Key Points
Expanded erythropoiesis strongly drives hepcidin suppression in severe transfusion-dependent HbE β-thalassemia.
β-thalassemia carriers, but not HbE carriers, have enhanced erythropoiesis associated with mildly suppressed hepcidin.
Abstract
Hemoglobin E (HbE) β-thalassemia is the most common severe thalassemia syndrome across Asia, and millions of people are carriers. Clinical heterogeneity in HbE β-thalassemia is incompletely explained by genotype, and the interaction of phenotypic variation with hepcidin is unknown. The effect of thalassemia carriage on hepcidin is also unknown, but it could be relevant for iron supplementation programs aimed at combating anemia. In 62 of 69 Sri Lankan patients with HbE β-thalassemia with moderate or severe phenotype, hepcidin was suppressed, and overall hepcidin inversely correlated with iron accumulation. On segregating by phenotype, there were no differences in hepcidin, erythropoiesis, or hemoglobin between severe or moderate disease, but multiple linear regression showed that erythropoiesis inversely correlated with hepcidin only in severe phenotypes. In moderate disease, no independent predictors of hepcidin were identifiable; nevertheless, the low hepcidin levels indicate a significant risk for iron overload. In a population survey of Sri Lankan schoolchildren, β-thalassemia (but not HbE) trait was associated with increased erythropoiesis and mildly suppressed hepcidin, suggesting an enhanced propensity to accumulate iron. In summary, the influence of erythropoiesis on hepcidin suppression associates with phenotypic disease variation and pathogenesis in HbE β-thalassemia and indicates that the epidemiology of β-thalassemia trait requires consideration when planning public health iron interventions.
Introduction
Hemoglobin E (HbE) β-thalassemia is the most common severe thalassemia syndrome in South and Southeast Asia, with more than 19 000 births annually.1,2 HbE β-thalassemia exhibits marked clinical heterogeneity: patients with identical β-thalassemia mutations show considerable phenotypic differences ranging from severe anemia associated with increasing splenomegaly, growth retardation, and functional defects to a much milder condition with normal growth and development, mild or modest splenomegaly, and lack of bone deformity and functional impairment.3,4 Iron overload resulting in cardiomyopathy is the most common cause of death in patients with transfusion-dependent thalassemia,5 but those with nontransfusion-dependent thalassemia are also at high risk for iron overload and its complications, especially hepatic and endocrine disease.6 Patients with severe thalassemia phenotypes require transfusion to suppress erythropoiesis and maintain function, whereas patients with moderate and milder forms can be managed conservatively. All forms of HbE β-thalassemia are associated with increased body iron, but the interaction of phenotypic variation with iron regulation in this condition has not been described.3
Hepcidin regulates systemic iron status by controlling dietary iron absorption and iron release from macrophages.7 Hepcidin synthesis is suppressed by erythropoiesis but is upregulated by iron accumulation and inflammation.8 Normally, these signals are balanced to provide an appropriate level of iron to meet erythropoietic demand, but in thalassemia, excessive erythropoiesis suppresses hepcidin, increasing iron absorption.9,10 Iron from transfusions compounds iron loading. Hepcidin concentrations are suppressed in β-thalassemia,11,12 as is hepatic HAMP mRNA expression in patients with thalassemia major (TM)13,14 and in the C57Bl/6 Hbbth3/+ thalassemia mouse model.15 However, patients with thalassemia intermedia exhibit lower liver HAMP mRNA than those with TM,14 probably because of the transfusions that are required to stabilize TM disease.16 Liver hepcidin mRNA expression in patients with TM and thalassemia intermedia correlates inversely with soluble transferrin receptor (sTfR) and erythropoietin (EPO), but not with iron stores.13 Hepcidin levels have not thus far been measured in HbE β-thalassemia.
The very high prevalence of anemia in Asia, making up 37.5% of the global anemia burden,17 has led to recommendations for universal iron supplementation or fortification of food with iron in these settings.18 However, the clinical burden of HbE β-thalassemia in Asia implies that β-thalassemia and HbE trait are also extremely common, and indeed, 6.6% of the Asian population carries a significant hemoglobinopathy.19 Individuals with thalassemia trait have increased erythropoiesis,20 but the effect of this phenomenon on hepcidin in the general population has not been reported. Effects of these genotypes on iron absorption could have important implications for the safety of iron intervention programs.
We evaluated the regulation of hepcidin in patients with HbE β-thalassemia and in carriers for this condition in 2 cross-sectional studies in Sri Lanka. First, in 69 patients with HbE β-thalassemia with diverse, well-characterized clinical phenotypes managed at a specialist clinic,4 we investigated associations between serum hepcidin and parameters of erythropoiesis, iron loading, and inflammation. Second, using samples from a national population-based hemoglobinopathy screening study, we compared erythropoiesis and hepcidin regulation between iron-replete adolescents who carry β-thalassemia or HbE mutations and those without these conditions. Our findings demonstrate that erythropoietic suppression of hepcidin varies with disease phenotype (despite similar or identical β-globin gene mutations), suggesting that variable regulation of hepcidin may be an important disease modifier in HbE β-thalassemia. Furthermore, we show that mild hepcidin suppression is present at population level in individuals with β-thalassemia trait.
Methods
HbE β-thalassemia study
Patients.
Patients in this sample (n = 69; 27/69 male) are part of a cohort of more than 200 participants with HbE β-thalassemia followed for up to 15 years at the National Thalassaemia Centre, District Hospital, Kurunegala, Sri Lanka, who were the first to undergo hepatic iron estimation by magnetic resonance imaging when this option became locally available. The diagnosis of HbE β-thalassemia had been confirmed by hemoglobin analysis and sequencing of the HBB genes, as previously described.21 All patients in this group had either β0 or severe β+ thalassemia mutations.
Long-term follow-up of this cohort has enabled classification of patients into moderate and severe clinical phenotypes, as previously detailed.4,22 Briefly, the moderate group included patients who either had grown and developed normally without regular transfusions (apart from episodic transfusions during infection or pregnancy), or had been started on transfusions elsewhere for uncertain reasons and in whom transfusion had been safely stopped. The severe group comprised patients who had increasing splenomegaly, retarded growth, bone deformity, or poor exercise tolerance, necessitating treatment with regular transfusion; for economic and related reasons, transfusions were dosed to control these complications, rather than achieve a particular pretransfusion hemoglobin level.
Standard chelation therapy (deferoxamine) was given when serum ferritin exceeded 1000 ng/mL, although because of a period of lack of availability or variable compliance, the control of iron accumulation was inadequate in some cases. Because previously it had been standard of care to splenectomize patients with significant splenomegaly, a large proportion of the group has undergone splenectomy.
Controls.
Ferritin, Hb, and hepcidin were measured in 25 iron-replete, nonthalassemic Sri Lankan individuals (9 male) to provide data matched for ethnicity and setting.
β-thalassemia trait study
Participants.
From a recent survey of ∼7000 Sri Lankan schoolchildren for hemoglobin variants and iron deficiency, a sample from 1612 children with low red cell indices (mean cell volume [MCV] <80 fL, mean cell hemoglobin [MCH] <27 pg, or both) and 209 control participants with normal indices was analyzed. Children with low MCV/MCH were slightly younger but had a similar sex ratio compared with control children. The low MCV/MCH population had a higher prevalence of hemoglobinopathy carriage (6.8% β-thalassemia trait and 1.7% HbE trait vs 0% in controls) and were used to assess the effect of thalassemia trait on hepcidin and iron indices.
Assays
Liver iron concentration (LIC) was measured using spin-density projection-assisted R2-magnetic resonance imaging (FerriScan).23
Laboratory measurements.
Hemoglobin values represented the mean hemoglobin values taken during the previous year. Five-milliliter venous pretransfusion blood was collected into plain, heparin, and EDTA tubes. Hematological indices were measured in the EDTA sample (Coulter Electronics). All samples were centrifuged, and plasma or sera was separated and stored at −20°C until shipment to Oxford on dry ice. Serum ferritin was measured by chemiluminescence using a VITROS ECi immunodiagnostic system (Ortho Clinical Diagnostics). Plasma erythropoietin (Epo) was measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems). Serum alanine aminotransferase (ALT) was measured using a UV kinetic method on a BS300 clinical chemistry analyzer (Mindray). Transferrin saturation was determined using the urea gel method of Evans and Williams24 and calculated from band quantitation performed using Scion Image software. Transferrin saturation reference range in normal participants by this method: 16% to 56%. Nontransferrin bound iron (NTBI) was measured by the method of Singh et al,25 using nitrilotriacetate as a collecting ligand for serum NTBI with high-performance liquid chromatography detection for the Fe-nitrilotriacetate complex. The NTBI reference range was −2.29 to −0.35 µM. Labile plasma iron, considered the redox-active component of NTBI rapidly accessible by chelators, was measured as described26; reference range, −0.102 to 0.178 µM (from Le Lan et al27). Serum Growth Differentiation Factor-15 (GDF-15), a measure of erythropoiesis known to be elevated in thalassemia and sensitive to modulation of erythropoiesis,16 was measured by ELISA (R&D Systems; reference ranges, 278-1064 pg/mL). sTfRs were measured by ELISA (HbE β-thalassemia study; 2B Scientific, Oxford Biosystems; reference ranges, 0.9-3.3 nmol/L; β-thalassemia trait study: R&D Systems, reference range, 8.7-27.1 nmol/L, both from manufacturer). High-sensitivity C-reactive protein (CRP) was measured by latex agglutination, using the Abbott Architect c16000 with a Multigent CRP-Vario reagent kit (reportable range, 0.1-160 mg/L). Plasma hepcidin was determined using the hepcidin-25 (human) enzyme immunoassay kit (Bachem)28 for the HbE β-thalassemia study and using the DRG hepcidin immunoassay for the population study.
Statistical analysis
Variables were inspected for normality and data log-transformed if necessary for parametric analysis. Arithmetic means for normally distributed (age, hemoglobin, fetal hemoglobin [HbF], total transfusions, transferrin saturation, NTBI, labile plasma iron) and geometric means for skewed data (hepcidin, ferritin, sTfR, LIC, EPO, GDF-15, CRP, ALT) were calculated, and ranges for data were reported. Statistical significance was defined as P < .05. Comparisons between means were made using the Student t-test. Pearson product-moment correlation was used to assess associations between indices of interest according to hypothesized interactions. Univariate linear regression analysis was performed to evaluate coefficients of relationships between hepcidin and erythropoietic, iron, and inflammatory variables. Forward stepwise regression, considering all variables and including those in which association with hepcidin was P < .05, was used to identify a parsimonious multiple regression model for associations with hepcidin. All analyses were done using Stata13 and GraphPad Prismv5.0.
Ethics
Approval for this research program was obtained from the Ethical Committee of the University of Kelaniya, Colombo, Sri Lanka; The University Health Network, Toronto, Canada; the Human Research Ethics Committee of the University of Western Australia; and the Oxford Tropical Research Ethical Committee, Oxford, United Kingdom. The study was conducted in accordance with the Declaration of Helsinki.
Results
HbE β-thalassemia study
Patient characteristics.
Demographic and laboratory parameters for the 69 patients are summarized in Table 1; data for each individual are shown in supplemental Table 1, available on the Blood Web site. Patients were anemic (mean Hb, 6.32g/dL), with relatively little variation, despite large differences across the patient group in terms of severity and corresponding transfusion requirement, characteristic of HbE β-thalassemia in Sri Lanka, and distinguishing it from other β-thalassemia syndromes.4 In the overall group, erythropoiesis, as indicated by GDF-15 and sTfR, was increased. Iron loading was substantial: mean LIC was 7.21 mg/g, and 30/67 (45%) had ferritin concentrations exceeding 1000 ng/mL. Among 23 patients who had received 20 or fewer lifetime transfusions, LIC ranged from 1.0 to 33 mg/g (mean, 8.7 mg/g), and 6 (26%) had serum ferritin values exceeding 1000 ng/mL. When compared by sex, there were no significant differences for any parameter measured except GDF-15 (6676 and 12,033 pg/mL in females and males, respectively) (supplemental Table 2). Hepcidin was suppressed below levels previously reported in healthy male adults, using this assay (mean, 14 ng/mL)29 in 62 (90%) of 69 patients analyzed (ie, 25/27 males and 37/42 females). The mean hepcidin level was 2.07 ng/mL (Table 1), and hepcidin was below the detection limit in 11 patients. When compared with nonthalassemic, nonanemic local controls, patients with HbE β-thalassemia had suppressed hepcidin, lower Hb, and higher ferritin (Table 1).
Table 1.
Demographic, iron, erythropoietic, and inflammatory indices for patients with HbE β-thalassemia overall and stratified by severe and moderate phenotype
| Parameter | All patients (n = 69; 42 female) | Severe (n = 28; 17 female) | Moderate (n = 41; 25 female) | P for difference between moderate/severe | Local controls (n = 25) |
|---|---|---|---|---|---|
| Age, years* | 26.6 (3-61) | 17.5 (3-28) | 32.8 (9-61) | <.0001† | 16.6 (16.0, 17.2) |
| Hb, g/dL* | 6.2 (4.4-8.3) | 5.8 (4.4-7.6) | 6.4 (4.9-8.3) | .1397 | 15.0 (14.5, 15.5)‡ |
| HbF, %* | 26.0 (6.2-51.2) | 24.1 (6.2-42.4) | 27.3 (6.4-51.2) | .3226 | 0.27 (0.19, 0.39)‡ |
| Total transfusions* | 47.6 (0, 143) | 59.3 (3, 143) | 39.7 (0, 123) | .0377† | |
| Ferritin, mg/L** | 947 (143, 9790) | 1356 (328, 9790) | 732 (143, 3260) | .0021† | 48.2 (38, 61.2)‡ |
| Liver iron, mg/gdwt** | 7.21 (0.7, 54.2) | 11.3 (1, 54.2) | 5.30 (0.7, 29.0) | .0006† | |
| NTBI, µM/L* | 4.68 (−2.67, 23.75) | 5.16 (−1.67, 10.27) | 4.36 (−2.67, 23.75) | .4456 | |
| Hepcidin, ng/mL** | 2.06 (0.1, 51.8) | 2.24 (0.1, 51.8) | 1.95 (0.1, 36.3) | .7441 | 28.23 (18.83, 42.3)‡ |
| sTfR, nM/L** | 7.83 (1, 35.3) | 6.51 (1, 35.3) | 8.87 (1.4, 35.3) | .1472 | |
| EPO, mg/L** | 158 (28.7, 2196.7) | 159 (28.7, 2196.7) | 158 (34.5, 1296.5) | .9915 | |
| CRP, mg/L** | 1.61 (0.20, 34.23) | 1.66 (0.23, 34.23) | 1.58 (0.20, 13.01) | .8433 | |
| GDF-15, pg/mL,** | 8407 (1920, 60 229) | 7011 (2125, 19 031) | 9517 (1920, 60 229) | .0549 | |
| Labile plasma iron, µM/L* | 4.01 (−1.07, 13.61) | 3.60 (0.1, 7.7) | 4.29 (−1.07, 13.61) | .9320 | |
| Transferrin SAT, %* | 90.9 (31.9, 100.0) | 97.3 (48.3, 100.0) | 86.6 (31.9, 100.0) | .0319† | |
| ALT, mg/L** | 37.2 (5, 213) | 48.2 (5, 213) | 31.1 (5, 79) | .0170† |
Values are *mean (range) or **geometric mean (range).
Significant difference between moderate and severe patients (P < .05).
Significant difference between controls and overall patient group, severe patients, and moderate patients.
Associations with hepcidin in all patients.
Supplemental Figure 1 summarizes hypothesized and subsequently observed associations between indices of erythropoiesis, iron loading, and hepcidin in the overall patient group. Hb correlated inversely with EPO, which in turn, was correlated with both sTfR and GDF-15, which were both inversely correlated with hepcidin. No associations between hepcidin and inflammation (measured by CRP) or between iron stores (ferritin, liver iron) and hepcidin were observed. However, there was an inverse correlation between hepcidin and NTBI, and NTBI correlated with iron stores; these also correlated with ALT, an index of liver damage. Associations with hepcidin were next evaluated by linear regression analyses. sTfR and also splenectomy were independently associated with hepcidin (Table 2). Taken together, these findings indicate that in patients with HbE β-thalassemia, hepcidin is regulated by erythropoiesis, with suppression of hepcidin inducing excess iron absorption and mobilization, thereby resulting in iron loading, increased free iron, and risk for organ damage.
Table 2.
Univariate and multiple linear regression for associations with hepcidin overall and in moderate and severe groups
| Variable | Overall group | Moderate phenotype | Severe phenotype | |||
|---|---|---|---|---|---|---|
| β Coefficient* | P | β Coefficient | P | β Coefficient | P | |
| Univariate linear regression | ||||||
| Age | −0.05 | .683 | 0.05 | .744 | −0.24 | .217 |
| Sex | 0.06 | .623 | −0.05 | .775 | 0.19 | .331 |
| Lifetime transfusions | −0.16 | .183 | −0.30 | .061 | −0.04 | .855 |
| Splenectomy† | −0.48 | <.001‡ | −0.53 | <.001‡ | −0.42 | .025‡ |
| Severity¶ | 0.04 | .744 | — | — | — | — |
| Interaction between splenectomy and severity | 0.00 | .988 | — | — | ||
| Erythropoiesis | ||||||
| Hemoglobin | 0.13 | .308 | −0.06 | .738 | 0.44 | .037‡ |
| Erythropoietin§ | −0.08 | .516 | 0.17 | .297 | −0.39 | .062 |
| sTfR§ | −0.36 | .003‡ | −0.22 | .165 | −0.52 | .006‡ |
| GDF-15§ | −0.23 | .057 | −0.03 | .853 | −0.49 | .008‡ |
| Iron indices | ||||||
| Ferritin§ | 0.00 | .993 | −0.33 | .043‡ | 0.31 | .114 |
| Liver iron loading§ | 0.11 | .381 | −0.04 | .824 | 0.26 | .184 |
| Transferrin saturation | −0.19 | .131 | −0.27 | .098 | −0.12 | .565 |
| NTBI | −0.42 | <.001‡ | −0.46 | .003‡ | −0.45 | .018‡ |
| Labile plasma iron | −0.26 | .034‡ | −0.33 | .043‡ | −0.16 | .412 |
| ALT§ | 0.05 | .687 | −0.18 | .279 | 0.23 | .244 |
| CRP§ | −0.13 | .297 | −0.20 | .231 | −0.08 | .707 |
| Multiple linear regression | ||||||
| sTfR | −0.34‖ | .001 | — | −0.45** | .010 | |
| Splenectomy | −0.47‖ | <.001 | −0.53 | <.001 | −0.36** | .037 |
β coefficient was performed on variables standardized to have a variance of 1, enabling comparison of coefficients between variables.
Splenectomy status coded as: splenectomized = 1, non-splenectomized = 0.
Significant association (P < 0.05).
Severity coded as Severe = 1, Moderate = 0.
Log-transformed.
R2 = 0.35.
R2 = 0.39.
Effect of splenectomy on erythropoiesis and iron regulation.
Forty-two (60.9%) of the patients had been splenectomized (supplemental Table 3). Splenectomized patients with HbE β-thalassemia had received more transfusions, potentially explaining the rationale for their procedure. The inflammatory marker CRP was higher in splenectomized patients, as were NTBI and transferrin saturation. Both inflammation and transferrin bound iron are known to induce hepcidin, but despite this, hepcidin levels were lower in splenectomized patients. There was no apparent difference in erythropoiesis (GDF-15, sTfR, and EPO). By multiple regression (Table 2), splenectomy was independently associated with lower hepcidin.
Analysis of parameters in moderate and severe phenotypes and comparison with controls.
Patient characteristics and laboratory indices between moderate and severe phenotypes were compared (Table 1). Patients with severe phenotypes were younger, reflecting their earlier age of presentation. Mean Hb levels in moderate patients (6.35 g/dL) did not differ significantly from the pretransfusion levels in the severe group (5.79 g/dL; P = .140), as observed previously.4,22 Likewise, HbF% did not differ between the 2 groups. Compared with patients with moderate phenotypes, patients with severe phenotypes had significantly greater iron loading and higher ALT but did not show evidence of increased erythroid drive or erythropoiesis (EPO, sTfR, and GDF15). Mean hepcidin concentrations were similar between groups. Compared with 25 healthy iron-replete Sri Lankan control participants, hepcidin and Hb were decreased and serum ferritin was increased in patients with both moderate and severe phenotype (Table 1; supplemental Figure 2).
A similar proportion of moderate and severe phenotype patients had been splenectomized (moderate, 24/41 [58.5%]; severe, 18/28 [64.3%]; P = .802 Fisher’s exact). Differences between splenectomized and nonsplenectomized patients stratified by phenotype were similar to the differences observed analyzing the overall group (supplemental Table 4).
Analysis of relationships between parameters in severe vs moderate phenotypes.
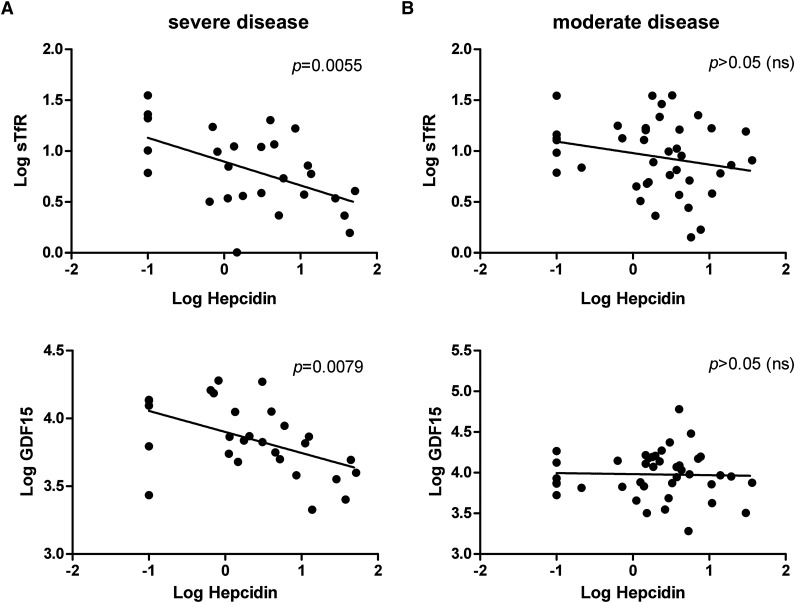
To further explore the underlying basis for the difference in moderate and severe phenotypes, we repeated analysis of correlations between indices stratified by severity (Figure 1). Among patients with severe phenotype, GDF15 and sTfR were inversely correlated with hepcidin (Figure 1A). In addition, hepcidin and NTBI were inversely correlated (r = −0.45; P = .018) in this group. Ferritin concentrations correlated with the lifetime number of blood transfusions that patients had received (r = 0.39; P = 0.046). These data indicate that erythropoiesis is the dominant regulator of hepcidin in severe-phenotype patients, which in turn contributes to NTBI accumulation and iron loading. Transfusion likely disrupts associations between Hb and erythropoiesis (EPO, sTfR, and GDF15), as well as contributing to iron stores (reflected by a relationship between transfusion number and serum ferritin). The interrelationships between these parameters in patients with severe phenotype are summarized in supplemental Figure 3A.
Figure 1.
Correlations between hepcidin and sTfR and GDF15 in patients with either (A) severe or (B) moderate HbE β-thalassemia. Scatter plots show individual data points for log hepcidin against either log sTfR or log GDF15. Hepcidin is inversely correlated with sTfR and GDF15 in patients with severe clinical phenotype, but there is no significant correlation among patients with moderate disease (despite the 2 patient groups carrying similar or identical β-globin gene mutations). P values indicate significance in the correlation. ns, not significant.
In patients with moderate phenotype (Figure 1B; supplemental Figure 3B), Hb was inversely correlated with EPO and GDF15, but unlike patients with severe phenotype, there were no associations between hepcidin and these indices of erythropoiesis. We observed inverse associations between hepcidin and NTBI (r = −0.46; P = .003) and ferritin (r = −0.33; P = .043; unlike the overall group). Associations between NTBI and indices of iron stores (LIC: r = −0.44, P = .005; ferritin: r = 0.45, P = .005), as seen in the overall group, were evident. No association between the number of transfusions and iron loading was identified.
We next performed linear regression analysis to identify factors associated with hepcidin stratified by severity (Table 2). In both groups, splenectomy was associated with reduced hepcidin; in patients with severe disease, multiple regression showed that sTfR was independently associated with hepcidin, but this was not seen in patients with moderate disease. Splenectomy was not responsible for the difference in hepcidin regulation observed between moderate and severe phenotypes (Table 2).
Effect of β thalassemia and HbE traits on hepcidin
To evaluate the effects of β-globin gene hemoglobinopathy carrier states on erythropoiesis and hepcidin at the population level, we first assessed hematologic and iron indices and thalassemia carriage in a community study of 1821 Sri Lankan schoolchildren aged 8 to 18 years (48.3% males), comprising 1612 children with low MCV and/or MCH and 209 children from the same population with normal red cell indices. Of the 1821 children (of whom 107 were β-thalassemia carriers and 25 HbE carriers), we then considered only children who were iron-replete (ferritin >15 μg/L; n = 1264; 71.5% of the cohort), and within this subpopulation, we compared affected children (carrying β-thalassemia trait [n = 82] or HbE trait [n = 19]) with control children with normal MCV/MCH (noncarriers, n = 176); this approach removed potential interactions between hepcidin and iron deficiency from the analysis. As shown in Table 3, children carrying β-thalassemia had evidence of increased erythropoiesis, a small but significant reduction in hepcidin, and suppression of hepcidin out of proportion to their iron stores (ie, hepcidin-ferritin ratio) compared with controls. This pattern was not seen in children carrying HbE trait. After adjusting for hemoglobin concentrations, differences in sTfR between carriers and noncarriers of β-thalassemia trait remained evident.
Table 3.
Comparison of hepcidin and hepcidin-ferritin ratio among iron-replete individuals with and without heterozygous β-gene mutations
| Normal population (N = 176) | β-thalassemia trait (N = 82) | HbE trait (N = 19) | P for difference between normal and β thalassemia trait | P for difference between normal and HbE trait | |
|---|---|---|---|---|---|
| Hemoglobin* | 15.2 (13.0-19.7) | 12.0 (9.6-17.6) | 13.3 (11.4-17.2) | <.001 | <.001 |
| Ferritin† | 39.7 (15.5-240.7) | 39.5 (15.4-205.5) | 40.9 (17.9-94.4) | .960 | .806 |
| sTfR† | 25.7 (10.4-93.2) | 35.8 (18.3-95.2) | 20.0 (11.0-32.7) | <.001 | .003 |
| Serum iron* | 17.3 (2.8-33.7) | 15.7 (2.7-30.5) | 14.7 (5.7-26.1) | .036 | .071 |
| Hepcidin† | 5.1 (1.2-44.6) | 4.1 (1.1-25.4) | 5.24 (4.0-6.8) | .007 | .837 |
| Hepcidin-ferritin ratio† | 0.13 (0.02-0.82) | 0.10 (0.02-0.44) | 0.13 (0.05-0.69) | .011 | .997 |
Arithmetic means (range); P values calculated from Student t test between arithmetic means of normal and individuals with heterozygote β-gene mutations of interest.
Geometric means (range); P values calculated from Student t test between log-transformed means of normal and individuals with heterozygote β-gene mutations of interest.
Next, we studied the effect of coinheritance of α-thalassemia mutations on phenotype among β-thalassemia carriers, irrespective of iron status. Of the 107 individuals with β-thalassemia trait, 15 coinherited a heterozygous α+-thalassemia mutation and 1 a homozygous α+-thalassemia mutation. In these 107 individuals, there were no differences in hemoglobin (heterozygous single gene α-thalassemia mutation vs no α-thalassemia mutation, 11.9 vs 11.8g/dL; P = .719), serum iron (14.1 vs 15.6; P = .364), MCH (20.5 vs 20.3 pg/cell; P = .570), or serum hepcidin (3.3 vs 3.8 ng/mL; P = .342). However, sTfR was reduced in children who had coinherited a heterozygous α-gene mutation along with β-thalassemia trait compared with children who had not (28.0 vs 37.1 nmol/L; P = .003), and MCV was higher (64.2 vs 61.4 fL; P = .002). There were also nonstatistically significant lower ferritin (19.1 vs 22.6 μg/L; P = .062) and higher hepcidin-ferritin ratio (0.19 vs 0.13; P = .094) levels compared with children without coinheritance of an α-globin mutation. Of the 25 individuals overall with HbE trait, only one had coinherited α-thalassemia trait, precluding further analysis. Taken together, these data indicate that at the population level, compared with controls, children with β-thalassemia trait exhibit an increase in erythropoiesis and associated hepcidin suppression, which is ameliorated by coinheritance of an α-globin mutation.
Discussion
Patients with HbE β-thalassemia, who account for at least half the cases of severe β-thalassemia worldwide, manifest a particularly heterogeneous clinical phenotype30 and consistently demonstrate increased iron absorption and iron loading.31,32 As shown in our data and previous studies,4,22 the phenotypic heterogeneity occurs within a narrow range of hemoglobin levels, with the pretransfusion hemoglobin of the severe group being only slightly lower than the steady-state hemoglobin levels in the moderate groups.4 For economic and related reasons, including compliance, it has not been possible to maintain the pretransfusion Hb levels above the 9 g/dL range, as is more usual practice in severe β-thalassemia. Nevertheless, these lower pretransfusion levels have successfully prevented important complications such as growth failure, bone deformity, and unacceptable quality of life. This practice reflects an approach achievable in many low-income settings, where this disease is prevalent. Phenotypic variation in HbE β-thalassemia can be a result of the β-globin mutation inherited in trans with HbE, coinheritance of an α-thalassemia deletion, mutations associated with HbF synthesis and bile conjugation, splenectomy, and exposure to malaria.4,21,33,34 We find that regulation of hepcidin may also be a key independent contributor. In patients with the severe phenotype, hepcidin is suppressed by erythropoiesis; conversely, in patients with moderate disease, hepcidin regulation is not subjugated entirely by erythropoiesis.
We observed a clear inverse association between hepcidin and NTBI across all patients, a relationship that has been previously reported in TM13 and that perhaps reflects the increased release of iron into the plasma that occurs when suppressed hepcidin levels permit enhanced iron absorption and mobilization. NTBI also correlated with ferritin and LIC, suggesting that a high level of NTBI is associated with hepatic iron accumulation. Iron loading correlated with ALT, a marker of hepatic damage. Thus, hepcidin suppression in this condition is associated with iron loading, saturation of iron binding proteins, and consequently, organ damage.
Erythropoietic drive can dominate the regulation of hepcidin, potentially acting via an erythroid-derived hormone, such as the recently described erythroferrone. In mouse models of thalassemia, bone marrow erythroferrone expression is increased.35 These discoveries propose a model that globin chain imbalance causes ineffective erythropoiesis, resulting in anemia and increased Epo production, which produces expanded erythropoiesis and enhanced erythroferrone production, which acts on the liver to suppress hepcidin. Our data indirectly support this hypothesis: patients with HbE β-thalassemia experience anemia, increased Epo, and enhanced erythropoiesis (potentially resulting in increased erythroferrone) that suppresses hepcidin, causing increased iron use, saturation of plasma iron transport mechanisms, tissue iron loading, and toxicity. Therefore, therapeutic interventions aimed at limiting hepcidin suppression may be of benefit, particularly in severe disease. Furthermore, the difference in erythropoietic suppression of hepcidin observed between severe and moderate disease (despite similar or identical β-globin gene mutations) suggests that genetic variation that affects hepcidin regulation may be an important disease modifier. Identification of the genes involved may aid diagnostic precision and enable more accurate prediction of prognostic outcomes.
Previous studies have indicated that suppression of hepcidin is greater in patients with thalassemia intermedia than TM,14 suggesting that by suppressing erythropoiesis, transfusion permits hepcidin levels to increase12,13; this effect occurs dynamically across the transfusion cycle in patients with TM.16 However, in our population, hepcidin concentrations were similarly suppressed in patients with both severe and moderate clinical phenotypes. Among the severe patients, the correlation between Hb and EPO was lost, likely as a result of transfusion; however, erythropoiesis remained inversely correlated with hepcidin. Conversely, in patients with moderate phenotype, hepcidin was not associated with indices of erythropoiesis, even though erythropoietic activity was similarly increased (raised EPO, GDF-15, and sTfR), and hepcidin was similarly suppressed. A potential explanation for this observation may be that patients with moderate phenotypes have stably increased erythropoiesis (unlike the dynamic changes in transfused patients), with slowly progressive iron loading, and hence their hepcidin set-points have achieved an equilibrium between erythropoiesis and iron loading, masking evidence of statistically independent associations between either of these stimuli and hepcidin. These observations suggest that moderate-phenotype patients may have a hitherto unrecognized increased ability to adapt more effectively to chronic anemia.
Splenectomy was independently associated with lower hepcidin levels, regardless of disease phenotype; differences in hepcidin were not mediated by a phenotype-splenectomy interaction. Higher NTBI and transferrin saturation in splenectomized patients may have resulted at least in part from the increased number of transfusions these individuals received, and the relatively decreased hepcidin could contribute by elevating the release of cellular iron (eg, from Kupffer cells) after erythrophagocytosis. Similar to a previous study,36 we found no difference in Hb, ferritin, or sTfR between splenectomized and nonsplenectomized patients.
Individuals carrying β-thalassemia trait have a mild globin chain imbalance and ineffective erythropoiesis,37 resulting in increased erythroid activity accompanied by a variable but usually mild microcytic anemia,20 associated with a mild increase in Epo.38 Our data show that β-thalassemia carriers have suppressed hepcidin concentrations overall and out of proportion to their iron stores. Hepcidin concentrations predict iron absorption and use,39 and thus, even moderate suppression of hepcidin, as seen here, can enhance iron absorption. The plausible explanation is that the mild anemia and increase in Epo and erythropoiesis seen in this condition mediate hepcidin suppression. Epo levels are approximately 30% higher than controls in β-thalassemia trait,38 which likely reflects the 20% reduction in hepcidin levels in this group. A recent study did not find decreased hepcidin in Brazilian β-thalassemia carriers,40 but the 20 individuals investigated were patients attending a hemoglobinopathy clinic, and so may not represent the manifestation of β-thalassemia in the general population. The phenotype of β-thalassemia trait can be ameliorated by the inheritance of α-thalassemia. Our data demonstrate that coinheritance of α-thalassemia results in a reduction in erythropoiesis and ameliorates hepcidin suppression. These data fit with the overall model correlating ineffective erythropoiesis with hepcidin suppression, as discussed earlier. In contrast, we did not identify differences in hepcidin in patients carrying HbE trait, reflecting previous studies that did not observe enhanced iron use in these individuals.20
Carriage of β-thalassemia trait is especially prevalent in regions of the world where the overall burden of anemia is high; for example, South and Southeast Asia and the Middle East. International anemia control guidelines presently recommend that all children and women living in settings in which the prevalence of anemia exceeds 20% receive supplemental iron; additional strategies include universal fortification of staple foods with iron.18 Although coinheritance of β-thalassemia trait with heterozygous HFE mutations does not result in iron overload, β-thalassemia trait does appear to exacerbate homozygous HFE hemochromatosis.41 The effects of long-term iron administration to individuals with mild suppression of hepcidin resulting from β-thalassemia trait have not been evaluated. The possibility that widespread distribution of iron (eg, provision of supplements to adolescent boys, as is recommended in India, or fortification of staple foods such as wheat and rice) may increase the risk for detrimental iron overload in β-thalassemia carriers has not been excluded.
Overall, our findings emphasize the importance of regular monitoring for iron accumulation in HbE β-thalassemia because of the marked hepcidin suppression observed in moderate and severe disease. These results also suggest that potential future treatments that either counteract hepcidin suppression, perhaps by inhibiting the synthesis of action of the erythroid regulator of hepcidin or acting as direct hepcidin agonists, could ameliorate iron loading in this condition and improve the course of the disease in the long term.42 Our data also suggest that in settings in which carriage of β-thalassemia trait is common, universal iron distribution programs should bear in mind the risk of causing an inadvertent burden of iron overload.
Acknowledgments
This work was supported by grants from the Wellcome Trust and Medical Research Council UK, the US March of Dimes, the Anthony Cerami and Ann Dunne Foundation for World Health, and the National Institute for Health Research Biomedical Research Centre Oxford. S.-R.P. is supported by fellowships from the National Health and Medical Research Council Australia, the Haematology Society of Australia and New Zealand, and the Bill and Melinda Gates Foundation.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J., A.A., P.E., C.A.F., C.W., P.S., K.W., A.E.A., and T.S.P. performed the experiments; E.J., S.-R.P., C.A.F., H.D., and D.J.W. analyzed the data; A. Premawardhena, D.B., A. Perera, N.F.O., P.E., J.B.P., and T.S.P. collected and analyzed the clinical data on the patients; H.D. and D.J.W. designed the study; E.J., S.-R.P., H.D., and D.J.W. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Weatherall, Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford, OX3 9DS, United Kingdom; e-mail: liz.rose@imm.ox.ac.uk; and Hal Drakesmith, Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford, OX3 9DS, United Kingdom; e-mail: hdrakes@hammer.imm.ox.ac.uk.
References
- 1.Vichinsky E. Hemoglobin e syndromes. Hematology (Am Soc Hematol Educ Program) 2007:79–83. doi: 10.1182/asheducation-2007.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115(22):4331–4336. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fucharoen S, Weatherall DJ. Hemoglobin E thalassemia. In: Weatherall DJ, Schechter AN, Nathan DJ, editors. Hemoglobin and its disorders. Cold Spring Harbor Laboratory Press; 2013. pp. 229–244. [Google Scholar]
- 4.Premawardhena A, Fisher CA, Olivieri NF, et al. Haemoglobin E beta thalassaemia in Sri Lanka. Lancet. 2005;366(9495):1467–1470. doi: 10.1016/S0140-6736(05)67396-5. [DOI] [PubMed] [Google Scholar]
- 5.Borgna-Pignatti C, Cappellini MD, De Stefano P, et al. Survival and complications in thalassemia. Ann N Y Acad Sci. 2005;1054:40–47. doi: 10.1196/annals.1345.006. [DOI] [PubMed] [Google Scholar]
- 6.Musallam KM, Cappellini MD, Wood JC, Taher AT. Iron overload in non-transfusion-dependent thalassemia: a clinical perspective. Blood Rev. 2012;26(Suppl 1):S16–S19. doi: 10.1016/S0268-960X(12)70006-1. [DOI] [PubMed] [Google Scholar]
- 7.Ganz T, Nemeth E. The hepcidin-ferroportin system as a therapeutic target in anemias and iron overload disorders. Hematology Am Soc Hematol Educ Program. 2011;2011:538-542. [DOI] [PMC free article] [PubMed]
- 8.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Origa R, Galanello R, Ganz T, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007;92(5):583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 10.Gardenghi S, Marongiu MF, Ramos P, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109(11):5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papanikolaou G, Tzilianos M, Christakis JI, et al. Hepcidin in iron overload disorders. Blood. 2005;105(10):4103–4105. doi: 10.1182/blood-2004-12-4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearney SL, Nemeth E, Neufeld EJ, et al. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. 2007;48(1):57–63. doi: 10.1002/pbc.20616. [DOI] [PubMed] [Google Scholar]
- 13.Kattamis A, Papassotiriou I, Palaiologou D, et al. The effects of erythropoetic activity and iron burden on hepcidin expression in patients with thalassemia major. Haematologica. 2006;91(6):809–812. [PubMed] [Google Scholar]
- 14.Camberlein E, Zanninelli G, Détivaud L, et al. Anemia in beta-thalassemia patients targets hepatic hepcidin transcript levels independently of iron metabolism genes controlling hepcidin expression. Haematologica. 2008;93(1):111–115. doi: 10.3324/haematol.11656. [DOI] [PubMed] [Google Scholar]
- 15.Adamsky K, Weizer O, Amariglio N, et al. Decreased hepcidin mRNA expression in thalassemic mice. Br J Haematol. 2004;124(1):123–124. doi: 10.1046/j.1365-2141.2003.04734.x. [DOI] [PubMed] [Google Scholar]
- 16.Pasricha SR, Frazer DM, Bowden DK, Anderson GJ. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with β-thalassemia major: a longitudinal study. Blood. 2013;122(1):124–133. doi: 10.1182/blood-2012-12-471441. [DOI] [PubMed] [Google Scholar]
- 17.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Essential Nutrition Actions WHO. Improving maternal, newborn, infant and young child health and nutrition. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 19.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86(6):480–487. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann MB, Fucharoen S, Winichagoon P, et al. Iron metabolism in heterozygotes for hemoglobin E (HbE), alpha-thalassemia 1, or beta-thalassemia and in compound heterozygotes for HbE/beta-thalassemia. Am J Clin Nutr. 2008;88(4):1026–1031. doi: 10.1093/ajcn/88.4.1026. [DOI] [PubMed] [Google Scholar]
- 21.Fisher CA, Premawardhena A, de Silva S, et al. Sri Lanka Thalassaemia Study Group. The molecular basis for the thalassaemias in Sri Lanka. Br J Haematol. 2003;121(4):662–671. doi: 10.1046/j.1365-2141.2003.04346.x. [DOI] [PubMed] [Google Scholar]
- 22.Olivieri NF, Muraca GM, O’Donnell A, Premawardhena A, Fisher C, Weatherall DJ. Studies in haemoglobin E beta-thalassaemia. Br J Haematol. 2008;141(3):388–397. doi: 10.1111/j.1365-2141.2008.07126.x. [DOI] [PubMed] [Google Scholar]
- 23.St Pierre TG, Clark PR, Chua-anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105(2):855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 24.Evans RW, Williams J. The electrophoresis of transferrins in urea/polyacrylamide gels. Biochem J. 1980;189(3):541–546. doi: 10.1042/bj1890541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S, Hider RC, Porter JB. A direct method for quantification of non-transferrin-bound iron. Anal Biochem. 1990;186(2):320–323. doi: 10.1016/0003-2697(90)90088-q. [DOI] [PubMed] [Google Scholar]
- 26.Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102(7):2670–2677. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 27.Le Lan C, Loréal O, Cohen T, et al. Redox active plasma iron in C282Y/C282Y hemochromatosis. Blood. 2005;105(11):4527–4531. doi: 10.1182/blood-2004-09-3468. [DOI] [PubMed] [Google Scholar]
- 28.Pasricha SR, Atkinson SH, Armitage AE, et al. Expression of the iron hormone hepcidin distinguishes different types of anemia in African children. Sci Transl Med. 2014;6(235) doi: 10.1126/scitranslmed.3008249. 235re3. [DOI] [PubMed] [Google Scholar]
- 29.Talbot NP, Lakhal S, Smith TG, et al. Regulation of hepcidin expression at high altitude. Blood. 2012;119(3):857–860. doi: 10.1182/blood-2011-03-341776. [DOI] [PubMed] [Google Scholar]
- 30.Cappellini MD, Cohen A, Eleftheriou A, et al. Thalassaemia intermedia and HbE. In: Guidelines for the Clinical Management of Thalassaemia. 2nd ed. Nicosia, Cyprus: Thalassaemia International Federation; 2008:121-131. [PubMed] [Google Scholar]
- 31.Pootrakul P, Kitcharoen K, Yansukon P, et al. The effect of erythroid hyperplasia on iron balance. Blood. 1988;71(4):1124–1129. [PubMed] [Google Scholar]
- 32.Fouladi M, Macmillan ML, Nisbet-Brown E, et al. Hemoglobin E/beta thalassemia: the Canadian experience. Ann N Y Acad Sci. 1998;850:410–411. doi: 10.1111/j.1749-6632.1998.tb10506.x. [DOI] [PubMed] [Google Scholar]
- 33.Suresh S, Fisher C, Ayyub H, et al. Alpha thalassaemia and extended alpha globin genes in Sri Lanka. Blood Cells Mol Dis. 2013;50(2):93–98. doi: 10.1016/j.bcmd.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Allen A, Fisher C, Premawardhena A, et al. Adaptation to anemia in hemoglobin E-ß thalassemia. Blood. 2010;116(24):5368–5370. doi: 10.1182/blood-2010-06-289488. [DOI] [PubMed] [Google Scholar]
- 35.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratummo K, Jetsrisuparb A, Fucharoen S, Tripatara A. Hepcidin expression from monocyte of splenectomized and non-splenectomized patients with HbE-β-thalassemia. Hematology. 2014;19(3):175–180. doi: 10.1179/1607845413Y.0000000110. [DOI] [PubMed] [Google Scholar]
- 37.Thein SL. Pathophysiology of beta thalassemia—a guide to molecular therapies. Hematology (Am Soc Hematol Educ Program) 2005:31–37. doi: 10.1182/asheducation-2005.1.31. [DOI] [PubMed] [Google Scholar]
- 38.Tassiopoulos T, Konstantopoulos K, Tassiopoulos S, et al. Erythropoietin levels and microcytosis in heterozygous beta-thalassaemia. Acta Haematol. 1997;98(3):147–149. doi: 10.1159/000203609. [DOI] [PubMed] [Google Scholar]
- 39.Prentice AM, Doherty CP, Abrams SA, et al. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood. 2012;119(8):1922–1928. doi: 10.1182/blood-2011-11-391219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guimarães JS, Cominal JG, Silva-Pinto AC, et al. Altered erythropoiesis and iron metabolism in carriers of thalassemia [published online ahead of print October 11, 2014]. Eur J Haematol. doi: 10.1111/ejh.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piperno A, Mariani R, Arosio C, et al. Haemochromatosis in patients with beta-thalassaemia trait. Br J Haematol. 2000;111(3):908–914. [PubMed] [Google Scholar]
- 42.Gardenghi S, Ramos P, Marongiu MF, et al. Hepcidin as a therapeutic tool to limit iron overload and improve anemia in β-thalassemic mice. J Clin Invest. 2010;120(12):4466–4477. doi: 10.1172/JCI41717. [DOI] [PMC free article] [PubMed] [Google Scholar]