Abstract
In order to increase the cytotoxic activity of a Salmonella strain carrying a salicylate-inducible expression system that controls cytosine deaminase production, we have modified both, the vector and the producer bacterium. First, the translation rates of the expression module containing the Escherichia coli codA gene cloned under the control of the Pm promoter have been improved by using the T7 phage gene 10 ribosome binding site sequence and replacing the original GUG start codon by AUG. Second, to increase the time span in which cytosine deaminase may be produced by the bacteria in the presence of 5-fluorocytosine, a 5-fluorouracyl resistant Salmonella strain has been constructed by deleting its upp gene sequence. This new Salmonella strain shows increased cytosine deaminase activity and, after infecting tumour cell cultures, increased cytotoxic and bystander effects under standard induction conditions. In addition, we have generated a purD mutation in the producer strain to control its intracellular proliferation by the presence of adenine and avoid the intrinsic Salmonella cell death induction. This strategy allows the analysis and comparison of the cytotoxic effects of cytosine deaminase produced by different Salmonella strains in tumour cell cultures.
Introduction
Bacteria can be easily adapted to synthesize proteins with relevant biotechnological applications. Over the past decade, many genera of bacteria have been explored as cell factories for cancer therapy due to their ability to specifically target tumours (Pawelek et al., 1997), reviewed in (Forbes, 2010). Salmonella enterica serovar Typhimurium (S. Typhimurium) is probably the intracellular pathogen that has been most extensively studied as an anti-tumour vector due to its intrinsic properties. These bacteria preferentially colonize and proliferate in solid tumours at ratios greater than 1000/1 compared with normal target organs, a behaviour that usually results in tumour growth inhibition (Pawelek et al., 1997). In addition, as a facultative anaerobe, Salmonella can grow under aerobic and anaerobic conditions, which allows bacteria to accumulate in large solid tumours and invade metastases (Saltzman et al., 1996; Yam et al., 2010).
Administration of attenuated Salmonella strains expressing different anti-tumour agents has been attempted in recent years with promising results in tumour regression (Nemunaitis et al., 2003; Barnett et al., 2005; Zhao et al., 2006; Royo et al., 2007; Jeong et al., 2014). One of the therapeutic genes successfully expressed in S. Typhimurium is the Escherichia coli codA gene, encoding cytosine deaminase (CD). This enzyme, present in fungi and bacteria but absent in mammalian cells (Nishiyama et al., 1985), catalyses the conversion of cytosine to uracil and ammonia (Koechlin et al., 1966). Cytosine deaminase can also deaminate the non-toxic cytosine analog, 5-fluorocytosine (5-FC) to the toxic metabolite, 5-fluorouracil (5-FU) that is widely used as a chemotherapeutic agent. This metabolite is then converted by cellular enzymes into 5-FdUMP, which inhibits DNA synthesis by blocking the activity of thymidylate synthase, 5-FUTP and 5-FdUTP, which are incorporated into RNA and DNA, respectively (Meyers et al., 2003), thus leading to cell death (Polak and Scholer, 1975; Damon et al., 2011). In addition, 5-FU can freely diffuse across the cell membrane and produce its cytotoxic effects in neighbouring cells, a phenomenon known as the bystander effect (Kuriyama et al., 1998). Despite several co-administration studies that have demonstrated conversion of 5-FC to 5-FU and significant tumour growth reduction in animal models (King et al., 2002; Nemunaitis et al., 2003; Royo et al., 2007), its application in cancer patients has been limited (Nemunaitis et al., 2003). Clinical data suggest that the anti-tumour activity of 5-FU is directly related to both the duration of drug exposure and its concentration in the tumour (Nemunaitis et al., 2003). However, in order to achieve a significant amount of active metabolites and cell killing, the required dose of the apparently harmless 5-FC may be high enough to cause adverse effects (reviewed in (Vermes et al., 2000)). This 5-FC toxicity may be due, in part, to the conversion of 5-FC to 5-FU by human intestinal microflora (Harris et al., 1986). Increasing the anti-tumour activity and minimizing the systemic toxicity would circumvent these problems, but to achieve this, it is necessary to improve the selective production of CD into the tumour. We have previously validated an in vivo salicylate-inducible cascade expression system that allows the controlled cytosine deaminase production. This system combines a set of salicylate-regulated elements from Pseudomonas putida that work in cascade, containing a regulatory module (NahR and XylS2 transcription regulators coding sequences) integrated in the chromosome of attenuated S. Typhimurium aroA (SL7207 strain) and an expression module, consisting in a codA gene cloned under the control of the Pm promoter either in a plasmid or integrated in the chromosome (Royo et al., 2007). In the presence of salicylate, XylS2 promotes transcription from Pm. In order to increase the CD production rates, in this work we have improved the CD expression module by engineering codA to be translated from the T7 phage gene 10 ribosome binding site and changing the original CD GUG start codon to AUG in new salicylate induced expression vectors (Medina et al., 2011). Since the microbial uracil phosphoribosyltransferase, encoded by upp, directly converts 5-FU to the metabolite 5-FUMP, from which the other toxic metabolites are produced, strains lacking this activity are more tolerant to 5-FU (Lundegaard and Jensen, 1999). To prevent killing of the producing bacteria during accumulation of toxic 5-FU, thus increasing the time span in which Salmonella produces CD, we have also constructed a 5-FU resistant upp mutant. Finally, in order to assess the effects of CD produced by improved strains and plasmids in tumour cell cycle distribution and bystander activity in long-term cell cultures, a purD mutation has been generated in the producer strains to avoid cell death induced by intracellular Salmonella proliferation (Leung and Finlay, 1991; Mesa-Pereira et al., 2013).
Results and discussion
Construction of a Salmonella strain with high salicylate-induced CD production rates
In order to increase the amount of CD produced keeping standard induction conditions, we improved both the producing Salmonella strain and the CD expression plasmid. First, we transferred the new genome-integrated regulatory module previously developed in our laboratory (Medina et al., 2011) to the SL7207 Salmonella strain, thus generating the MPO375 strain (bacterial strains and plasmid are listed in Supporting information Table S1,). This regulatory module contains a constitutively expressed gfp gene to track Salmonella during the infection process. Second, we modified this strain with the aim of avoiding host cell death induced by Salmonella intracellular proliferation (see below). To that end, we transduced a ΔpurD mutation into MPO375 to get the strain MPO376. In this way, intracellular proliferation can be controlled by the amount of adenine in the culture medium (Leung and Finlay, 1991; Mesa-Pereira et al., 2013). On the other hand, we constructed new plasmids with higher CD expression rates than pMPO16, the vector previously used in our laboratory to express CD (Royo et al., 2007). The E. coli codA sequence cloned in this plasmid has the original GUG start codon and its own ribosome binding site (from now on, CDGUG sequence). To increase CD production, we changed the codA start codon to AUG and cloned the resulting sequence into the high copy number vector pMPO52 (Medina et al., 2011) to produce pMPO88. In this vector, codA expression is under the control of the Pm promoter and the T7 gene10 ribosome binding site, a strong ribosome binding site that achieves high translation levels (from now on, CD7AUG sequence). We have previously reported that the salicylate-induced expression levels of vectors based in pWSK29, a low copy number vector that is stable through the whole Salmonella infection cycle without selection pressure, are comparable to that of their corresponding high copy number vectors (Medina et al., 2011). To generate versions of the CD expression modules in low copy number plasmids, the engineered codA genes in pMPO88 and pMPO16 were subcloned in the pWSK29 derived vector pMPO20, thus generating plasmids pMPO90 and pMPO1088 respectively.
To compare the amount of CD produced by the different constructs, we analysed whole-cell protein extracts from cultures of the strain MPO376 carrying the low copy number vectors pMPO1088 (CDGUG) or pMPO90 (CD7AUG) by SDS-PAGE, in the presence or absence of salicylate. As shown in Fig. 1A (lanes 4 and 6), the pMPO90 vector produces more CD than pMPO1088 after salicylate induction. Afterwards, we determined the CD activity from these cell lysates by analysing the conversion of 5-FC to the cytotoxic agent 5-FU. The assays (Fig. 1B) revealed that, upon induction, the strain harbouring the pMPO90 (CD7AUG) vector reached an activity about 3.5-fold higher than the same strain bearing pMPO1088 (CDGUG). Thus, these results demonstrate that the new CD7AUG sequence produces higher amounts of CD and therefore 5-FU than CDGUG using the same Salmonella producer strain and concentrations of salicylate and 5-FC.
Fig 1.
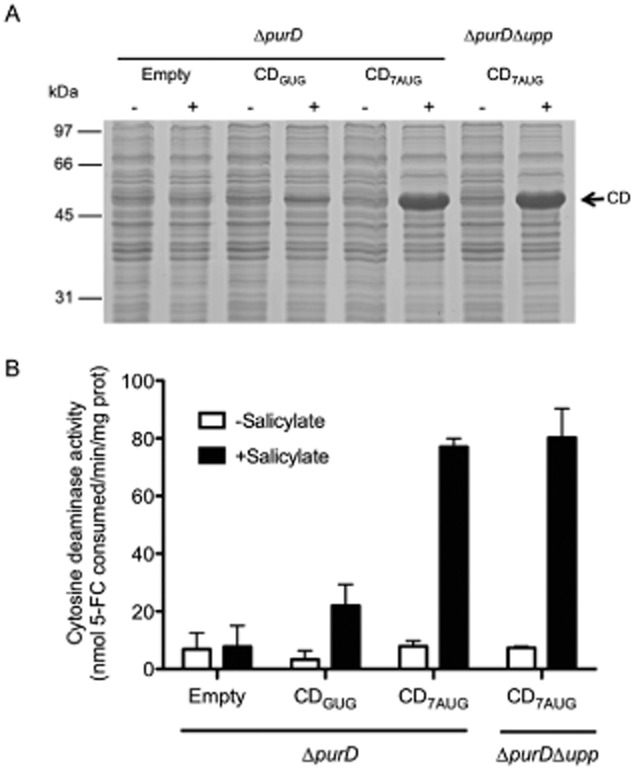
Production of CD in low copy number expression vectors.A. SDS-PAGE analysis of salicylate dependent overproduction of cytosine deaminase. Whole extracts of Salmonella MPO376 (ΔpurD) bearing pMPO54 (empty vector), pMPO1088 (CDGUG) or pMPO90 (CD7AUG) plasmids and Salmonella strain MPO378 (ΔpurDΔupp) bearing pMPO90 plasmid, either uninduced (-) or induced by salicylate for 4 h (+). Three μl of supernatant was loaded in each track.B. Analysis of conversion of 5-FC. Cytosine deaminase activity from cell extracts of Salmonella MPO376 bearing pMPO54, pMPO1088 or pMPO90 plasmids, and MPO378 bearing pMPO90 either induced by salicylate for 4 h, or not induced. Cytosine deaminase activity was assayed as previously described (Nishiyama et al., 1985). Each bar represents the average of three independent experiments ± SD.
Expression of CD with a 5-FU resistant Salmonella strain
As shown before, the new vector pMPO90 (CD7AUG) allows production of a larger amount of CD than the former construct under salicylate induction. However, since Salmonella is sensitive to 5-FU, the maximum rate of synthesis of this cytotoxic metabolite could also be limited by the maximum tolerated concentration and not only by the amount of CD present in the bacterium. To test this prediction, we first determined the growth of Salmonella carrying different plasmids on plates containing 5-FC (Fig. 2). Cultures of strain MPO376 bearing either the empty vector or one of the two CD-expressing plasmids (pMPO1088 or pMPO90) were spotted on plates in the presence or absence of salicylate and supplemented with two different concentrations, 0.5 or 5 μM, of 5-FC. Consistent with the results mentioned above, the Salmonella strain carrying the plasmid pMPO90 presents a more severe growth defect even at the low 5-FC concentration than the strain bearing pMPO1088 at the high 5-FC concentration, which correlates with its higher expression of CD and, consequently, higher production rate of 5-FU. This clearly showed that 5-FU production could be limited by the bacterium sensitivity to it.
Fig 2.
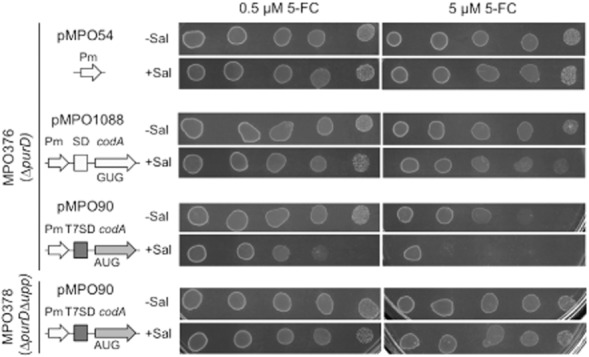
Effect of 5-FU produced on the bacterial growth. Serial dilutions (102 to 106) of cultures grown with or without salicylate were plated in supplemented minimal E medium in the presence of 0.5 μM or 5 μM of 5-FC and incubated for 24 h at 37°C.
This observation prompted us to obtain a Salmonella mutant resistant to 5-FU, strain that could produce higher amounts of this drug and for a longer time than the isogenic sensitive strain using the same 5-FC dosage. The mutant was constructed by deleting the upp gene sequence, whose product is involved in 5-FU sensitivity (Glaab et al., 2005), thus generating the strain MPO378 (ΔpurDΔupp). For the construction of upp mutant strain, the ‘One Step Deletion’ approach was used to replace target gene by the chloramphenicol resistance cassette (Datsenko and Wanner, 2000). We transformed this strain with the plasmid pMPO90 (CD7AUG) and performed the same experiments to determine the amount of CD produced and the activity of whole-cell extracts of salicylate induced cultures (Nishiyama et al., 1985). As shown in Fig. 1A and B, CD production and activity were independent of the upp mutation, since the strain behaved as its upp+ counterpart. Conversely, and as expected, the mutant was resistant to the 5-FU produced when grown on plates supplemented with 0.5 and 5 μM of 5-FC (Fig. 2) despite the high CD activity achieved. These results suggest that this strain and plasmid combination may represent an improvement in bacterial cancer therapy since it has the capacity of achieving a higher 5-FU concentration with a low 5-FC dosage, which, in turns, would reduce the deleterious effect of this compound in healthy eukaryotic cells.
A novel strategy to analyse the cytotoxic effect of Salmonella-producing 5-FU in tumour cell cultures
Next, we decided to compare the consequences of 5-FU-controlled production by the different plasmids and strains obtained in this work in eukaryotic cell cultures. To determine the effects of 5-FU in eukaryotic cells, it is necessary to analyse the evolution of cell cultures for 6 days after the addition of this compound (Erbs et al., 2000; Bourbeau et al., 2004). However, once Salmonella has infected the eukaryotic cells, bacterial proliferation and expression of certain bacterial proteins during the first hours of infection induce host cell death within 18–24 h, hindering the study of the effect of the 5-FU produced by Salmonella in cell cultures (Kim et al., 1998; Paesold et al., 2002; Mesa-Pereira et al., 2013). To analyse the effects of the 5-FU overproduced by Salmonella in cell cultures, we generated a mutation in the producer strain to prevent bacterial growth and protein production inside host cell. It has been previously reported that attenuated purD mutants are invasion proficient but unable to proliferate once inside the eukaryotic cell. Nevertheless, the addition of adenine to culture medium can temporally suppress this deficiency (Leung and Finlay, 1991); thus, in a purD- background, intracellular proliferation and CD overproduction can be controlled by the presence of adenine and salicylate respectively. This strategy has been recently exploited in our laboratory to study the role of SpvB Salmonella effector protein in the infection process (Mesa-Pereira et al., 2013). In the present work, we have used a similar experimental approach to study CD overproduction effects, and included a ΔpurD mutation in all the strains used in this work. The strain MPO376 (ΔpurD) bearing the empty vector, pMPO1088 (CDGUG) or pMPO90 (CD7AUG) and the strain MPO378 (ΔpurDΔupp) carrying pMPO90 were used to infect HeLa cells. After invasion, adenine was added to infected cell cultures. and 1 h later, once infection was established, codA expression was induced with salicylate. Five hours later, adenine concentration was reduced 40-fold to avoid bacterial proliferation, 50 μM of 5-FC was added and cells were incubated for 6 days in the presence of salicylate and 5-FC. As a control, uninfected cell cultures followed the same treatment but in the presence of 10 μM of 5-FU (Erbs et al., 2000). The effects of codA overexpression and 5-FU production were analysed by flow cytometry and microscopy.
Figure 3A shows the cell cycle distribution of HeLa cell cultures. As expected, most of the cells (67%) of the control HeLa cell cultures in presence of 5-FC and absence of 5-FU were in G0/G1 phase of the cell cycle. Similarly, treatment with 5-FU produced the expected effects on the cell cycle distribution (Pizzorno et al., 1995; Takeda et al., 1999; Yoshikawa et al., 2001; De Angelis et al., 2006): cells in G0/G1 phase were reduced down to 30%, while the dead cell population, represented as the percentage of cells in sub-G1 phase of the cell cycle, and cells arrested in S and G2/M phase were increased. Conversely, cell cycle of cells infected with the ΔpurD strain bearing the empty vector were not affected even 6 days after infection, which confirms that this mutant is unable to induce cell death in the absence of adenine. Interestingly, expression of CD by this proliferation deficient strain led to a substantial alteration of the cell cycle distribution in a way similar to 5-FU (increase in dead or arrested cells and decrease in normal cells). Consistent with the above experiments, infection with the ΔpurD strain bearing pMPO1088 (CDGUG) had a slight but observable effect on cell cycle distribution. However, the effect was much higher when the infecting bacteria harboured pMPO90 (CD7AUG). In this case, there even was an increase in > 4N cells indicative of aberrant endoreduplication. In addition, the maximum effect on cell cycle distribution was achieved when the strain bearing pMPO90 (CD7AUG) was the 5-FU resistant strain ΔpurDΔupp (more cells in sub-G1 than in G0/G1 phase), which was even more pronounced than that induced with 10 μM of 5-FU in the control culture. Accordingly, phase contrast microscopy showed much less proliferating HeLa cells when Salmonella expressed CD from pMPO90 (CD7AUG), an effect that was even more evident in these conditions than in 5-FU treated cultures (Fig. 3B). Additionally, to test whether the 5-FU produced from these vectors and strains has similar consequences on different tumour cell lines, we performed the same analysis to determine the effects on MCF-7 and HCT116 cell cycle distribution. As shown in Fig. S1 (Supporting information), CD controlled expression produced similar effects on MCF-7 and HCT116 cell cycle, thus showing they are not restricted to HeLa cells. Taken together, these results demonstrate that upon salicylate induction, Salmonella bearing the new codA expressing construct produces more CD and converts more 5-FU than the same strain transformed with the former construct, which subsequently correlates with a higher cytotoxicity in HeLa, MCF-7 and HCT116 cells. These high levels of 5-FU reached are also toxic to the producer strain, although using an upp mutant to produce CD circumvents this limitation. Finally, this experimental approach combining the salicylate induced expression system, and the purD mutant has proven to be effective to analyse and compare the effect of different CD producing strains on eukaryotic cell cycle. Thus, it can be a useful tool to investigate the consequences on cell physiology of any other cytotoxic protein produced by Salmonella, evaluate its potential as anticancer therapy agent in cell cultures and select the most appropriate combination of strains and plasmids prior to their study in animal models.
Fig 3.
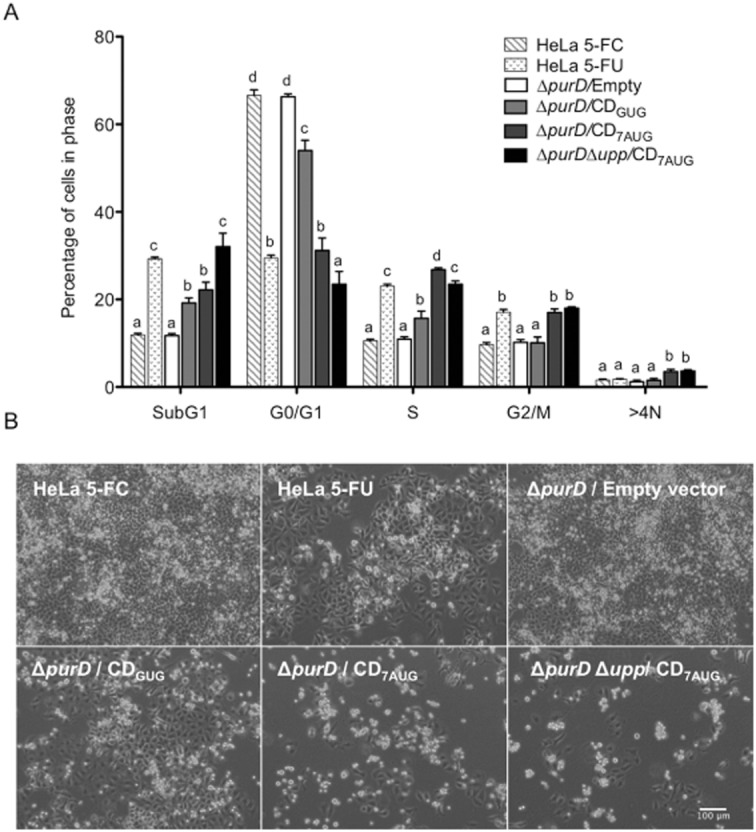
In vitro sensitivity to 5-FU produced by Salmonella on infected HeLa cells.A. Cell cycle distribution of HeLa cells infected with Salmonella MPO376 (ΔpurD) bearing pMPO54 (empty vector), pMPO1088 (CDGUG) or pMPO90 (CD7AUG) and MPO378 (ΔpurDΔupp) bearing pMPO90, at multiplicity of infection 50:1. The cells were cultured in the presence of 50 μM of 5-FC and harvested at 6 days post-induction. Ten thousand events were analysed by flow cytometry for each sample. Graphics represents the mean ± SD of three independent experiments. Non-infected HeLa cells treated with 50 μM of 5-FC or 10 μM of 5-FU were used as controls. One-way analysis of variance and Tukey HSD post hoc tests were applied to test for significant differences. Data from the same group marked with different alphabet are significantly different at P < 0.05.B. Phase contrast microscopy of infected HeLa cells as well as uninfected control and HeLa cells treated with 5-FU are shown at day 6.
Bystander activity of the 5-FU produced in cell cultures
Although Salmonella is able to invade tumour cells in vitro, there is some controversy regarding bacterial localization in vivo and some data indicate that bacteria also proliferates extracellulary in the necrotic region of solid tumour (Westphal et al., 2008; Crull et al., 2011). Therefore, the effectiveness of CD expressed by Salmonella in cancer therapy depends on the bystander activity of the produced drug. 5-fluorouracil has such bystander activity since it passively diffuses from cell to cell. For that reason, we compared the bystander effect on HeLa cell cultures infected with the strain MPO376 (ΔpurD) bearing the empty vector, pMPO90 (CD7AUG) or pMPO1088 (CDGUG) and the strain MPO378 (ΔpurDΔupp) carrying pMPO90. Cytosine deaminase expression was induced with salicylate as described above but, in this case, adenine was always present in the cultures at normal concentration. Forty hours after infection, supernatants of the different cultures were transferred to uninfected HeLa cells, and cell cycle distribution was analysed by flow cytometry 6 days later. Since supernatant transfer to fresh cultures resulted in a fivefold dilution, we used uninfected cultures treated with either 10 μM or 50 μM 5-FU as control, so they were also diluted to about 2 μM and 10 μM respectively.
The experiments, summarized in Fig. 4, revealed a bystander effect of the three CD-expressing Salmonella when compared with the strain carrying the empty vector. An increase in the sub-G1 population can be observed with these three strains. Although there is little difference between the effects detected with the ΔpurD strain carrying either pMPO90 (CD7AUG) or pMPO1088 (CDGUG), the higher effect was again achieved with the ΔpurDΔupp strain carrying pMPO90 plasmid. In fact, the consequences on cell cycle distribution caused by this combination of strain and plasmid were even greater than those generated in the control cultures.
Fig 4.
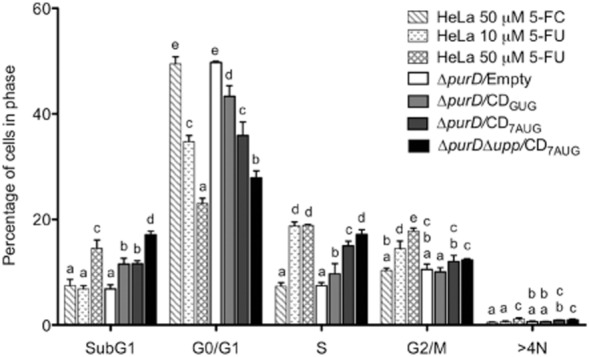
Bystander effect: Cytotoxicity of supernatants from infected HeLa cells treated with 5-FC. HeLa cells were infected at multiplicity of infection 50:1 and were grown in presence of 5-FC 250 μM in medium supplemented with adenine. Media were collected 40 h later, diluted 1:5 and added to uninfected HeLa cells. Effect of 5-FU produced was determined at day 6 by cell cycle analysis. Graphics represents mean ± SD of three independent experiments. Non-infected HeLa cells treated with 50 μM of 5-FC, 10 μM and 50 μM of 5-FU were used as controls. One-way analysis of variance and Tukey HSD post hoc tests were applied to test for significant differences. Data from the same group marked with different alphabet are significantly different at P < 0.05.
Current gene delivery systems have low efficiency targeting tumour tissues and can transduce only a small percentage of cells within a tumour. In consequence, the clinical use of cancer gene therapy is limited. Gene therapy approaches to express CD by transformed tumour cells could surpass this limitation due to the bystander effect of the CD/5-FU system. Given that CD has a cytosolic location, a possible limitation of this approach is that CD-expressing cells are killed before cytotoxic concentrations of extracellular 5-FU are reached, limiting in this way the bystander effect and, therefore, the anti-tumour efficiency (Lawrence et al., 1998). Better results would probably be obtained by expressing a secreted form of CD (Rehemtulla et al., 2004) or, specially, using Salmonella as a delivery vector, because it selectively targets tumours and preferentially colonizes extracellular compartments (Pawelek et al., 1997; Agorio et al., 2007; Loessner et al., 2007; Leschner and Weiss, 2010; Crull et al., 2011). Bacterial production of CD also has limitations since Salmonella is also sensitive to the high concentrations of 5-FU achieved. However, the upp- 5-FU resistance mutation in Salmonella would make the bacterial strain more competent in cancer therapy as it circumvents the suicide of the drug ‘factory’ and increases the bystander effect. The results of this work show that a Salmonella strain that combines high production levels of CD such as those achieved with pMPO90 with resistance to 5-FU due to the upp- mutation is a better candidate to be used to intra-tumourally delivered 5-FU in cancer treatment.
Acknowledgments
We are grateful to all members of the laboratory for their insights and helpful suggestions, and to Guadalupe Martín Cabello, Nuria Pérez Claros, Katherina García García and Corin Díaz Ramos for technical help. We also thank J. Casadesús, A. López-Rivas and D. Tuveson for the gift of HeLa, MCF-7 and HCT116 cell lines respectively.
Conflict of interest
None declared.
Supporting Information
Fig. S1. In vitro sensitivity to 5-FU produced by Salmonella on infected MCF-7 and HCT116 cells. A cell cycle distribution of MCF-7 (A) or HCT116 (B) cells infected with Salmonella MPO376 (ΔpurD) bearing pMPO54 (empty vector), pMPO1088 (CDGUG) or pMPO90 (CD7AUG) and MPO378 (ΔpurDΔupp) bearing pMPO90, at multiplicity of infection 50:1. The cells were cultured in the presence of 50 μM of 5-FC and harvested at 6 days post-induction. 10 000 events were analysed by flow cytometry for each sample. Graphics represents the mean ± SD of three independent experiments. Non-infected cells treated with 50 μM of 5-FC or 10 μM of 5-FU were used as controls. One-way ANOVA and Tukey HSD post hoc tests were applied to test for significant differences. Data from the same group marked with different alphabet are significantly different at P < 0.05.
Table S1. Bacterial strains and plasmids used in or constructed for this study.
References
- Agorio C, Schreiber F, Sheppard M, Mastroeni P, Fernandez M, Martinez MA. Chabalgoity JA. Live attenuated Salmonella as a vector for oral cytokine gene therapy in melanoma. J Gene Med. 2007;9:416–423. doi: 10.1002/jgm.1023. [DOI] [PubMed] [Google Scholar]
- Barnett SJ, Soto LJ, 3rd, Sorenson BS, Nelson BW, Leonard AS. Saltzman DA. Attenuated Salmonella typhimurium invades and decreases tumor burden in neuroblastoma. J Pediatr Surg. 2005;40:993–997. doi: 10.1016/j.jpedsurg.2005.03.015. discussion 997–998. [DOI] [PubMed] [Google Scholar]
- Bourbeau D, Lavoie G, Nalbantoglu J. Massie B. Suicide gene therapy with an adenovirus expressing the fusion gene CD: UPRT in human glioblastomas: different sensitivities correlate with p53 status. J Gene Med. 2004;6:1320–1332. doi: 10.1002/jgm.611. [DOI] [PubMed] [Google Scholar]
- Crull K, Bumann D. Weiss S. Influence of infection route and virulence factors on colonization of solid tumors by Salmonella enterica serovar Typhimurium. FEMS Immunol Med Microbiol. 62:75–83. doi: 10.1111/j.1574-695X.2011.00790.x. [DOI] [PubMed] [Google Scholar]
- Damon LE, Cadman E. Benz C. Enhancement of 5-fluorouracil antitumor effects by the prior administration of methotrexate. Pharmacol Ther. 1989;43:155–185. doi: 10.1016/0163-7258(89)90117-4. [DOI] [PubMed] [Google Scholar]
- Datsenko KA. Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis PM, Svendsrud DH, Kravik KL. Stokke T. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Mol Cancer. 2006;5:20. doi: 10.1186/1476-4598-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs P, Regulier E, Kintz J, Leroy P, Poitevin Y, Exinger F, et al. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813–3822. [PubMed] [Google Scholar]
- Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10:785–794. doi: 10.1038/nrc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaab WE, Mitchell LS, Miller JE, Vlasakova K. Skopek TR. 5-fluorouracil forward mutation assay in Salmonella: determination of mutational target and spontaneous mutational spectra. Mutat Res. 2005;578:238–246. doi: 10.1016/j.mrfmmm.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Harris BE, Manning BW, Federle TW. Diasio RB. Conversion of 5-fluorocytosine to 5-fluorouracil by human intestinal microflora. Antimicrob Agents Chemother. 1986;29:44–48. doi: 10.1128/aac.29.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, Kim K, Lim D, Jeong K, Hong Y, Nguyen VH, et al. Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium. PLoS ONE. 2014;9:e80050. doi: 10.1371/journal.pone.0080050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Eckmann L, Savidge TC, Lowe DC, Witthoft T. Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998;102:1815–1823. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I, Bermudes D, Lin S, Belcourt M, Pike J, Troy K, et al. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum Gene Ther. 2002;13:1225–1233. doi: 10.1089/104303402320139005. [DOI] [PubMed] [Google Scholar]
- Koechlin BA, Rubio F, Palmer S, Gabriel T. Duschinsky R. The metabolism of 5-fluorocytosine-2-14-C and of cytosine-14-C in the rat and the disposition of 5-fluorocytosine-2-14-C in man. Biochem Pharmacol. 1966;15:435–446. doi: 10.1016/0006-2952(66)90254-1. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Masui K, Sakamoto T, Nakatani T, Kikukawa M, Tsujinoue H, et al. Bystander effect caused by cytosine deaminase gene and 5-fluorocytosine in vitro is substantially mediated by generated 5-fluorouracil. Anticancer Res. 1998;18:3399–3406. [PubMed] [Google Scholar]
- Lawrence TS, Rehemtulla A, Ng EY, Wilson M, Trosko JE. Stetson PL. Preferential cytotoxicity of cells transduced with cytosine deaminase compared to bystander cells after treatment with 5-flucytosine. Cancer Res. 1998;58:2588–2593. [PubMed] [Google Scholar]
- Leschner S. Weiss S. Salmonella-allies in the fight against cancer. J Mol Med (Berl) 2010;88:763–773. doi: 10.1007/s00109-010-0636-z. [DOI] [PubMed] [Google Scholar]
- Leung KY. Finlay BB. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci USA. 1991;88:11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner H, Endmann A, Leschner S, Westphal K, Rohde M, Miloud T, et al. Remote control of tumour-targeted Salmonella enterica serovar Typhimurium by the use of L-arabinose as inducer of bacterial gene expression in vivo. Cell Microbiol. 2007;9:1529–1537. doi: 10.1111/j.1462-5822.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- Lundegaard C. Jensen KF. Kinetic mechanism of uracil phosphoribosyltransferase from Escherichia coli and catalytic importance of the conserved proline in the PRPP binding site. Biochemistry. 1999;38:3327–3334. doi: 10.1021/bi982279q. [DOI] [PubMed] [Google Scholar]
- Medina C, Camacho EM, Flores A, Mesa-Pereira B. Santero E. Improved expression systems for regulated expression in Salmonella infecting eukaryotic cells. PLoS ONE. 2011;6:e23055. doi: 10.1371/journal.pone.0023055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa-Pereira B, Medina C, Camacho EM, Flores A. Santero E. Novel tools to analyze the function of Salmonella effectors show that SvpB ectopic expression induces cell cycle arrest in tumor cells. PLoS ONE. 2013;8:e78458. doi: 10.1371/journal.pone.0078458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers M, Hwang A, Wagner MW, Bruening AJ, Veigl ML, Sedwick WD. Boothman DA. A role for DNA mismatch repair in sensing and responding to fluoropyrimidine damage. Oncogene. 2003;22:7376–7388. doi: 10.1038/sj.onc.1206941. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Cunningham C, Senzer N, Kuhn J, Cramm J, Litz C, et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003;10:737–744. doi: 10.1038/sj.cgt.7700634. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Kawamura Y, Kawamoto K, Matsumura H, Yamamoto N, Ito T, et al. Antineoplastic effects in rats of 5-fluorocytosine in combination with cytosine deaminase capsules. Cancer Res. 1985;45:1753–1761. [PubMed] [Google Scholar]
- Paesold G, Guiney DG, Eckmann L. Kagnoff MF. Genes in the Salmonella pathogenicity island 2 and the Salmonella virulence plasmid are essential for Salmonella-induced apoptosis in intestinal epithelial cells. Cell Microbiol. 2002;4:771–781. doi: 10.1046/j.1462-5822.2002.00233.x. [DOI] [PubMed] [Google Scholar]
- Pawelek JM, Low KB. Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- Pizzorno G, Sun Z. Handschumacher RE. Aberrant cell cycle inhibition pattern in human colon carcinoma cell lines after exposure to 5-fluorouracil. Biochem Pharmacol. 1995;49:553–557. doi: 10.1016/0006-2952(94)00444-q. [DOI] [PubMed] [Google Scholar]
- Polak A. Scholer HJ. Mode of action of 5-fluorocytosine and mechanisms of resistance. Chemotherapy. 1975;21:113–130. doi: 10.1159/000221854. [DOI] [PubMed] [Google Scholar]
- Rehemtulla A, Hamstra DA, Kievit E, Davis MA, Ng EY, Dornfeld K. Lawrence TS. Extracellular expression of cytosine deaminase results in increased 5-FU production for enhanced enzyme/prodrug therapy. Anticancer Res. 2004;24:1393–1399. [PubMed] [Google Scholar]
- Royo JL, Becker PD, Camacho EM, Cebolla A, Link C, Santero E. Guzman CA. In vivo gene regulation in Salmonella spp. by a salicylate-dependent control circuit. Nat Methods. 2007;4:937–942. doi: 10.1038/nmeth1107. [DOI] [PubMed] [Google Scholar]
- Saltzman DA, Heise CP, Hasz DE, Zebede M, Kelly SM, Curtiss R, 3rd, et al. Attenuated Salmonella typhimurium containing interleukin-2 decreases MC-38 hepatic metastases: a novel anti-tumor agent. Cancer Biother Radiopharm. 1996;11:145–153. doi: 10.1089/cbr.1996.11.145. [DOI] [PubMed] [Google Scholar]
- Takeda H, Haisa M, Naomoto Y, Kawashima R, Satomoto K, Yamatuji T. Tanaka N. Effect of 5-fluorouracil on cell cycle regulatory proteins in human colon cancer cell line. Jpn J Cancer Res. 1999;90:677–684. doi: 10.1111/j.1349-7006.1999.tb00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermes A, Guchelaar HJ. Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000;46:171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- Westphal K, Leschner S, Jablonska J, Loessner H. Weiss S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008;68:2952–2960. doi: 10.1158/0008-5472.CAN-07-2984. [DOI] [PubMed] [Google Scholar]
- Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, et al. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res. 2010;164:248–255. doi: 10.1016/j.jss.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama JI, Yamamura T. Hashimoto-Tamaoki T. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res. 2001;61:1029–1037. [PubMed] [Google Scholar]
- Zhao M, Yang M, Ma H, Li X, Tan X, Li S, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. In vitro sensitivity to 5-FU produced by Salmonella on infected MCF-7 and HCT116 cells. A cell cycle distribution of MCF-7 (A) or HCT116 (B) cells infected with Salmonella MPO376 (ΔpurD) bearing pMPO54 (empty vector), pMPO1088 (CDGUG) or pMPO90 (CD7AUG) and MPO378 (ΔpurDΔupp) bearing pMPO90, at multiplicity of infection 50:1. The cells were cultured in the presence of 50 μM of 5-FC and harvested at 6 days post-induction. 10 000 events were analysed by flow cytometry for each sample. Graphics represents the mean ± SD of three independent experiments. Non-infected cells treated with 50 μM of 5-FC or 10 μM of 5-FU were used as controls. One-way ANOVA and Tukey HSD post hoc tests were applied to test for significant differences. Data from the same group marked with different alphabet are significantly different at P < 0.05.
Table S1. Bacterial strains and plasmids used in or constructed for this study.


