Abstract
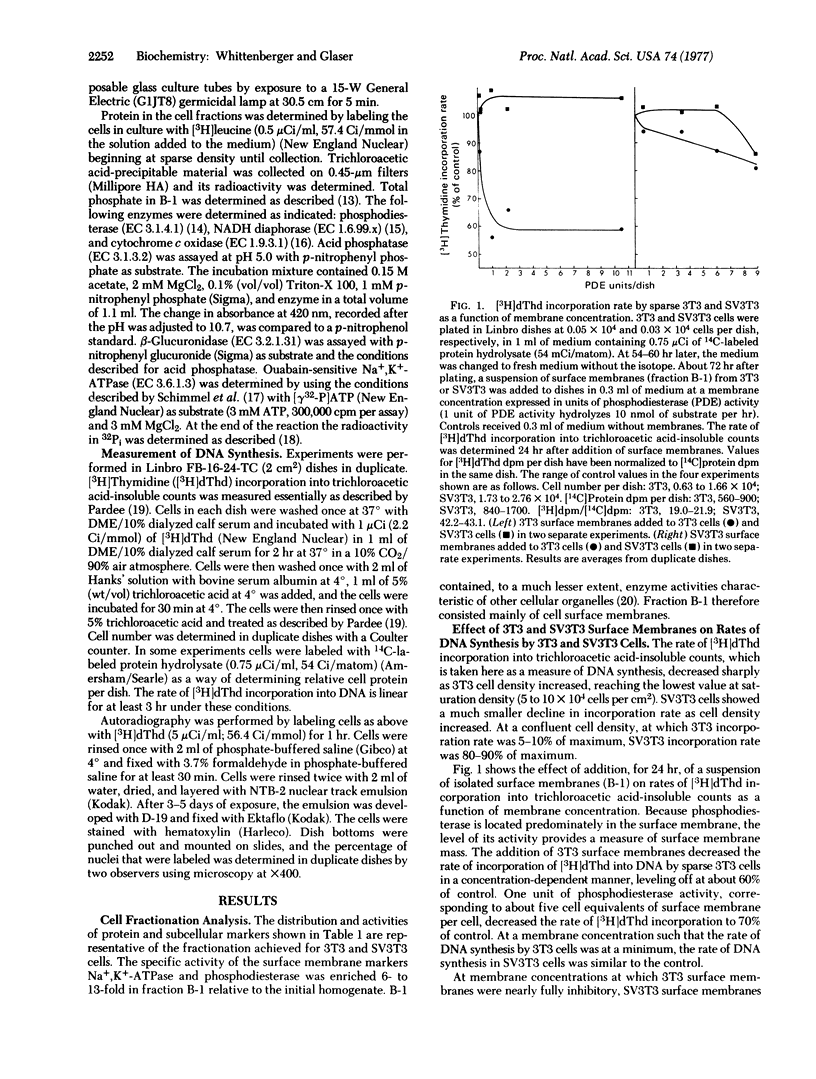
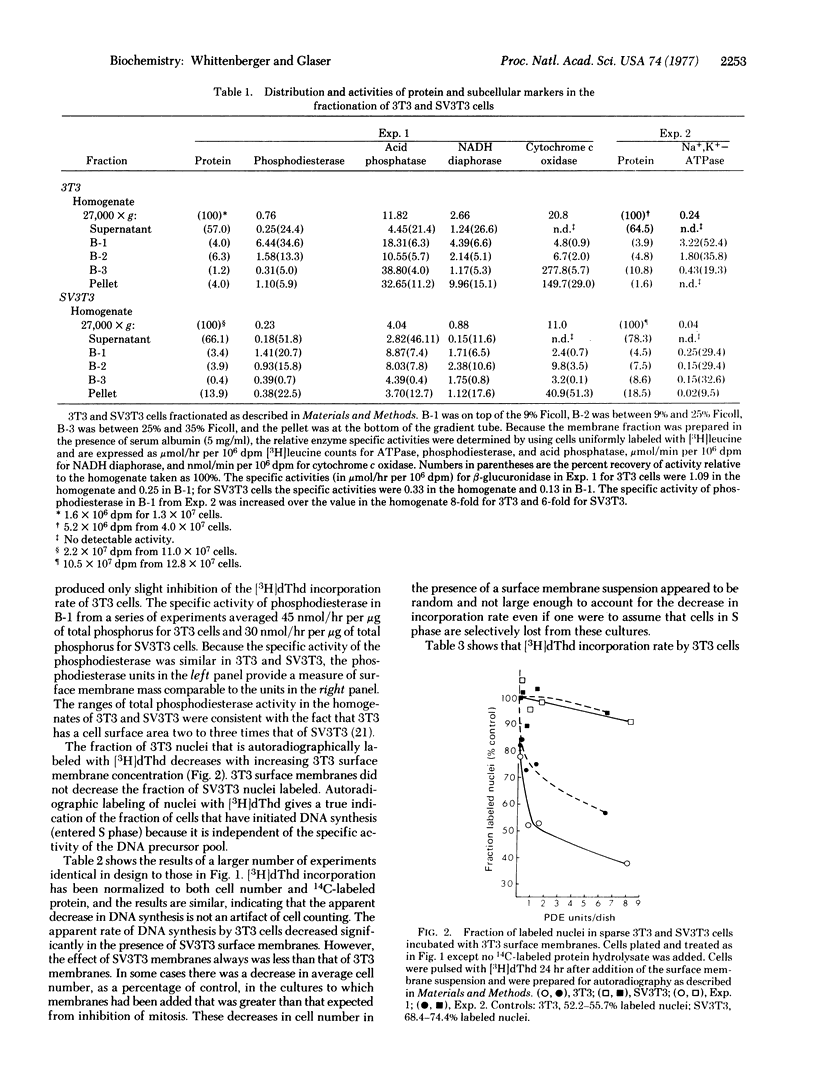
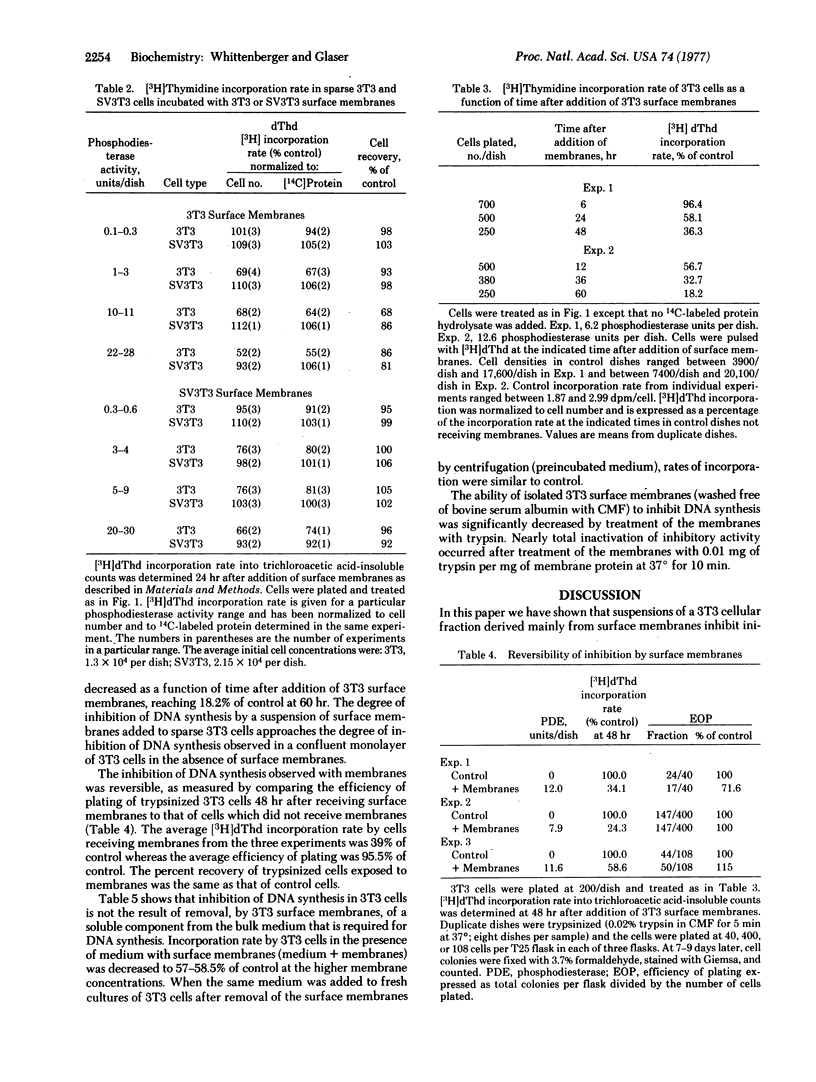
When added to a sparse culture of 3T3 cells, a surface membrane-enriched fraction from 3T3 cells inhibited the rate of DNA synthesis in a time- and concentration-dependent manner. The membrane preparation had no effect on the rate of DNA synthesis of simian virus 40-transformed 3T3 cells. A similar membrane preparation from transformed cells had a lesser inhibitory effect on 3T3 cells and no effect on transformed cells. The inhibition by membranes was reversible. The data suggest that, when added to growing 3T3 cells, 3T3 surface membranes can mimic the effect of increasing cell density on DNA synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borek C., Sachs L. The difference in contact inhibition of cell replication between normal cells and cells transformed by different carcinogens. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1705–1711. doi: 10.1073/pnas.56.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Stoker M. G. Conditions determining initiation of DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1970 May;66(1):204–210. doi: 10.1073/pnas.66.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Eagle H., Foley G. E., Koprowski H., Lazarus H., Levine E. M., Adams R. A. Growth characteristics of virus-transformed cells. Maximum population density, inhibition by normal cells, serum requirement, growth in soft agar, and xenogeneic transplantability. J Exp Med. 1970 Apr 1;131(4):863–879. doi: 10.1084/jem.131.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark A., Nathans A., Steiner D. F. Preparation and characterization of plasma membrane-enriched fractions from rat pancreatic islets. J Cell Biol. 1976 Nov;71(2):606–623. doi: 10.1083/jcb.71.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino R. J., Burger M. M. Growth inhibition of animal cells by succinylated concanavalin A. Nature. 1975 Jul 3;256(5512):19–22. doi: 10.1038/256019a0. [DOI] [PubMed] [Google Scholar]
- Nilausen K., Green H. Reversible arrest of growth in G1 of an established fibroblast line (3T3). Exp Cell Res. 1965 Oct;40(1):166–168. doi: 10.1016/0014-4827(65)90306-x. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. Binding of ( 3 H)concanavalin A to normal and transformed cells. J Biol Chem. 1973 Jun 25;248(12):4286–4292. [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel S. D., Kent C., Bischoff R., Vagelos P. R. Plasma membranes from cultured muscle cells: isolation procedure and separation of putative plasma-membrane marker enzymes. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3195–3199. doi: 10.1073/pnas.70.11.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M. G., Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967 Jul 8;215(5097):171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- Stoker M., Piggott D. Shaking 3T3 cells: further studies on diffusion boundary effects. Cell. 1974 Nov;3(3):207–215. doi: 10.1016/0092-8674(74)90133-0. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H., GOLDBERG B. D. TRANSFORMATION OF PROPERTIES OF AN ESTABLISHED CELL LINE BY SV40 AND POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:66–73. doi: 10.1073/pnas.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]



