A soluble variant of the monoglyceride lipase Yju3p was successfully expressed, purified and crystallized. Diffraction data were collected to 2.4 Å resolution.
Keywords: s-Yju3p, monoacylglycerol lipase, monoglyceride lipase
Abstract
The protein Yju3p is the orthologue of monoglyceride lipases in the yeast Saccharomyces cerevisiae. A soluble variant of this lipase termed s-Yju3p (38.3 kDa) was generated and purified to homogeneity by affinity and size-exclusion chromatography. s-Yju3p was crystallized in a vapour-diffusion setup at 293 K and a complete data set was collected to 2.4 Å resolution. The crystal form was orthorhombic (space group P212121), with unit-cell parameters a = 77.2, b = 108.6, c = 167.7 Å. The asymmetric unit contained four molecules with a solvent content of 46.4%.
1. Introduction
Lipases are enzymes that break down lipids by catalyzing the hydrolysis of ester bonds. Monoglyceride lipases (MGLs) specifically catalyze the breakdown of monoglycerides (MGs) into molecules of fatty acids and glycerol and have been identified in all kingdoms of life. They are involved in the digestive uptake and metabolism of nutritional lipids and in the synthesis and remodelling of membrane lipids. In intracellular lipolysis, stored triglycerides are mobilized from lipid droplets through the consecutive action of different lipases, where MGLs catalyze the last step (Lass et al., 2011 ▶). MGLs have high substrate specificity for MG, but have low stereoselectivity (Tornqvist & Belfrage, 1976 ▶; Heier et al., 2010 ▶; Imamura & Kitaura, 2000 ▶; Navia-Paldanius et al., 2012 ▶). MGLs have been shown to be involved in the endocannabinoid metabolism in the mammalian brain and also aid in bacterial cellular defence (Batovska et al., 2009 ▶; Dinh et al., 2004 ▶; Conley & Kabara, 1973 ▶; Kabara et al., 1978 ▶; Isaacs, 2001 ▶; Preuss et al., 2005 ▶).
Despite their ubiquitous expression and important physiological functions, only the three-dimensional structures of MGLs from Homo sapiens and Bacillus sp. H257 have been determined to date (Bertrand et al., 2010 ▶; Labar et al., 2010 ▶; Schalk-Hihi et al., 2011 ▶; Rengachari et al., 2012 ▶, 2013 ▶). The structures revealed an α/β hydrolase fold with a dynamic yet topologically conserved cap region. The amphiphilic nature of this cap region in eukaryotes might be necessary for the interaction with the lipid–water interface in order to extract MGs from lipid membranes (Schalk-Hihi et al., 2011 ▶).
This study focuses on Yju3p, the Saccharomyces cerevisiae orthologue of mammalian MGL (Heier et al., 2010 ▶). Studies using mass spectroscopy have shown that Yju3p binds to lipid droplets and is not found in cytosolic cell fractions (Athenstaedt et al., 1999 ▶; Athenstaedt & Daum, 2005 ▶). These findings imply that triglycerides stored in lipid droplets can be completely degraded to free fatty acids and glycerol on the lipid droplet surface. In this study, we report the crystallization of a soluble variant of the S. cerevisiae MGL, s-Yju3p, in an orthorhombic space group and preliminary diffraction analysis at 2.6 Å resolution, and describe the data quality and other properties of the crystal.
2. Materials and methods
2.1. Macromolecule production
Overexpression of Yju3p in Escherichia coli did not result in sufficient soluble target protein after cell lysis without adding detergents. A soluble variant of Yju3p was generated from the previously described construct by employing site-directed mutagenesis (Heier et al., 2010 ▶). The details and the rationale for this solubility-enhancement mutation L175S will be described elsewhere. The soluble variant containing this mutation will be referred to as s-Yju3p.
E. coli BL21 (DE3) cells harbouring a pProExHtb vector (LifeTechnologies) subcloned with s-Yju3p were grown in LB (Luria–Miller) broth (Carl Roth GmbH, Karlsruhe, Germany) at 37°C from an overnight seed culture until they reached an optical density (OD600) of 0.7. Gene expression was induced using 1 mM IPTG at 37°C for 4 h. The cells were harvested and lyzed by sonication in lysis buffer (20 mM Tris–HCl pH 8.0, 100 mM NaCl). The lysate was centrifuged at 22 000g for 30 min and the soluble fraction was loaded onto an Ni–NTA agarose resin column (Qiagen, Hilden, Germany). The protein was eluted with 20 mM Tris–HCl pH 8.0, 100 mM NaCl, 250 mM imidazole, 5% glycerol and was dialyzed against buffer A (20 mM Tris–HCl pH 8.0, 100 mM NaCl, 5% glycerol, 1 mM EDTA, 1 mM DTT). Subsequently, the protein was concentrated and loaded onto a Superdex 200 column (GE Healthcare) in buffer A at a flow rate of 2 ml min−1. The purity of the protein was examined using SDS–PAGE and the protein concentration was determined by UV spectroscopy using an extinction coefficient of 44 810 M −1 cm−1. Macromolecule-production information is summarized in Table 1 ▶.
Table 1. Macromolecule-production information.
| Source organism | S. cerevisiae |
| DNA source | pYEX-4T-1; insert cut out using BamHI and EcoRI |
| Cloning vector | pET21a; insert cut out using BamHI and XhoI |
| Expression vector | pProExHtb |
| Expression host | E. coli |
| Complete amino-acid sequence of the construct produced | MSYYHHHHHHDYDIPTTENLYFQGAMGSAPYPYKVQTTVPELQYENFDGAKFGYMFWPVQNGTNEVRGRVLLIHGFGEYTKIQFRLMDHLSLNGYESFTFDQRGAGVTSPGRSKGVTDEYHVFNDLEHFVEKNLSECKAKGIPLFMWGHSMGGGICLNYACQGKHKNEISGYIGSGPLIILHPHTMYNKPTQIIAPLLAKFSPRVRIDTGLDLKGITSDKAYRAFLGSDPMSVPLYGSFRQIHDFMQRGAKLYKNENNYIQKNFAKDKPVIIMHGQDDTINDPKGSEKFIRDCPSADKELKLYPGARHSIFSLETDKVFNTVFNDMKQWLDKHTTTEAKP |
2.2. Crystallization
Initial crystallization trials were performed with a 16.4 mg ml−1 s-Yju3p solution using the sitting-drop vapour-diffusion method with a 1:1 ratio of protein and reservoir solution (0.5 µl each). Initial crystals were obtained from the Morpheus screen (Molecular Dimensions, Suffolk, England) in a drop consisting of 0.1 M MOPS/HEPES-Na pH 7.5, 10%(w/v) PEG 20 000, 20%(v/v) PEG MME 550, 0.03 M sodium nitrate, 0.03 M disodium hydrogen phosphate, 0.03 M ammonium sulfate. This crystal was used to prepare a micro-seeding stock (D’Arcy et al., 2007 ▶). The seeding stock was diluted 1:1000 and used to set up the Morpheus screen again with a drop ratio of 0.4:0.4:0.2 µl protein solution (14 mg ml−1), reservoir solution and seeding stock, respectively. A crystal diffracting to 9 Å resolution was obtained from a drop consisting of 0.1 M bicine/Trizma base pH 8.5, 10%(w/v) PEG 20 000, 20%(v/v) PEG MME 550, 0.03 M sodium nitrate, 0.03 M disodium hydrogen phosphate, 0.03 M ammonium sulfate. Upon further optimization using the hanging-drop method, a crystal diffracting to 2.4 Å resolution was obtained from a drop containing the same conditions except that the pH of the bicine/Trizma buffer stock was 8.7. A 5 µl drop of 2:2:1 ratio of protein solution (14 mg ml−1), reservoir solution and seeding stock (1:100), respectively, was used for a second optimization using the hanging-drop method with the same conditions (Table 2 ▶). All crystallization experiments were performed at a temperature of 293 K.
Table 2. Crystallization.
| Method | Vapour diffusion: sitting/hanging-drop method |
| Plate type | Linbro (24-well) |
| Temperature (K) | 293 |
| Protein concentration (mgml1) | 14 |
| Buffer composition of protein solution | 20mM TrisHCl pH 8.0, 100mM NaCl, 5% glycerol, 1mM EDTA, 1mM DTT |
| Composition of reservoir solution | 0.1M bicine/Trizma base pH 8.7, 10%(w/v) PEG 20 000, 20%(v/v) PEG MME 550, 0.03M sodium nitrate, 0.03M disodium hydrogen phosphate, 0.03M ammonium sulfate |
| Volume and ratio of drop | 5l; 2:2:1 ratio of protein, reservoir solution and seeding stock |
| Volume of reservoir (ml) | 0.5 |
2.3. Data collection and processing
Diffraction data for the s-Yju3p crystals were collected on the PXIII beamline at Swiss Light Source (SLS), Villigen, Switzerland. The 270-image data set (oscillation angle 1°) was processed by XDS and scaled using AIMLESS (Kabsch, 2010 ▶; Evans & Murshudov, 2013 ▶). Details of the data collection and processing and statistics describing the quality of the data are listed in Table 3 ▶.
Table 3. Data collection and processing.
Values in parentheses are for the highest resolution shell.
| Diffraction source | PXIII beamline, SLS |
| Wavelength () | 0.999900 |
| Temperature (K) | 100 |
| Detector | MAR 225 CCD |
| Crystal-to-detector distance (mm) | 250 |
| Rotation range per image () | 1.00 |
| Total rotation range () | 270 |
| Space group | P212121 |
| a, b, c () | 77.2, 108.6, 167.7 |
| , , () | 90, 90, 90 |
| Mosaicity () | 0.10 |
| Resolution range () | 45.272.60 (2.702.60) |
| Total No. of reflections | 391577 |
| No. of unique reflections | 43595 |
| Completeness (%) | 99.1 (98.9) |
| Multiplicity | 9.0 (9.1) |
| I/(I) | 11.6 (2.5) |
| R meas | 0.191 (1.116) |
| R p.i.m. | 0.063 (0.365) |
| CC1/2 | 0.992 (0.712) |
| Overall B factor from Wilson plot (2) | 30.5 |
3. Results and discussion
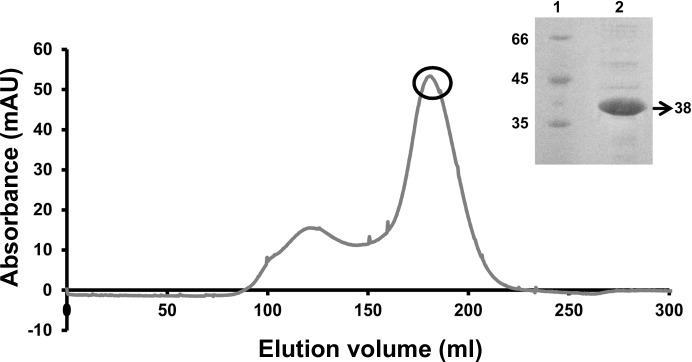
s-Yju3p was purified to apparent homogeneity by metal-affinity chromatography followed by size-exclusion chromatography. The major elution volume peak corresponds to monomeric s-Yju3p with an apparent molcular weight of 38 kDa (Fig. 1 ▶).
Figure 1.
Size-exclusion chromatography and SDS–PAGE analysis of s-Yju3p. The purified and monomeric fraction of s-Yju3p from the size-exclusion column was used for crystallization trials.
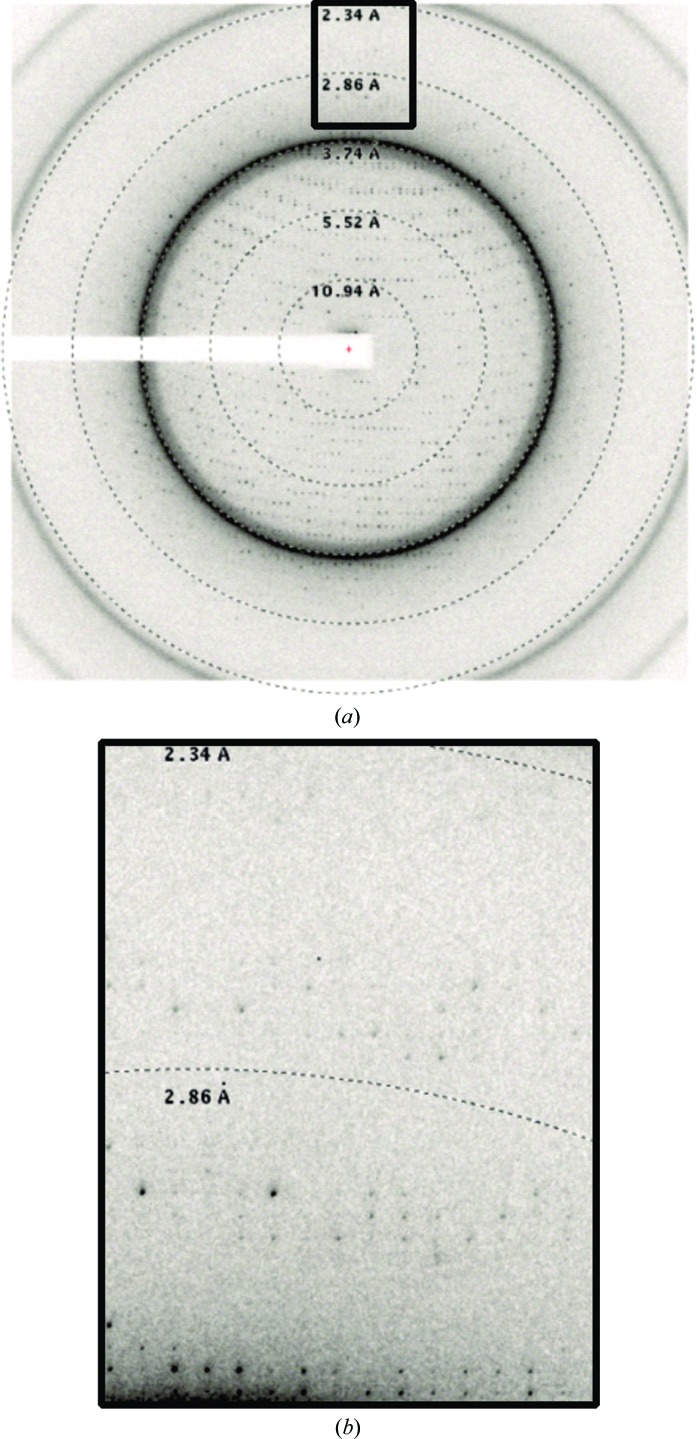
s-Yju3p crystallized as a cluster of platelets and crystals were detected after 7 days (Fig. 2 ▶). Single crystals from the cluster were harvested and flash-cooled in liquid nitrogen without the addition of further cryoprotectants (the addition of glycerol as a cryoprotectant led to the disintegration of the crystals). 270 diffraction images (oscillation angle 1°) were collected at 100 K from the best crystal diffracting to 2.4 Å resolution (Fig. 3 ▶), but the data set was cut at 2.6 Å during scaling based on the CC1/2 and R merge values. The data set was integrated in the primitive orthorhombic space group P212121 as verified by POINTLESS (Evans, 2006 ▶). The unit-cell parameters were a = 77.2, b = 108.6, c = 167.7 Å. According to the Matthews coefficient, the asymmetric unit contained four molecules with 46.4% solvent content [2.29 Å3 Da−1, P(tot) = 0.70; Matthews, 1968 ▶]. Phenix.xtriage revealed that the crystal form contained neither twinning nor pseudo-translational symmetry (Adams et al., 2010 ▶). Attempts to derive the phases of s-Yju3p by molecular replacement failed owing to a lack of high-identity structures. According to a BLAST search, the protein of known structure that has the highest sequence identity is MGL from H. sapiens, with a sequence identity of 24% (based on 97% query coverage). Hence, approaches leading to experimental phase determination are being pursued, i.e. the use of selenomethionine-incorporated protein for SAD and the use of heavy-atom soaking for MIR/MAD.
Figure 2.

Crystals of s-Yju3p obtained by the hanging-drop method at 293 K in 0.1 M bicine/Trizma base pH 8.7, 10%(w/v) PEG 20 000, 20%(v/v) PEG MME 550, 0.03 M sodium nitrate, 0.03 M disodium hydrogen phosphate, 0.03 M ammonium sulfate. The scale bar is 200 µm in length.
Figure 3.
X-ray diffraction image of s-Yju3p; the inset shows a close-up of high-resolution spots extending to 2.4 Å.
MGLs are a relevant class of enzymes which have been heavily studied with respect to their biochemical and pharmacological properties for industrial applications. Our understanding of the structural restraints determining the enzyme specificity is still vague, yet will aid our knowledge of substrate specificity. The structure of Yju3p will thus pave the way for a deeper understanding of the structure–function relationship of MGLs.
Acknowledgments
This work was supported by the Doctoral School ‘DK Molecular Enzymology’ Grant W901-B12 and by Project FWF P24857 funded by the Austrian Science Fund. We acknowledge the help of the staff at the PXIII beamline at Swiss Light Source (SLS) for their help during diffraction data collection.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Athenstaedt, K. & Daum, G. (2005). J. Biol. Chem. 280, 37301–37309. [DOI] [PubMed]
- Athenstaedt, K., Zweytick, D., Jandrositz, A., Kohlwein, S. D. & Daum, G. (1999). J. Bacteriol. 181, 6441–6448. [DOI] [PMC free article] [PubMed]
- Batovska, D. I., Todorova, I. T., Tsvetkova, I. V. & Najdenski, H. M. (2009). Pol. J. Microbiol. 58, 43–47. [PubMed]
- Bertrand, T., Augé, F., Houtmann, J., Rak, A., Vallée, F., Mikol, V., Berne, P. F., Michot, N., Cheuret, D., Hoornaert, C. & Mathieu, M. (2010). J. Mol. Biol. 396, 663–673. [DOI] [PubMed]
- Conley, A. J. & Kabara, J. J. (1973). Antimicrob. Agents Chemother. 4, 501–506. [DOI] [PMC free article] [PubMed]
- D’Arcy, A., Villard, F. & Marsh, M. (2007). Acta Cryst. D63, 550–554. [DOI] [PubMed]
- Dinh, T. P., Kathuria, S. & Piomelli, D. (2004). Mol. Pharmacol. 66, 1260–1264. [DOI] [PubMed]
- Evans, P. (2006). Acta Cryst. D62, 72–82. [DOI] [PubMed]
- Evans, P. R. & Murshudov, G. N. (2013). Acta Cryst. D69, 1204–1214. [DOI] [PMC free article] [PubMed]
- Heier, C., Taschler, U., Rengachari, S., Oberer, M., Wolinski, H., Natter, K., Kohlwein, S. D., Leber, R. & Zimmermann, R. (2010). Biochim. Biophys. Acta, 1801, 1063–1071. [DOI] [PMC free article] [PubMed]
- Imamura, S. & Kitaura, S. (2000). J. Biochem. 127, 419–425. [DOI] [PubMed]
- Isaacs, C. E. (2001). Adv. Nutr. Res. 10, 271–285. [DOI] [PubMed]
- Kabara, J. J., Lynch, P., Krohn, K. & Schemmel, R. (1978). The Pharmacological Effects of Lipids, edited by J. J. Kabara, pp. 25–36. Champaign: The Americal Oil Chemists’ Society.
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Labar, G., Bauvois, C., Borel, F., Ferrer, J.-L., Wouters, J. & Lambert, D. M. (2010). Chembiochem, 11, 218–227. [DOI] [PubMed]
- Lass, A., Zimmermann, R., Oberer, M. & Zechner, R. (2011). Prog. Lipid Res. 50, 14–27. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Navia-Paldanius, D., Savinainen, J. R. & Laitinen, J. T. (2012). J. Lipid Res. 53, 2413–2424. [DOI] [PMC free article] [PubMed]
- Preuss, H. G., Echard, B., Enig, M., Brook, I. & Elliott, T. B. (2005). Mol. Cell. Biochem. 272, 29–34. [DOI] [PubMed]
- Rengachari, S., Aschauer, P., Schittmayer, M., Mayer, N., Gruber, K., Breinbauer, R., Birner-Gruenberger, R., Dreveny, I. & Oberer, M. (2013). J. Biol. Chem. 288, 31093–31104. [DOI] [PMC free article] [PubMed]
- Rengachari, S., Bezerra, G. A., Riegler-Berket, L., Gruber, C. C., Sturm, C., Taschler, U., Boeszoermenyi, A., Dreveny, I., Zimmermann, R., Gruber, K. & Oberer, M. (2012). Biochim. Biophys. Acta, 1821, 1012–1021. [DOI] [PMC free article] [PubMed]
- Schalk-Hihi, C. et al. (2011). Protein Sci. 20, 670–683.
- Tornqvist, H. & Belfrage, P. (1976). J. Biol. Chem. 251, 813–819. [PubMed]