Abstract
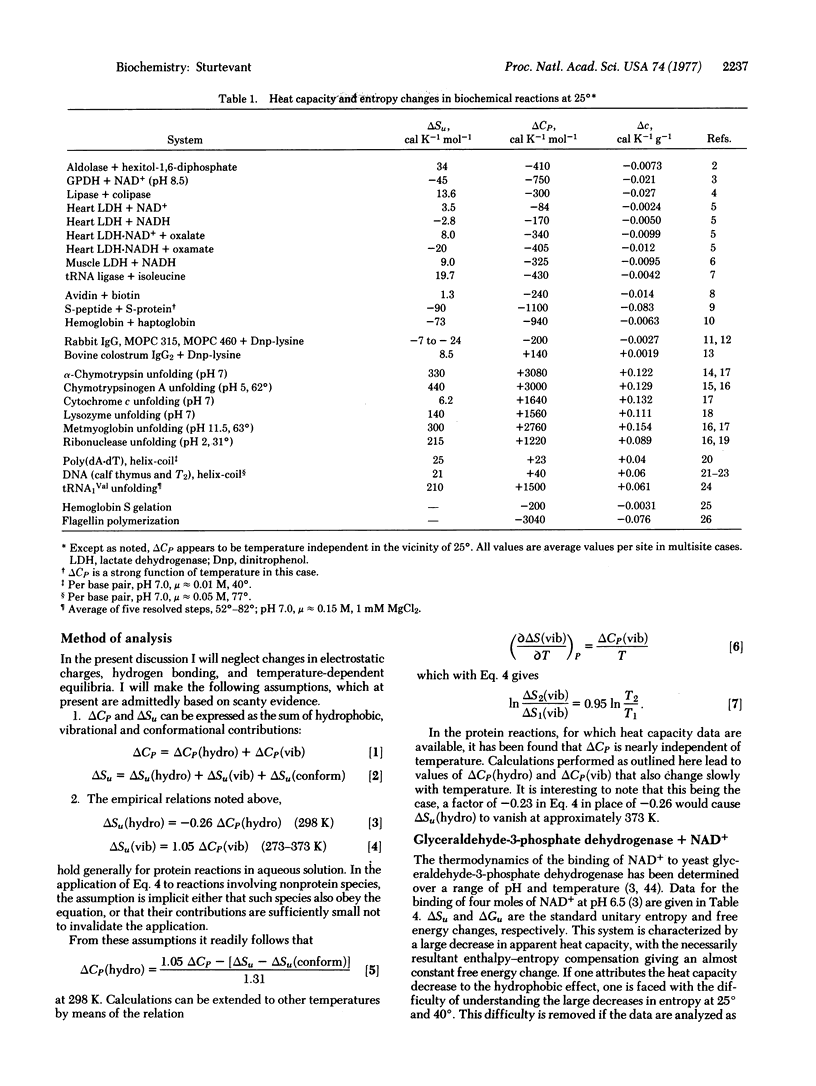
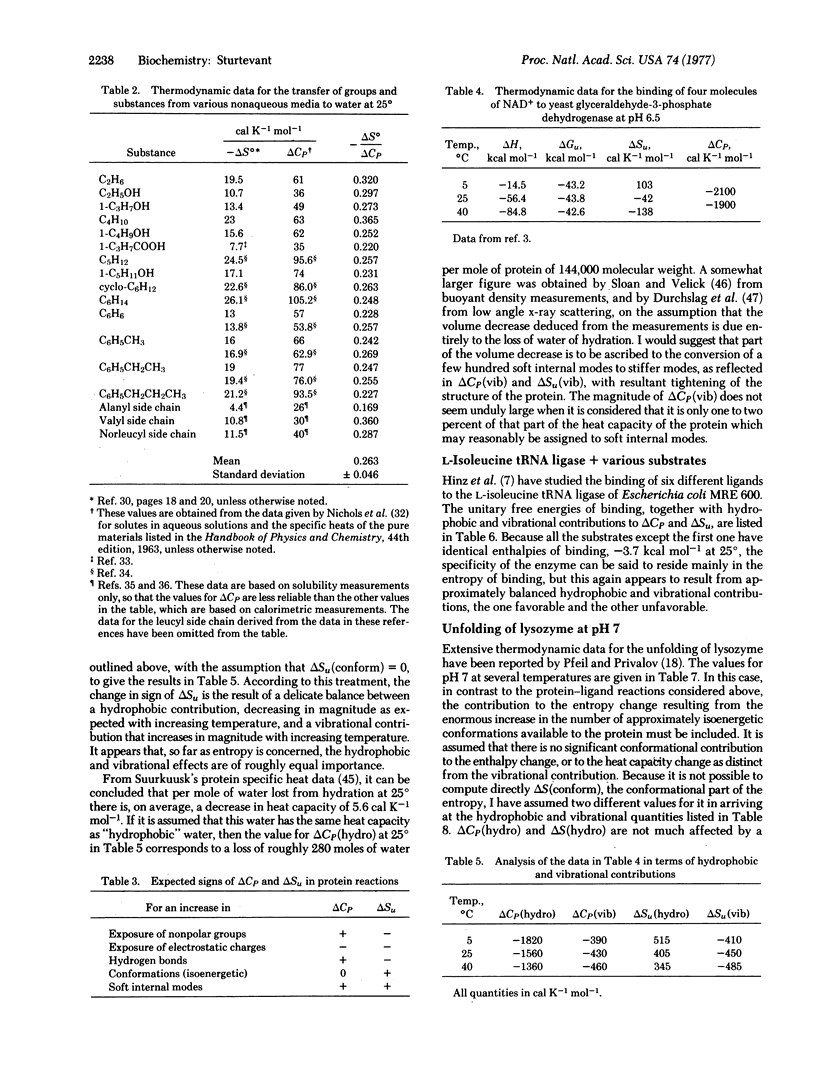
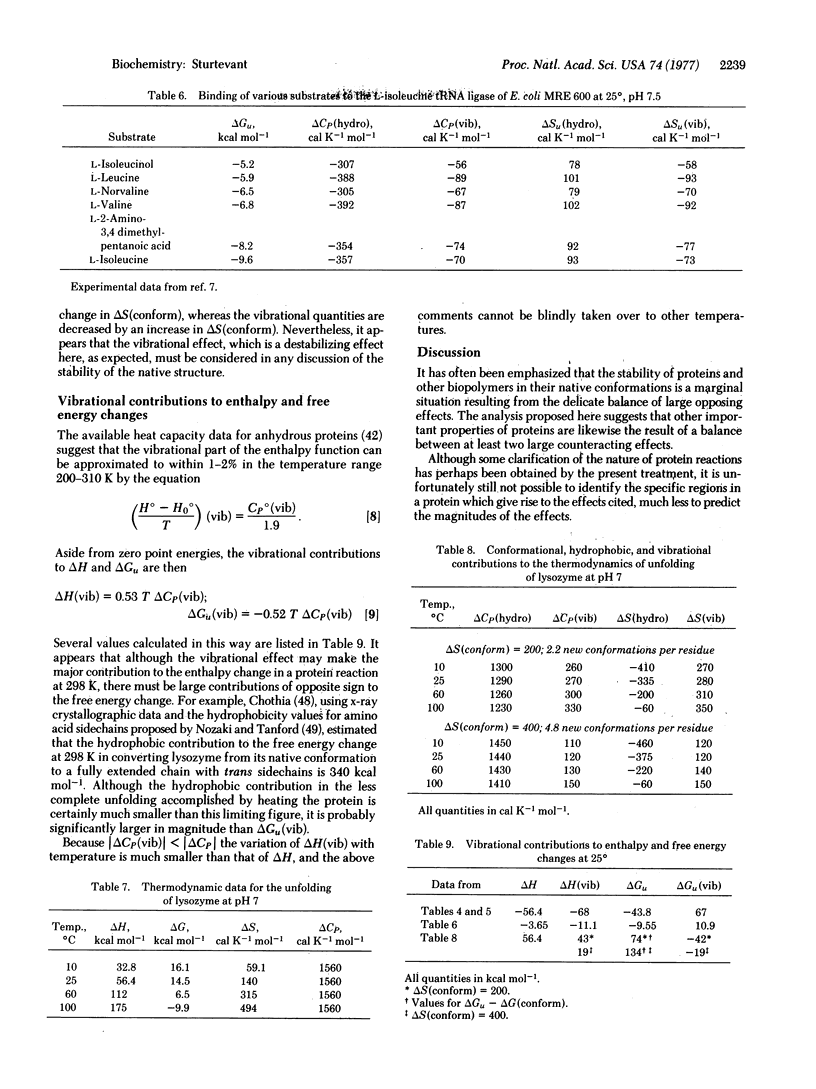
Six possible sources of the large heat capacity and entropy changes frequently observed for processes involving proteins are identified. Of these the conformational, hydrophobic, and vibrational effects seem likely to be of greatest importance. A method is proposed for estimating the magnitudes of the hydrophobic and vibrational contributions. Application of this method to several protein processes appears to achieve significant clarification of previously confusing and apparently contradictory data.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barisas B. G., Singer S. J., Sturtevant J. M. Thermodynamics of the binding of 2,4-dinitrophenyl and 2,4,6-trinitrophenyl haptens to the homologous and heterologous rabbit antibodies. Biochemistry. 1972 Jul 18;11(15):2741–2744. doi: 10.1021/bi00765a001. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Oliveira R. J., Westort C. Thermodynamics of protein denaturation. Effect of pressu on the denaturation of ribonuclease A. Biochemistry. 1970 Feb 17;9(4):1038–1047. doi: 10.1021/bi00806a045. [DOI] [PubMed] [Google Scholar]
- Brown K. G., Erfurth S. C., Small E. W., Peticolas W. L. Conformationally dependent low-frequency motions of proteins by laser Raman spectroscopy. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1467–1469. doi: 10.1073/pnas.69.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C. Structural invariants in protein folding. Nature. 1975 Mar 27;254(5498):304–308. doi: 10.1038/254304a0. [DOI] [PubMed] [Google Scholar]
- Donnér J., Spink C. H., Borgstöm B., Sjöholm I. Interactions between pancreatic lipase, co-lipase, and taurodeoxycholate in the absence of triglyceride substrate. Biochemistry. 1976 Nov 30;15(24):5413–5417. doi: 10.1021/bi00669a031. [DOI] [PubMed] [Google Scholar]
- Durchschlag H., Puchwein G., Kratky O., Schuster I., Kirschner K. X-ray small-angle scattering of yeast glyceraldehyde-3-phosphate dehydrogenase as a function of saturation with nicotinamide-adenine-dinucleotide. Eur J Biochem. 1971 Mar 1;19(1):9–22. doi: 10.1111/j.1432-1033.1971.tb01282.x. [DOI] [PubMed] [Google Scholar]
- Fanconi B., Finegold L. Vibrational states of the biopolymer polyglycine II: theory and experiment. Science. 1975 Oct 31;190(4213):458–460. doi: 10.1126/science.1166312. [DOI] [PubMed] [Google Scholar]
- Hearn R. P., Richards F. M., Sturtevant J. M., Watt G. D. Thermodynamics of the binding of S-peptide to S-protein to form ribonuclease S.. Biochemistry. 1971 Mar 2;10(5):806–817. doi: 10.1021/bi00781a013. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Jaenicke R. Thermodynamics of complex formation between nicotinamide adenine dinucleotide and pig skeletal muscle lactate dehydrogenase. Biochemistry. 1975 Jan 14;14(1):24–27. doi: 10.1021/bi00672a005. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Weber K., Flossdorf J., Kula M. R. Thermodynamic studies on the specificity of L-isoleucine-tRNA ligase of Escherichia coli MRE 600. Calorimetric investigations on binding of amino acids and isoleucinol to the enzyme. Eur J Biochem. 1976 Dec 11;71(2):437–442. doi: 10.1111/j.1432-1033.1976.tb11131.x. [DOI] [PubMed] [Google Scholar]
- Hutchens J. O., Cole A. G., Stout J. W. Heat capacities from 11 to 305 degrees K and entropies of hydrated and anhydrous bovine zinc insulin and bovine chymotrypsinogen A. Entropy change for formation of peptide bonds. J Biol Chem. 1969 Jan 10;244(1):26–32. [PubMed] [Google Scholar]
- Hvidt A. A discussion of pressure-volume effects in aqueous protein solutions. J Theor Biol. 1975 Mar;50(1):245–252. doi: 10.1016/0022-5193(75)90035-1. [DOI] [PubMed] [Google Scholar]
- Jackson W. M., Brandts J. F. Thermodynamics of protein denaturation. A calorimetric study of the reversible denaturation of chymotrypsinogen and conclusions regarding the accuracy of the two-state approximation. Biochemistry. 1970 May 26;9(11):2294–2301. doi: 10.1021/bi00813a011. [DOI] [PubMed] [Google Scholar]
- Johnston M. F., Barisas B. G., Sturtevant J. M. Thermodynamics of hapten binding to MOPC 315 and MOPC 460 mouse myeloma proteins. Biochemistry. 1974 Jan 15;13(2):390–396. doi: 10.1021/bi00699a026. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Klump H., Ackermann T. Experimental thermodynamics of the helix--random coil transition. IV. Influence of the base composition of DNA on the transition enthalpy. Biopolymers. 1971;10(3):513–522. doi: 10.1002/bip.360100307. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Lavialle F., Rogard M., Alfsen A. Calorimetric determination of the enthalpy and heat capacity changes for the association of haptoglobin with hemoglobin. I. Demonstration of two interacting systems. Biochemistry. 1974 May 7;13(10):2231–2234. doi: 10.1021/bi00707a032. [DOI] [PubMed] [Google Scholar]
- Niekamp C. W., Sturtevant J. M., Velick S. F. Energetics of the cooperative and noncooperative binding of nicotinamide adenine dinucleotide to yeast glyceraldehyde-3-phosphate dehydrogenase at pH 6.5 and pH 8.5. Equilibrium and calorimetric analysis over a range of temperature. Biochemistry. 1977 Feb 8;16(3):436–445. doi: 10.1021/bi00622a015. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Pfeil W., Privalov P. L. Thermodynamic investigations of proteins. III. Thermodynamic description of lysozyme. Biophys Chem. 1976 Jan;4(1):41–50. doi: 10.1016/0301-4622(76)80005-1. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Filimonov V. V., Venkstern T. V., Bayev A. A. A calorimetric investigation of tRNAVal1 melting. J Mol Biol. 1975 Sep 25;97(3):279–288. doi: 10.1016/s0022-2836(75)80041-6. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Khechinashvili N. N. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol. 1974 Jul 5;86(3):665–684. doi: 10.1016/0022-2836(74)90188-0. [DOI] [PubMed] [Google Scholar]
- Privalov P. L., Khechinashvili N. N., Atanasov B. P. Thermodynamic analysis of thermal transitions in globular proteins. I. Calorimetric study of chymotrypsinogen, ribonuclease and myoglobin. Biopolymers. 1971 Oct;10(10):1865–1890. doi: 10.1002/bip.360101009. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Hofrichter J., Eaton W. A. Calorimetric and optical characterization of sickle cell hemoglobin gelation. J Mol Biol. 1975 Aug 5;96(2):239–253. doi: 10.1016/0022-2836(75)90345-9. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E., Sturtevant J. M. Thermodynamics of the helix-coil transition of the alternating copolymer of deoxyadenylic acid and deoxythymidylic acid. J Mol Biol. 1969 Jun 28;42(3):577–580. doi: 10.1016/0022-2836(69)90244-7. [DOI] [PubMed] [Google Scholar]
- Schmid F., Hinz H. J., Jaenicke R. Thermodynamic studies of binary and ternary complexes of pig heart lactate dehydrogenase. Biochemistry. 1976 Jul 13;15(14):3052–3059. doi: 10.1021/bi00659a018. [DOI] [PubMed] [Google Scholar]
- Shiao D. D., Sturtevant J. M. Heats of thermally induced helix-coil transitions of DNA in aqueous solution. Biopolymers. 1973;12(8):1829–1836. doi: 10.1002/bip.1973.360120810. [DOI] [PubMed] [Google Scholar]
- Sloan D. L., Velick S. F. Protein hydration changes in the formation of the nicotinamide adenine dinucleotide complexes of glyceraldehyde 3-phosphate dehydrogenase of yeast. I. Buoyant densities, preferential hydrations, and fluorescence-quenching titrations. J Biol Chem. 1973 Aug 10;248(15):5419–5423. [PubMed] [Google Scholar]
- Suurkuusk J. Specific heat measurements on lysozyme, chymotrypsinogen, and ovalbumin in aqueous solution and in solid state. Acta Chem Scand B. 1974;28(4):409–417. doi: 10.3891/acta.chem.scand.28b-0409. [DOI] [PubMed] [Google Scholar]
- Suurkuusk J., Wadsö I. Thermochemistry of the avidin-biotin reaction. Eur J Biochem. 1972 Jul 24;28(3):438–441. doi: 10.1111/j.1432-1033.1972.tb01930.x. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Hearn R. P., Wrathall D. P., Sturtevant J. M. A calorimetric study of thermally induced conformational transitions of ribonuclease A and certain of its derivatives. Biochemistry. 1970 Jun 23;9(13):2666–2677. doi: 10.1021/bi00815a015. [DOI] [PubMed] [Google Scholar]
- Velick S. F., Baggott J. P., Sturtevant J. M. Thermodynamics of nicotinamide-adenine dinucleotide addition to the glyceraldehyde 3-phosphate dehydrogenases of yeast and of rabbit skeletal muscle. An equilibrium and calorimetric analysis over a range of temperatures. Biochemistry. 1971 Mar 2;10(5):779–786. doi: 10.1021/bi00781a009. [DOI] [PubMed] [Google Scholar]
- Zipp A., Kauzmann W. Pressure denaturation of metmyoglobin. Biochemistry. 1973 Oct 9;12(21):4217–4228. doi: 10.1021/bi00745a028. [DOI] [PubMed] [Google Scholar]


