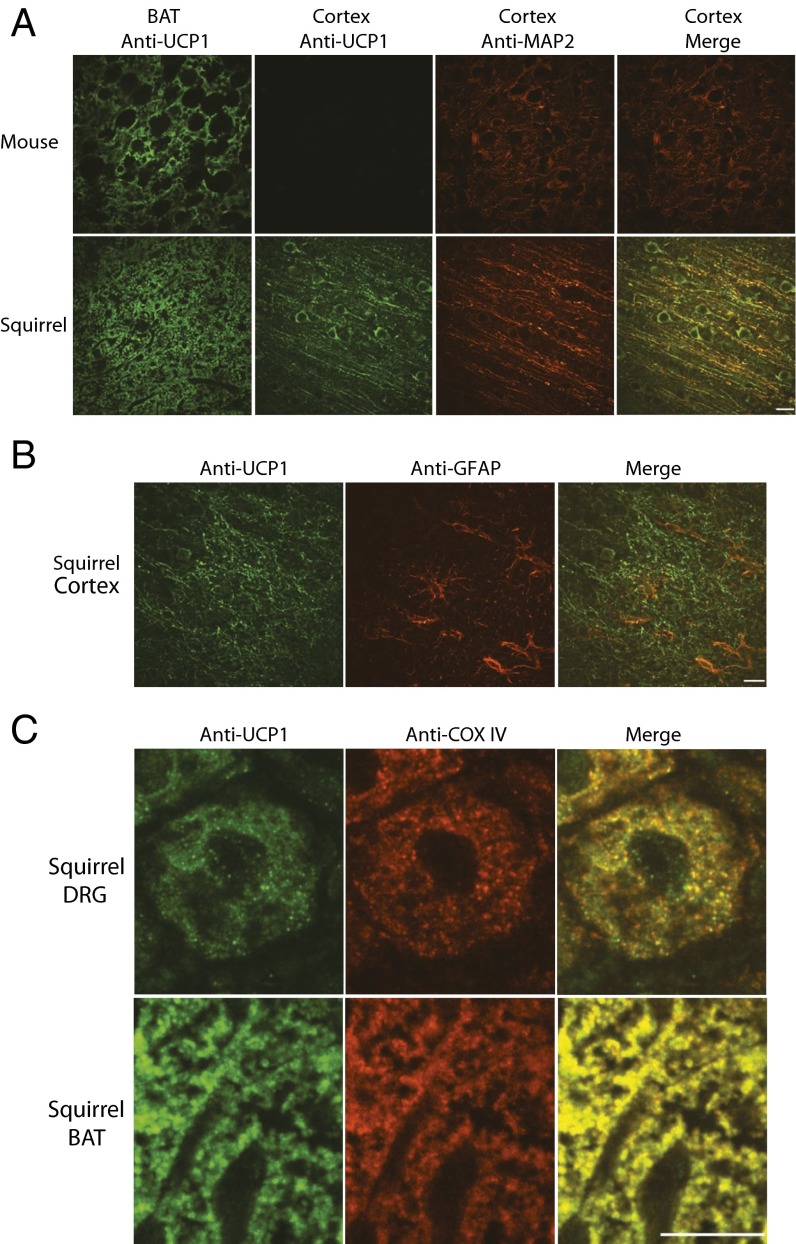
Having established the presence of UCP1 transcripts in torpid squirrel nervous tissues, we next performed immunohistochemical analyses using an antibody targeting a conserved region between mouse and squirrel UCP1. As expected, we observed UCP1 labeling in both squirrel and mouse BAT. Strikingly, we also detected UCP1 protein in squirrel, but not mouse, cortex, hippocampus, TG, and DRG (Fig. 2A and Fig. S1). In squirrel cortex, UCP1 expression partially overlapped with the neuron-specific microtubule-associated protein 2 (MAP2) (28). We observed the same labeling pattern using a different antibody raised against a conserved UCP1 region (Fig. S2). Notably, we did not detect overlap between UCP1 and astrocyte-specific glial fibrillary acidic protein (Fig. 2B). Together, these data demonstrate the presence of UCP1 protein in squirrel neurons.
Fig. 2.
UCP1 protein is expressed in the mitochondria of squirrel neurons. (A) Representative images of mouse and torpid ground squirrel BAT and cortex sections immunolabeled for UCP1 protein (ab10983, green) and for the neuronal marker MAP2 (red). (B) Representative images of torpid ground squirrel cortex sections immunolabeled for UCP1 (ab10983, green) and the astrocyte marker glial fibrillary acidic protein (red). (C) Representative images of torpid squirrel DRG and BAT sections immunolabeled for UCP1 (ab10983, green) and the mitochondrial marker COX IV (red). (Scale bars, 20 µm.) See also Figs. S1 and S2. n ≥ 14 sections for each condition.
To achieve its thermogenic capabilities by uncoupling the electron transport chain, UCP1 must be present in mitochondria (29). Therefore, we conducted colocalization studies in dorsal root ganglia and BAT, using antibodies to both UCP1 and cytochrome c oxidase (COX IV), a major component of the inner mitochondrial membrane electron-transport chain (30). We observed mitochondrial localization of UCP1 in both squirrel neurons and BAT (Fig. 2C).
We tested the presence of UCP1 protein in mitochondrial lysates from different tissues by immunoblot analysis, using an antibody raised against a conserved epitope between mouse and squirrel UCP1. We detected several bands migrating at positions close to the predicted molecular weights of both proteins at 33 kDa in mitochondrial lysates from mouse and torpid and active squirrel BAT (Fig. 3A). We also detected robust staining in mitochondria from squirrel, but not mouse, cortex. These results agree with our immunohistochemical analyses, using the same (Fig. 2 and Fig. S1) or an alternative (Fig. S2) UCP1 antibody. We also detected robust staining in mitochondria from torpid squirrel whole-brain lysate, spinal cord, and pons (Fig. 3A). In contrast, we did not detect any signal in mouse whole brain or spinal cord or in squirrel kidney, liver, or lung (Fig. 3A and Fig. S3A). Together, these results are in agreement with the pattern of UCP1 mRNA expression (Fig. 1) and immunohistochemical analyses (Fig. 2 and Figs. S1 and S2) and strongly suggest the detected bands correspond to different UCP1 species, which are known to migrate on immunoblot at slightly different levels in different preparations and tissues, possibly as a result of posttranslational modifications (31–35). Notably, compared with tissues from summer active squirrels, we detected significant up-regulation of UCP1 protein in mitochondrial lysates from torpid brain, cortex, and spinal cord (P = 0.0194, P = 0.0158, and P = 0.016, respectively; unpaired t test; n ≥ 3 experiments) (Fig. 3B).
Fig. 3.
UCP1 protein is seasonally expressed and present in mitochondrial fractions of lysates from squirrel nervous tissues. (A) Immunoblot analysis of UCP1 and COX IV in mitochondrial fractions isolated from summer active squirrel, torpid squirrel, or mouse tissues. Forty micrograms total protein per sample, n ≥ 5 animals. See also Fig. S3. (B) Quantification of UCP1 levels in torpid versus active squirrels normalized to COX IV loading control (brain, P = 0.0194; cortex, P = 0.0158; spinal cord, P = 0.016; unpaired t test; n = 3 animals for each conditions, n ≥ 3 experiments).
We tested the subcellular localization of squirrel UCP1, using a mitochondria-enrichment assay. A comparison of the total and mitochondria-enriched preparations from squirrel spinal cord showed substantial increases in UCP1 protein, together with the mitochondria-specific ATP synthase (ATP5A). In contrast, we did not detect UCP1 in mitochondrial preparations from mouse spinal cord (Fig. S3B). Thus, immunohistologic and biochemical data demonstrate the expression of UCP1 protein in the mitochondria of squirrel neurons.
Functional Characterization of Squirrel UCP1 Protein.
Squirrel UCP1 cloned from nervous tissue showed 84% and 80% identity to its mouse and human orthologs, respectively (Fig. S4). Because UCP1 function in squirrel neurons is unknown, we investigated its activity in vitro through studying uncoupling properties of sqUCP1 in comparison with its mouse ortholog. Toward this end, we generated tetracycline-inducible HEK293 cell lines with stable genomic integration of squirrel and mouse UCP1 with an in-frame HA-tag at the N terminus (sqUCP1-HA and mUCP1-HA, respectively). Immunoblot analysis confirmed the mitochondrial expression of sqUCP1-HA and mUCP1-HA proteins in these cell lines (Fig. 4A).
Fig. 4.
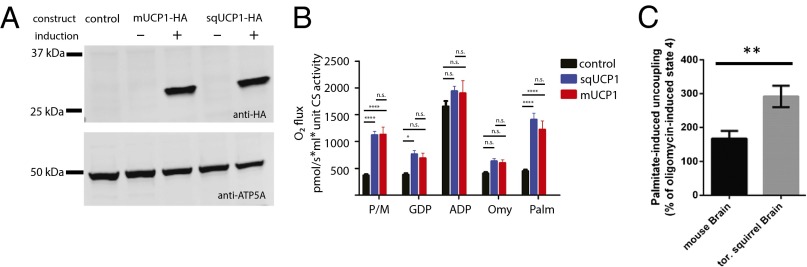
Squirrel UCP1 uncouples oxidative phosphorylation. (A) Immunoblot analysis of HA-tagged mouse (m) and squirrel (sq) UCP1 in total lysates from nontransfected control and stably transfected tetracycline-inducible HEK293 cells. (B) Polarographic respirometry measurements of mitochondria isolated from HEK293 cells stably expressing mouse or squirrel UCP1. Oxygen flux normalized to citrate synthase activity was measured in response to the sequential addition of the following reagents [final concentrations on addition: 5 mM pyruvate/2 mM malate (P/M), 0.75 mM GDP, 1 mM ADP, 2 µg/mL oligomycin (Omy), 0.1 mM palmitate (Palm)]. Data shown as mean ± SEM (n ≥ 7; two-way ANOVA with Tukey’s post hoc test; *P < 0.05; ****P < 0.0001). (C) Fatty-acid (palmitate)-induced uncoupling in mitochondria isolated from mouse and torpid squirrel whole brain. Data are presented as percentage increase above oligomycin-induced state 4 respiration (mean ± SEM; n ≥ 3; unpaired t test; **P < 0.01).
To test uncoupling properties, we isolated mitochondria from HEK293 cells expressing squirrel or mouse UCP1 and analyzed their function using a high-sensitivity polarographic respirometry assay. Mitochondria were incubated in an isolated chamber, and the rates of oxygen consumption in response to the sequential addition of various reaction components were measured (Fig. 4B). We found that UCP1-containing mitochondria exhibited significantly higher basal oxygen consumption rates compared with nontransfected (control) cells when incubated in nonphosphorylating (state 2) conditions in ADP-free media containing pyruvate/malate [normalized O2 flux (pmol), mean ± SEM (n ≥ 7); sqUCP1-HA, 1,088 ± 68; mUCP1-HA, 1,166 ± 157.3; control, 367.7 ± 24.2; sqUCP1 vs. control, P < 0.0001; mUCP1 vs. control, P < 0.0001; two-way ANOVA with Tukey’s post hoc test].
Mouse and human UCP1 are activated by free fatty acids and inhibited by purine nucleotides such as GDP (12, 29, 36–38). Along these lines, addition of GDP specifically reduced the oxygen flux of UCP1 containing mitochondria to such an extent that mUCP1-HA became indistinguishable from control, which remained unchanged (Fig. 4B; sqUCP1-HA, 740.8 ± 65.4; mUCP1-H, 705.4 ± 101.9; control, 381.9 ± 29.9). When ADP was added to induce phosphorylating (state 3) respiration, oxygen consumption rates for all groups increased to similar levels, suggesting the presence of active mitochondria in all three samples (sqUCP1-HA, 1,899 ± 92.9; mUCP1-HA, 1,943 ± 269.3; control, 1,654 ± 102.6). Subsequent titration of oligomycin was sufficient to poison ATP synthase and return the system to nonphosphorylating respiration levels (state 4O) (Fig. 4B; sqUCP1-HA, 625.8 ± 46.5; mUCP1-HA, 606 ± 66.8; control, 406.9 ± 25.3). To test whether squirrel UCP1 is activated by free fatty acids, we finally added 100 μM palmitate, which significantly increased respiration rates of UCP1-containing mitochondria over that of the control (sqUCP1-HA, 1,362 ± 113.5; mUCP1-HA, 1,254 ± 177.5; control, 448 ± 32.3; sqUCP1 vs. control, P < 0.0001; mUCP1 vs. control, P < 0.0001).
When ATP synthase is not active, such as during state 2 or in the presence of oligomycin, respiration rates mainly reflect attempts by the electron transport chain to maintain the proton motive force in the face of proton leak back into the matrix (leak respiration). Thus, the significantly higher oxygen fluxes observed in UCP1-containing mitochondria under basal state 2 conditions and after palmitate addition likely result from increased proton leak through UCP1 and subsequent attempts by the electron transport chain to compensate for this leak. Together, these data show that squirrel UCP1 is indistinguishable from the mouse ortholog and functions as a mitochondrial uncoupling protein.
To analyze the uncoupling abilities of mitochondria isolated from mouse and torpid squirrel brains, we performed a respirometry assay to quantify palmitate-induced uncoupling. Although we did not detect the presence of UCP1 in the mouse brain (Figs. 2A and 3A and Figs. S1 and S2), we detected some level of palmitate-induced uncoupling in this tissue, which likely reflects the presence of other uncoupling proteins, such as UCP2 (39). However, we found that mitochondria isolated from squirrels displayed significantly higher levels of uncoupling compared with mouse brain [291.8 ± 31.8 and 167.1 ± 22.5, respectively (percentage of oligomycin-induced state 4 conditions); P = 0.0075, unpaired t test, mean ± SEM; n ≥ 3; Fig. 4C], supporting the notion that UCP1 is present in mitochondria from torpid squirrel brain.
Squirrel Cortex Has a BAT-Independent Heat Source.
Our findings that UCP1 is expressed in neurons of hibernating torpid squirrels and is capable of decoupling mitochondria led us to hypothesize that UCP1 generates heat in the nervous system. This hypothesis is in accord with an earlier study suggesting that UCP1 contributes to thermogenesis in the brain of cold-acclimated carp (19). To directly test this hypothesis, we compared the temperatures of BAT, cortex, and white adipose tissue (WAT) by decapitation and rapid insertion of a thermocoupled probe in torpid squirrels hibernating at an ambient temperature of 3.8 °C (Fig. 5). Consistent with previous observations in arctic ground squirrels (Spermophilus parryii) (9), we found that BAT and cortex were markedly warmer than WAT and the environment (BAT, 4.2 ± 0.14 °C; cortex, 4.6 ± 0.20 °C; WAT, 3.9 ± 0.16; mean ± SEM, n = 14). Pair-wise comparisons within each animal showed that cortex was consistently warmer than BAT (P = 0.009, ANOVA with Holm-Sidak’s multiple comparisons test; n = 14) and WAT (P = 0.002; Fig. 5B), which agrees with the hypothesis that UCP1 expression in neurons contributes to heat generation during torpor.
Fig. 5.
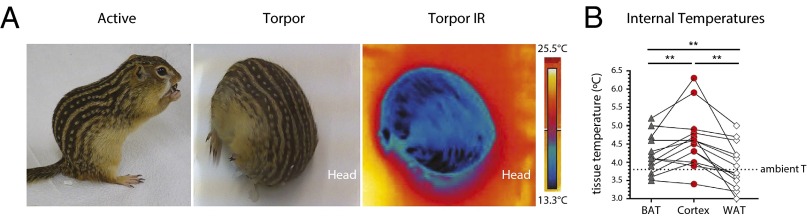
Brain temperature is elevated in torpid hibernating squirrels. (A) Photographs of active and torpid thirteen-lined ground squirrel, and infrared image of torpid squirrel. Infrared photos were taken at room temperature to highlight the difference and increase contrast between ambient temperature and that of the surface of a torpid squirrel moved from the hibernaculum. Scale represents minimum and maximum temperature. (B) Internal temperatures of cortex, BAT, and WAT in torpid squirrels. Lines connecting symbols denote temperature profiles of individual animals. Horizontal line denotes ambient temperature of the hibernaculum (n = 14 animals; mean ± SEM; ANOVA with Holm-Sidak’s multiple comparisons test; **P < 0.01). IR, infrared.
Discussion
Here, we demonstrate the expression of a functional UCP1 protein in neuronal mitochondria of ground squirrels. We show that UCP1 expression is significantly up-regulated during hibernation. These findings provide a molecular explanation for the heat production in the nervous systems of hibernators during torpor. Until now, the prevailing view was that BAT was the sole significant source of heat. However, this notion is inconsistent with our observation that cortex temperature is significantly elevated compared with BAT and other tissues during torpor. Furthermore, the dramatic reductions in heart rate [down to three beats per minute (4)] and cerebral blood flow [∼90% reduction, from 62 ± 16 mL/100 g per minute to 7 ± 4 mL/100 g per minute (40)] in torpid animals pose formidable obstacles to efficient heat exchange between BAT and nervous tissue. Although we do not exclude a significant contribution of BAT-mediated heating, our findings offer an additional nervous tissue-autonomous mechanism. We hypothesize that mammalian hibernators make use of neuronal UCP1 to supply heat, which aids in maintaining basal activity of both central and peripheral nervous systems during hibernation. Our study demonstrates seasonal changes in neuronal UCP1 expression. In the future, it would be interesting to investigate the dynamics of UCP1 levels in different intrahibernation cycles: interbout arousal and early and late torpor, as well as arousal phases.
It is tempting to speculate that other mammalian hibernators may use a similar mechanism. For example, extreme hibernators such as arctic ground squirrels experience torpor at very low ambient temperatures (≤−10 °C). These unique animals experience zero to subzero temperatures in several internal organs and tissues (41), but brain temperature is maintained at a constantly elevated level of 4 °C (9). Similar to thirteen-lined ground squirrels (their close relative), arctic ground squirrels may potentially rely on UCP1 to support the integrity and function of the nervous system during deep torpor. In this case, however, UCP1 expression should be seasonal, as shown here for the thirteen-lined ground squirrels, because an earlier study did not detect UCP1 transcripts in the brain of active arctic ground squirrels (42). It will be interesting, therefore, to evaluate the expression of UCP1 transcripts and protein in torpid animals.
During arousal, hibernators significantly increase oxygen consumption rates and rapidly perfuse torpid tissues. In contrast, there may be danger of ischemia during entrance into and arousal from torpor (2). How mammalian hibernators are able to adapt to rapidly fluctuating oxygen levels without incurring oxidative damage, and how they avoid reperfusion injury in the brain and other sensitive tissues, is not well understood. Multiple studies have shown that hibernators display resistance to ischemia/reperfusion injury and, during torpor, increase expression of molecules that help regulate oxidative stress, such as superoxide dismutase 1 and 2, catalase, and glutathione peroxidase (2, 43–46). Interestingly, uncoupling proteins have been linked to reduction of reactive oxygen species formation (47). Although such a role for UCP1 is more controversial than for some other uncoupling proteins, there is still evidence to suggest that UCP1 may indeed function to regulate reactive oxygen species levels (48–50). Further studies are needed to explore the role of UCP1 in regulation of reactive oxygen species level during hibernation cycles.
Notably, we did not detect BAT-specific molecular markers other than UCP1 in the squirrel nervous tissue (Table S1) (15, 25–27). This suggests that ground squirrels evolved alternative molecular mechanisms for the regulation of UCP1 expression in their neurons that differ from those in BAT. However, it has been demonstrated that levels of nonesterified serum fatty acids are elevated during torpor in different mammalian hibernators, including ground squirrels (9, 51), hamsters (52), and black bears (53). This pool of free fatty acids in the blood may promote activation of UCP1 in the torpid brain. Recently, adipocyte-specific fatty acid binding protein 4 (FABP4) was identified in different regions of the thirteen-lined ground squirrel brain. Moreover, FABP4 was found to be up-regulated in nervous tissue during hibernation, offering a potential pathway for the uptake of fatty acids by torpid brain (54). This study, however, did not identify UCP1 mRNA in torpid brain, even though we detected significant amounts of UCP1 protein in this tissue.
Identification of UCP1 protein in the ground squirrel brain is interesting from an evolutionary point of view. To our knowledge, this report is the first demonstration of UCP1 protein in neuronal mitochondria of a mammal. Earlier reports did not identify UCP1 in mouse TG and DRG (23) and detected only trace amounts of UCP1 transcript in mouse cortex (∼0.01% of UCP1 level in BAT) (21, 22), suggesting neuronal UCP1 expression could be a specific feature pertaining to animals that have to withstand prolonged periods of extreme hypothermia.
In support of this hypothesis, UCP1 was previously identified in the brain of common carp (C. carpio) (19). In carp, UCP1 transcripts are twofold up-regulated in different brain regions after cold acclimation. Similar to the conclusions drawn here, this molecular adaptation was suggested to support nervous tissue function and thermal adaptation of brain metabolism in cold water, and potentially to support winter dormancy.
Cranial endothermy was also discovered in lamnid sharks, billfishes, tunas, and opah and was proposed as a physiologic adaptation to deep dives in cold water (Fig. 6) (55–57). However, these divergent groups of fish evolved this capability through UCP1-independent mechanisms. Sharks use a vascular heat exchange system (56), whereas billfish developed heater organs derived from modified, noncontractile extraocular muscles that can increase local cranial temperature 13 °C above ambient water condition (58).
Fig. 6.

Cranial endothermy in vertebrates. Examples of different strategies for cranial endothermy in three groups of fish and a hibernating mammal. Images of fish were obtained from Wikimedia Commons.
Recently, localized heat production was described in the Lesser hedgehog tenrec (Echinops telfairi), a protoendothermic mammal that regulates its core body temperature only during the reproductive season. UCP1-containing fat deposits were identified in the abdomen of these animals, just adjacent to the gonads. Therefore, tenrecs may support their reproductive function using the strategy of localized endothermy (18).
Our findings provide a molecular explanation for the increased heat production in the nervous system of thirteen-lined ground squirrels during torpor, supporting the notion that localized endothermy is an essential prerequisite for survival despite cold core body temperatures, a strategy that has evolved independently multiple times over the course of vertebrate evolution.
Methods
Animals were housed in a pathogen-free facility at Yale University. All animal procedures were performed in compliance with the Institutional Animal Care and Use Committee of Yale University. Animal handling, tissue collection, temperature measurements, deep sequencing, cloning, in situ hybridization, immunohistochemistry, generation of stable cell lines, mitochondrial isolation, western blotting, and respirometry were carried out following the detailed protocols described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Eve Schneider for comments throughout the study, Margaretha Blignaut for help with animal imaging, and Joseph DePonte for coordinating the construction of the hibernaculum. We are grateful to Oroboros Instruments for the loan of an Oxygraph-2k. This work was supported by fellowships from the Beckman Foundation and Alfred P. Sloan Foundation (to E.O.G.), National Institutes of Health Grants T32 HG-3198-10 (to W.J.L.) and R01 DK-40936 (to G.I.S.), and startup funds from Yale University (to S.N.B. and E.O.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421419112/-/DCSupplemental.
References
- 1.Merriman DK, Lahvis G, Jooss M, Gesicki JA, Schill K. Current practices in a captive breeding colony of 13-lined ground squirrels (Ictidomys tridecemlineatus) Lab Anim (NY) 2012;41(11):315–325. doi: 10.1038/laban.150. [DOI] [PubMed] [Google Scholar]
- 2.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: Cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83(4):1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 3.Lyman CP, O’Brien RC. Sensitivity to low temperature in hibernating rodents. Am J Physiol. 1972;222(4):864–869. doi: 10.1152/ajplegacy.1972.222.4.864. [DOI] [PubMed] [Google Scholar]
- 4.Lyman CP. The myocardium—its biochemistry and biophysics. III. Hibernation in animals. Hibernation in mammals. Circulation. 1961;24(2):434–445. doi: 10.1161/01.cir.24.2.434. [DOI] [PubMed] [Google Scholar]
- 5.Heller HC, Colliver GW. CNS regulation of body temperature during hibernation. Am J Physiol. 1974;227(3):583–589. doi: 10.1152/ajplegacy.1974.227.3.583. [DOI] [PubMed] [Google Scholar]
- 6.Chatfield PO, et al. Effects of cooling on nerve conduction in a hibernator, golden hamster, and non-hibernator, albino rat. Am J Physiol. 1948;155(2):179–185. doi: 10.1152/ajplegacy.1948.155.2.179. [DOI] [PubMed] [Google Scholar]
- 7.Kehl TH, Morrison P. Peripheral nerve function and hibernation in the thirteen-lined ground squirrel, Spsrmophilus tridecemlineaius. Bulletin of the Museum of Comparative Zoology Harvard. 1960;124:387–403. [Google Scholar]
- 8.Paintal AS. Block of conduction in mammalian myelinated nerve fibres by low temperatures. J Physiol. 1965;180(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- 9.Barger JL, Barnes BM, Boyer BB. Regulation of UCP1 and UCP3 in arctic ground squirrels and relation with mitochondrial proton leak. J Appl Physiol (1985) 2006;101(1):339–347. doi: 10.1152/japplphysiol.01260.2005. [DOI] [PubMed] [Google Scholar]
- 10.Kozak LP, Britton JH, Kozak UC, Wells JM. The mitochondrial uncoupling protein gene. Correlation of exon structure to transmembrane domains. J Biol Chem. 1988;263(25):12274–12277. [PubMed] [Google Scholar]
- 11.Nicholls DG, Bernson VS, Heaton GM. The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl. 1978;32:89–93. doi: 10.1007/978-3-0348-5559-4_9. [DOI] [PubMed] [Google Scholar]
- 12.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151(2):400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricquier D. Uncoupling protein 1 of brown adipocytes, the only uncoupler: A historical perspective. Front Endocrinol (Lausanne) 2011;2:85. doi: 10.3389/fendo.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013;27(3):234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enerbäck S, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387(6628):90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 17.Hughes DA, Jastroch M, Stoneking M, Klingenspor M. Molecular evolution of UCP1 and the evolutionary history of mammalian non-shivering thermogenesis. BMC Evol Biol. 2009;9:4. doi: 10.1186/1471-2148-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oelkrug R, et al. Brown fat in a protoendothermic mammal fuels eutherian evolution. Nat Commun. 2013;4:2140. doi: 10.1038/ncomms3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jastroch M, Buckingham JA, Helwig M, Klingenspor M, Brand MD. Functional characterisation of UCP1 in the common carp: Uncoupling activity in liver mitochondria and cold-induced expression in the brain. J Comp Physiol B. 2007;177(7):743–752. doi: 10.1007/s00360-007-0171-6. [DOI] [PubMed] [Google Scholar]
- 20.Jastroch M, Wuertz S, Kloas W, Klingenspor M. Uncoupling protein 1 in fish uncovers an ancient evolutionary history of mammalian nonshivering thermogenesis. Physiol Genomics. 2005;22(2):150–156. doi: 10.1152/physiolgenomics.00070.2005. [DOI] [PubMed] [Google Scholar]
- 21.Lengacher S, Magistretti PJ, Pellerin L. Quantitative rt-PCR analysis of uncoupling protein isoforms in mouse brain cortex: Methodological optimization and comparison of expression with brown adipose tissue and skeletal muscle. J Cereb Blood Flow Metab. 2004;24(7):780–788. doi: 10.1097/01.WCB.0000122743.72175.52. [DOI] [PubMed] [Google Scholar]
- 22.Mehler-Wex C, et al. Microarray analysis reveals distinct gene expression patterns in the mouse cortex following chronic neuroleptic and stimulant treatment: Implications for body weight changes. J Neural Transm. 2006;113(10):1383–1393. doi: 10.1007/s00702-005-0425-y. [DOI] [PubMed] [Google Scholar]
- 23.Manteniotis S, et al. Comprehensive RNA-Seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in Trigeminal ganglia. PLoS ONE. 2013;8(11):e79523. doi: 10.1371/journal.pone.0079523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otis JP, Ackermann LW, Denning GM, Carey HV. Identification of qRT-PCR reference genes for analysis of opioid gene expression in a hibernator. J Comp Physiol B. 2010;180(4):619–629. doi: 10.1007/s00360-009-0430-9. [DOI] [PubMed] [Google Scholar]
- 25.Kajimura S, et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 2008;22(10):1397–1409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seale P, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matus A, Bernhardt R, Hugh-Jones T. High molecular weight microtubule-associated proteins are preferentially associated with dendritic microtubules in brain. Proc Natl Acad Sci USA. 1981;78(5):3010–3014. doi: 10.1073/pnas.78.5.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricquier D, Gaillard JL, Turc JM. Microcalorimetry of isolated mitochondria from brown adipose tissue. Effect of guanosine-di-phosphate. FEBS Lett. 1979;99(1):203–206. doi: 10.1016/0014-5793(79)80279-3. [DOI] [PubMed] [Google Scholar]
- 30.Capaldi RA. Structure and function of cytochrome c oxidase. Annu Rev Biochem. 1990;59:569–596. doi: 10.1146/annurev.bi.59.070190.003033. [DOI] [PubMed] [Google Scholar]
- 31.Zaninovich AA, Raíces M, Rebagliati I, Ricci C, Hagmüller K. Brown fat thermogenesis in cold-acclimated rats is not abolished by the suppression of thyroid function. Am J Physiol Endocrinol Metab. 2002;283(3):E496–E502. doi: 10.1152/ajpendo.00540.2001. [DOI] [PubMed] [Google Scholar]
- 32.Harper JA, et al. Artifactual uncoupling by uncoupling protein 3 in yeast mitochondria at the concentrations found in mouse and rat skeletal-muscle mitochondria. Biochem J. 2002;361(Pt 1):49–56. doi: 10.1042/0264-6021:3610049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mzilikazi N, Jastroch M, Meyer CW, Klingenspor M. The molecular and biochemical basis of nonshivering thermogenesis in an African endemic mammal, Elephantulus myurus. Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R2120–R2127. doi: 10.1152/ajpregu.00427.2007. [DOI] [PubMed] [Google Scholar]
- 34.Carroll AM, Porter RK, Morrice NA. Identification of serine phosphorylation in mitochondrial uncoupling protein 1. Biochim Biophys Acta. 2008;1777(7-8):1060–1065. doi: 10.1016/j.bbabio.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Clarke KJ, et al. A role for ubiquitinylation and the cytosolic proteasome in turnover of mitochondrial uncoupling protein 1 (UCP1) Biochim Biophys Acta. 2012;1817(10):1759–1767. doi: 10.1016/j.bbabio.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 36.Rial E, Poustie A, Nicholls DG. Brown-adipose-tissue mitochondria: The regulation of the 32000-Mr uncoupling protein by fatty acids and purine nucleotides. Eur J Biochem. 1983;137(1-2):197–203. doi: 10.1111/j.1432-1033.1983.tb07815.x. [DOI] [PubMed] [Google Scholar]
- 37.Rial E, Nicholls DG. The regulation of the proton conductance of brown fat mitochondria. Identification of functional and non-functional nucleotide-binding sites. FEBS Lett. 1983;161(2):284–288. doi: 10.1016/0014-5793(83)81026-6. [DOI] [PubMed] [Google Scholar]
- 38.Shabalina IG, Jacobsson A, Cannon B, Nedergaard J. Native UCP1 displays simple competitive kinetics between the regulators purine nucleotides and fatty acids. J Biol Chem. 2004;279(37):38236–38248. doi: 10.1074/jbc.M402375200. [DOI] [PubMed] [Google Scholar]
- 39.Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: In support of function and survival. Nat Rev Neurosci. 2005;6(11):829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- 40.Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab. 1994;14(2):193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- 41.Barnes BM. Freeze avoidance in a mammal: Body temperatures below 0 degree C in an Arctic hibernator. Science. 1989;244(4912):1593–1595. doi: 10.1126/science.2740905. [DOI] [PubMed] [Google Scholar]
- 42.Boyer BB, Barnes BM, Lowell BB, Grujic D. Differential regulation of uncoupling protein gene homologues in multiple tissues of hibernating ground squirrels. Am J Physiol. 1998;275(4 Pt 2):R1232–R1238. doi: 10.1152/ajpregu.1998.275.4.R1232. [DOI] [PubMed] [Google Scholar]
- 43.Allan ME, Storey KB. Expression of NF-κB and downstream antioxidant genes in skeletal muscle of hibernating ground squirrels, Spermophilus tridecemlineatus. Cell Biochem Funct. 2012;30(2):166–174. doi: 10.1002/cbf.1832. [DOI] [PubMed] [Google Scholar]
- 44.Vucetic M, et al. The impact of cold acclimation and hibernation on antioxidant defenses in the ground squirrel (Spermophilus citellus): An update. Free Radic Biol Med. 2013;65:916–924. doi: 10.1016/j.freeradbiomed.2013.08.188. [DOI] [PubMed] [Google Scholar]
- 45.Osborne PG, Hashimoto M. Brain antioxidant levels in hamsters during hibernation, arousal and cenothermia. Behav Brain Res. 2006;168(2):208–214. doi: 10.1016/j.bbr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Bogren LK, Olson JM, Carpluk J, Moore JM, Drew KL. Resistance to systemic inflammation and multi organ damage after global ischemia/reperfusion in the arctic ground squirrel. PLoS ONE. 2014;9(4):e94225. doi: 10.1371/journal.pone.0094225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011;51(6):1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 48.Adjeitey CN, Mailloux RJ, Dekemp RA, Harper ME. Mitochondrial uncoupling in skeletal muscle by UCP1 augments energy expenditure and glutathione content while mitigating ROS production. Am J Physiol Endocrinol Metab. 2013;305(3):E405–E415. doi: 10.1152/ajpendo.00057.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Echtay KS, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415(6867):96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 50.Oelkrug R, Goetze N, Meyer CW, Jastroch M. Antioxidant properties of UCP1 are evolutionarily conserved in mammals and buffer mitochondrial reactive oxygen species. Free Radic Biol Med. 2014;77:210–216. doi: 10.1016/j.freeradbiomed.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Nelson CJ, Otis JP, Carey HV. Global analysis of circulating metabolites in hibernating ground squirrels. Comp Biochem Physiol Part D Genomics Proteomics. 2010;5(4):265–273. doi: 10.1016/j.cbd.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Weitten M, Robin JP, Oudart H, Pévet P, Habold C. Hormonal changes and energy substrate availability during the hibernation cycle of Syrian hamsters. Horm Behav. 2013;64(4):611–617. doi: 10.1016/j.yhbeh.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 53.LeBlanc PJ, et al. Correlations of plasma lipid metabolites with hibernation and lactation in wild black bears Ursus americanus. J Comp Physiol B. 2001;171(4):327–334. doi: 10.1007/s003600100180. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz C, Hampton M, Andrews MT. Seasonal and regional differences in gene expression in the brain of a hibernating mammal. PLoS ONE. 2013;8(3):e58427. doi: 10.1371/journal.pone.0058427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Runcie RM, Dewar H, Hawn DR, Frank LR, Dickson KA. Evidence for cranial endothermy in the opah (Lampris guttatus) J Exp Biol. 2009;212(Pt 4):461–470. doi: 10.1242/jeb.022814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Block BA, Carey FG. Warm brain and eye temperatures in sharks. J Comp Physiol B. 1985;156(2):229–236. doi: 10.1007/BF00695777. [DOI] [PubMed] [Google Scholar]
- 57.Carey FG. A brain heater in the swordfish. Science. 1982;216(4552):1327–1329. doi: 10.1126/science.7079766. [DOI] [PubMed] [Google Scholar]
- 58.Block BA. Endothermy in fish: Thermogenesis, ecology and evolution. Biochemistry and Molecular Biology of Fishes. 1991;1:269–311. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.