Abstract
Aim
Anorectal melanoma (ARM) is a very uncommon and highly lethal malignancy. Due to its rarity and non-specific symptoms, preoperative diagnosis may be often erroneous and subsequent treatment inappropriate. We report a case of primary rectal melanoma and discuss the current diagnostic and therapeutic challenges.
Case report
An 87-year-old man was admitted to our surgical unit with a history of progressive constipation, tenesmus, rectal bleeding and transanal mucous discharge. Preoperative investigations, including CT scan and colonoscopy with biopsy, were suggestive for locally advanced low rectal sarcoma and therefore the patient underwent abdominoperineal resection (APR). However, histopathological examination and immunohistochemistry resulted in a postoperative diagnosis of primary rectal melanoma. The patient died 6 months later due to local and systemic recurrence.
Conclusion
ARM should always be considered when unusual anorectal lesions are discovered. Regardless of the pathological stage and the extent of surgery, prognosis of ARM remains poor. Thus, whenever feasible, wide local excision is now the preferred treatment, since it is associated with lower postoperative morbidity and better quality of life compared to APR. In our case, although the initial diagnosis was incorrect, APR was justified by the local invasiveness and large size of the tumor.
Keywords: Anorectal melanoma, Wide local excision, Abdominoperineal resection
Introduction
Anorectal melanoma (ARM) is a highly lethal malignancy, accounting for only 1–2% of all lower gastrointestinal cancers and fewer than 2% of all melanomas (1). Regardless of the tumor stage and the extent of surgery, prognosis remains poor (1, 2); thus, whenever feasible, a wide local excision (WLE) is now preferred to a more extensive abdominoperineal resection (APR) (3, 4). However, due to its rarity, preoperative diagnosis may often be erroneous (5)and subsequent treatment inappropriate (6). The aim of this paper is to describe a well-documented case of primary rectal melanoma and to discuss our findings in light of the more recent literature.
Case report
An 87-year-old man was admitted to our surgical unit with a 3-month history of progressive constipation, tenesmus, rectal bleeding and transanal mucous discharge. Digital rectal examination revealed a hard mass arising from the left postero-lateral rectal wall. No palpable inguinal lymphadenopaties were found. Serum levels of tumour markers, including AFP, CA19.9, CEA, CA 125, were in the normal range. Colonoscopy showed a bluish-grey polypoid mass just above the dentate line, from which multiple biopsies were taken (Figure 1). Histopathological and immunohistochemical examinations (S-100 protein, CD45, cytokeratins, vimentin) were suggestive of undifferentiated non-epithelial neoplasia. The CT scan showed an intraluminal fungating mass, measuring 9 cm in maximum diameter, with intense and irregular contrast enhancement, obscuring the distal rectal lumen, infiltrating the perirectal fat and the levator ani muscle. No evidence of lymph nodes or systemic metastasis was found. Subsequent endoluminal ultrasound confirmed the local spreading of the tumor and the absence of perirectal lymphadenopaties.
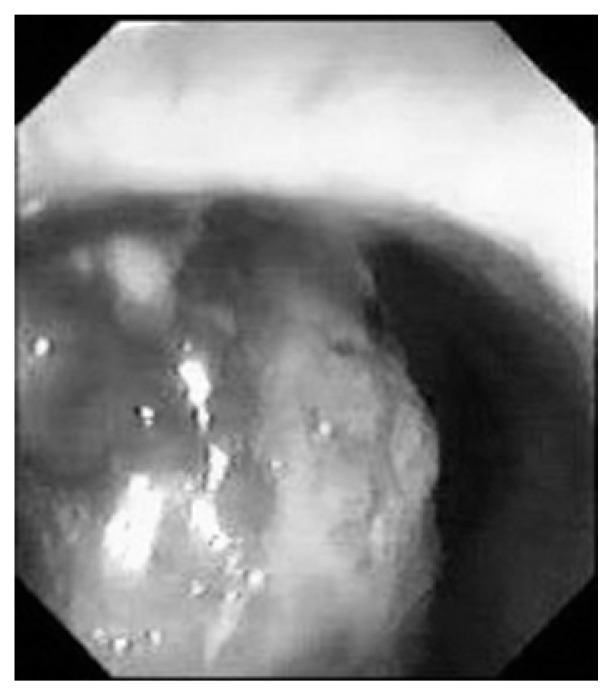
Fig. 1.
Colonoscopy reveals a bluish-grey polypoid mass in the lower rectum, just above the dentate line.
A presumptive diagnosis of undifferentiated low rectal sarcoma was made, and the patient underwent APR. The gross aspect of the resected specimen was that of a black, partially ulcerated tumour, 9×6×4 cm in size, located in the lower third of the rectum, abutting the dentate line (Figure 2). Histopathological examination confirmed full-thickness infiltration of the rectal wall to the perirectal fat and levator ani muscle, while it ruled out direct involvement of the dentate line. The tumour, covered with normal columnar epithelium, was composed of epithelioid cells with diffuse growth pattern (Figure 3a). Cytoplasmic melanin granules were seen in several neoplastic cells. Mitotic rate was 10/10 HPF. Immunohistochemistry showed intense diffuse staining with HMB-4 (Figure 3b) and focal staining for Vimentin and Melan-A. Positive reaction for Ck20 was observed only at the overlying columnar epithelium. These findings resulted in a diagnosis of rectal melanoma, classified as stage IIC under the AJCC staging system 2009. The postoperative course was uneventful and the patient was discharged 14 days post-operatively in good condition. At the 4-month follow-up, liver, lung and paraaortic lymph node metastases, together with massive pelvic recurrence were documented on CT scan. Two months later the patient died of neoplastic cachexia.
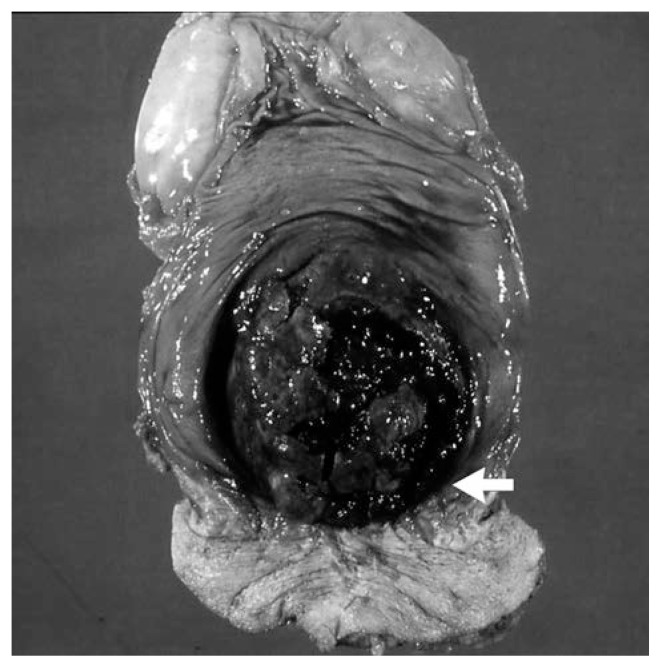
Fig. 2.
Specimen from abdominoperineal resection shows a large black ulcerated mass contiguous to the dentate line (arrow).
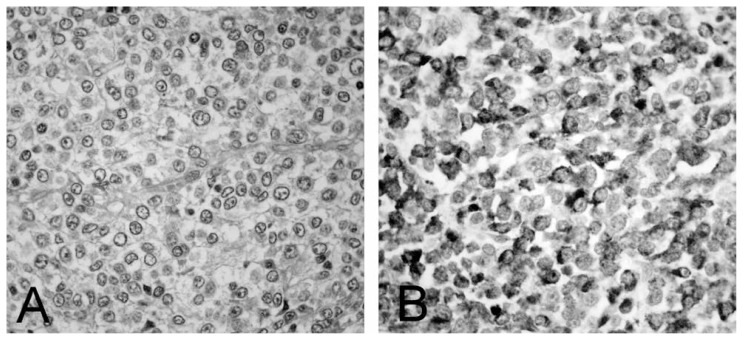
Fig. 3.
Histopathological examination: A) The tumour was composed of epithelioid cells with diffuse growth pattern (HE × 40). B) At immunohistochemistry, neoplastic cells showed intense diffuse staining with HMB-45 (Anti-HMB-45 antibody ×40).
Discussion
ARM is a very uncommon malignancy with an estimated incidence of 1.7 cases/1 million/year (7). Generally, it involves the dentate line, but a purely rectal location is reported in up to 24–35% of cases(2, 8). It predominantly affects the elderly (6th–8th decades of life) and is more frequent in women (male/female ratio of 1:1–1:4) (1, 5). Diagnosing ARM may be difficult for several reasons: the lack of clinical suspicion due to its very low incidence, the late-onset of non-specific symptoms, the frequent absence of the typical melanin pigmentation (up to 87% of cases), and their histological features overlapping those of other tumours, including sarcoma, lymphoma and undifferentiated carcinoma (1, 5, 9). Thus, although several immunohistochemical stains (the most common being HMB-45 and Melan-A), may help the correct evaluation of preoperative biopsy (9), misdiagnosis is reported to occur in over 50% of cases with diagnostic delay ranging between 3 and 8 months (5). Nevertheless, when diagnosis is eventually made, more than 60% of patients are found to have a localized disease that would seem to be potentially curable (2, 10). This notwithstanding, prognosis remains very poor, regardless of the tumour stage and of the extent of surgery, with an overall 5-years survival of no more than 10–20% (1, 2). Thus, while until recently APR was considered to be the standard treatment (11), less aggressive WLE is now increasingly recommended, since it is associated with lower postoperative morbidity and better quality of life (3, 4). APR is still considered appropriate for tumours not allowing local excision or for palliative treatment of extensive lesions, although in some instances it may be performed as a consequence of erroneous preoperative diagnosis (3, 4, 12). In our case, the preoperative diagnosis was incorrect, but the decision to perform an APR primarily came from the local invasiveness and large size of the tumor, and ultimately it may be regarded as consistent with current recommendations. However, a recent survey in the USA failed to demonstrate a significant trend toward less aggressive surgery for ARM (6). With the exception of the major melanoma centers, it is likely that inaccurate preoperative diagnosis together with limited experience in managing this rare malignancy are the main causes of still inadequate treatment.
The downside of WLE may be the higher rate of local recurrence (up to 50%), which may require salvage APR and compromise the primary purpose of sphincter-sparing resection (10). The use of radiation therapy in combination with WLE is reported by some authors to be beneficial (8), but this needs to be confirmed by further studies.
Conclusions
The case presented confirms that preoperative diagnosis of ARM is difficult and prognosis remains poor even in early stage tumours and after extensive surgery. ARM should always be considered when unusual anorectal lesions are discovered, and surgical management should be the least invasive, although APR may be required for locally advanced lesions.
References
- 1.Meguerditchian AN, Meterissian SH, Dunn KB. Anorectal melanoma: diagnosis and treatment. Dis Colon Rectum. 2011;54:638–44. doi: 10.1007/DCR.0b013e31820c9b1b. [DOI] [PubMed] [Google Scholar]
- 2.Bello DM, Smyth E, Perez D, Khan S, Temple LK, Ariyan CE, Weiser MR, Carvajal RD. Anal versus rectal melanoma: does site of origin predict outcome? Dis Colon Rectum. 2013;56:150–7. doi: 10.1097/DCR.0b013e31827901dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yap LB, Neary P. A comparison of wide local excision with abdominoperineal resection in anorectal melanoma. Melanoma research. 2004;14:147–150. doi: 10.1097/00008390-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Yeh JJ, Shia J, Hwu WJ, Busam KJ, Paty PB, Guillem JG, Coit DG, Wong WD, Weiser MR. The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Ann Surg. 2006;244:1012–1017. doi: 10.1097/01.sla.0000225114.56565.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falch C, Stojadinovic A, Hannvon-Weyhern C, Protic M, Nissan A, Faries MB, Daumer M, Bilchik AJ, Itzhak A, Brücher BL. Anorectal malignant melanoma: extensive 45-year review and proposal for a novel staging classification. J Am Coll Surg. 2013;217:324–35. doi: 10.1016/j.jamcollsurg.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Iddings DM, Fleisig AJ, Chen SL, Faries MB, Morton DL. Practice patterns and outcomes for anorectal melanoma in the USA, reviewing three decades of treatment: is more extensive surgical resection beneficial in all patients? Ann Surg Oncol. 2010;17:40–4. doi: 10.1245/s10434-009-0705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstock MA. Epidemiology and prognosis of anorectal melanoma. Gastroenterology. 1993;104:174–178. doi: 10.1016/0016-5085(93)90849-8. [DOI] [PubMed] [Google Scholar]
- 8.Kelly P, Zagars GK, Cormier JN, Ross MI, Guadagnolo BA. Sphincter-sparing local excision and hypofractionated radiation therapy for anorectal melanoma: a 20-year experience. Cancer. 2011;117:4747–55. doi: 10.1002/cncr.26088. [DOI] [PubMed] [Google Scholar]
- 9.Chute JD, Cousar JB, Mills SE. Anorectal malignant melanoma. Morphologic and immunohistochemical features. Am J Clin Pathol. 2006;126:93–100. doi: 10.1309/DVWL-TV8F-FKC3-L80H. [DOI] [PubMed] [Google Scholar]
- 10.Pessaux P, Pocard M, Elias D, Duvillard P, Avril M_F, Zimmerman P, Lasser P. Surgical management of primary anorectal melanoma. Br J Surg. 2004;91:1183–1187. doi: 10.1002/bjs.4592. [DOI] [PubMed] [Google Scholar]
- 11.Brady MS, Kavolius JP, Quan SH. Anorectal melanoma. A 64-year experience at memorial Sloan-Kettering Cancer center. Dis Colon Rectum. 1995;38:146–151. doi: 10.1007/BF02052442. [DOI] [PubMed] [Google Scholar]
- 12.Terada R, Ito S, Kobayashi M, Akama F, Tsujimura M, Ooe H. Anorectal melanoma: successful treatment by surgical excision and combination chemoimmunotherapy. Hepatogastroenterology. 2002;49:1545–8. [PubMed] [Google Scholar]