Abstract
Aim
Purpose of this retrospective comparative study is to evaluate the results of reconstruction of diabetic feet by split thickness skin graft (STSG) and by dermal substitute Integra® covered by STSG in terms of vascularity of the reconstructed wound-bed by measurements of tissue oxygenation (TcPO2).
Patients and Methods
23 patients were included into the study (12 were reconstructed by STSG only and 11 with Integra® and STSG three weeks later). In each patient TcPO2 measurements were performed at the same spot of the reconstructed area at 14 days, one month, 3 months, 6 months, 12 months and 24 months after reconstruction.
Results
Wound beds reconstructed by Integra® showed on average 10 mmHg higher TcPO2.
Conclusions
Our study estimated in an objective way, by TcPO2 value measurements, the oxygenation of the wound bed in diabetic feet after reconstruction by STSG only and after adding dermal substitute Integra® to the wound bed before final STSG coverage.
During first month after reconstruction no statistically significant differences were found. After 3 months TcPO2 studies revealed statistically significant higher oxygen tissue pressure in diabetic feet covered by Integra® plus STSG. These findings endorse in an objective way the clinical findings already reported while using the dermal substitute. It remains to explain the role of this increase of oxygen tissue pressure in redefine the indications for the use of dermal substitutes in reconstruction of poor vascularized regions.
Keywords: TcPO2, Dermal substitutes, Diabetic foot, Skin graft
Introduction
Diabetic foot is any infection, ulceration or destruction of deep tissues of the foot associated with neuropathy and/or peripheral arterial disease in the lower extremity of people with diabetes (1). The major adverse outcomes of diabetic foot problems are foot ulcers and amputations. Signs of peripheral arterial disease (PAD) (2) can be found in approximately half of patients with a foot ulcer. Given the uncertainties of medical history and clinical examination, more objective measurements of skin perfusion are frequently needed. Commonly used techniques include ankle pressure, toe pressure and transcutaneous oxygen pressure (TcPO2) measurements (1). The technique of transcutaneous oxymetry (TcPO2) allows the estimation of the partial pressure of oxygen on the skin surface. This technique (3) utilizes a sensor containing a Clark polarographic electrode that is placed on the skin at the area of interest, avoiding callous areas, edema, and bony prominences. The sensor warms the surrounding skin, causing localized hyperemia and facilitating oxygen diffusion. The measured PO2 in the dermis is displayed in millimeters of mercury, with a normal healthy value in the foot being > 50 mmHg (4). A value of < 40 mmHg is thought to represent sufficient hypoxia to impair wound healing, while patients with critical limb ischemia typically have a TcPO2 value of < 30 mmHg. All patients with CLI, in accordance with Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) criteria (2), are referred for angiographic study. If an obstruction greater than 50% of vessel diameter is present, peripheral transluminal angioplasty (PTA) is the first choice revascularization procedure during the same session, if possible. When PTA was not feasible, a bypass graft (BPG) is considered. Only after the revascularization the reconstruction is possible. Reconstruction of large foot wounds in patients with diabetes was uncommon until the mid-1980s (5). Recently, a number of studies have reported managing large-tissue defects of the diabetic foot with local random or pedicle flaps and microsurgical grafts. However, these surgical approaches are technically demanding and involve the creation of another major wound in the patients with diabetes, who typically has compromised healing abilities. The use of local flaps is limited by the availability of healthy, well vascularized tissue and the size of the defect. Bioengineered tissues, such as Apligraf® and Dermagraft® (6) have been used primarily for full-thickness wounds. A permanent skin substitute that contains a dermal regenerate template (Integra® Dermal Regeneration Template; Integra Life-Sciences, Plainsboro, NJ) provides an option for treating soft-tissue defects in diabetic feet as described by Clerici et al. (7). This product can be placed over exposed tendon and fascia in the operative setting. Since it was first described, Integra® has now became part of the armamentarium of plastic surgeon. However, there are disadvantages to its use; it is relatively expensive, difficult to use, and prone to infection. One of the major advantages of Integra® is considered to be the improved scar elasticity when compared to STSG alone (7–9). Despite Integra® being the most commonly used dermal skin substitute worldwide, a review of the current literature demonstrates that there are very few clinical trials that are based on an objective evaluation of outcome (10). Purpose of this retrospective comparative study is to evaluate the results of reconstruction of diabetic feet by split thickness skin graft (STSG) and by dermal substitute IntegraV covered by STSG in terms of vascularity of the reconstructed wound-bed by measurements of tissue oxygenation (TcPO2).
Patients
Subject
All patients with diabetic foot ulcers treated from January 2009 to March 2013 using STSG ± Integra® at the Department of Plastic and Reconstructive Surgery, Hospital of Cattinara, “A.O.U. Ospedali Riuniti”, Trieste, Italy were considered eligible for the study.
Materials and apparatus
Dermal substitute
The dermal substitute Integra® is a bilayer membrane made by using a porous co-precipitate of type I bovine collagen and glycosaminoglycan (chondroitin-6-sulfate) and covered using a temporary epidermal substitute made of a silicone membrane. It is available in a hydrated sterile package and can be used after being rinsed in a saline solution. Our protocol (10) calls for the application of the dermal substitute to a well vascularized wound bed that is free from infection, fastening it to the substrate using staples. On day 21, the silicone foil is removed, and the neodermis is covered using a thin graft (0.2–0.4 mm) (11).
Transcutaneous oxygen tension assessment
Greenhalgh (12, 13) and Meier described the TcPO2 method to evaluate skin graft take and skin graft maturity. Each skin graft was evaluated after a 20-min acclimatization period in a warm environment (room temperature 23–24°C). Transcutaneous oxygen tension was determined using Clark-type oxygen-sensing electrode (Multi-channel TcPO2/PCO2 Periflux System). The electrode was attached to the skin graft of the foot and the controlled heating element around the electrode was warmed to 44°C. Measurements were performed with the patient resting in supine position, covered by a light blanket, and at a room temperature of 21–23°C. The instrument was calibrated against a known standard before each use and the probe was left in place for 20 mins before a final reading was obtained. The study protocol was approved by the Institutional Review Board (IRB) and a written consent form was obtained from all participating volunteers.
Methods
23 patients with diabetic foot ulcers were included into the study. In all patients the reconstruction starts when local and systemic infections were under control as indicated by subsidence of fever, normalization of leukocyte count and disappearance of clinical signs of inflammation in the foot. In each patient TcPO2 measurements were performed at the same spot of the reconstructed area at 14 days, one month, 3 months, 6 months, 12 months and 24 months after reconstruction.
Statistical methods
For continuous variable (TcPO2), mean were calculated; the difference between the values of the two group was evaluated by the T Student test.
Results
From January 2009 to March 2013 we treated with skin graft 23 patients. Of this 23 patients 12 were reconstructed by STSG only and 11 with Integra® and STSG.
The clinical characteristics of two subgroups of patients are summarized in Table 1.
Table 1.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF FOLLOWED PATIENTS (N = 23).
| Variables | Patients (N = 23) |
|---|---|
| Age (years) | 62.2±12.2 |
| Female (n) | 5 |
| %HbA1c | 8.5±2.1 |
| Critical limb ischaemia (n) | 16 |
| IDSA Class. 3 | 11 |
| IDSA Class. 4 | 12 |
| Antibiotics use ev | 23 |
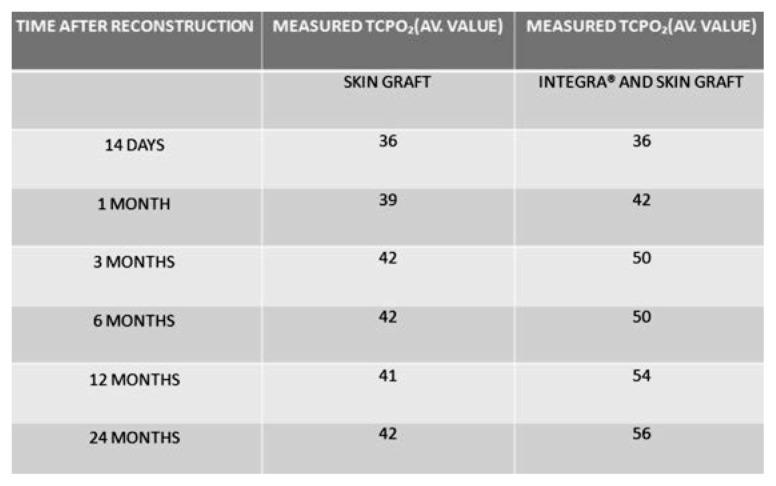
Median TcPO2 skin graft at 1 month was 39 mmHg; it increased after 2 months to 42 mmHg, and did not change 24 months after transplantation.
Median TcPO2 dermal substitute Integra® plus skin graft at 1 month was 42 mmHg; it increased after 2 months to 50 mmHg, and to 56 mm Hg 24 months after transplantation.
The wound beds reconstructed by Integra® showed on average 10 mmHg higher TcPO2 (p<0.03).
The results are summarized in Figures 1 and 2.
Fig. 1.
TcPO2 average value: skin graft vs dermal substitute Integra® plus skin graft.
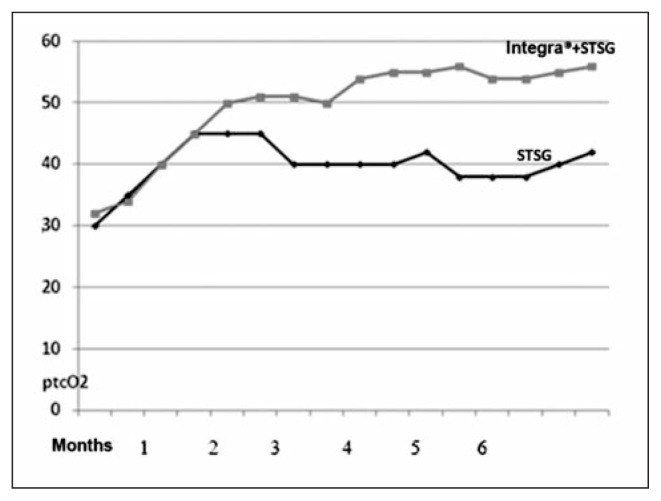
Fig. 2.
Curve of values.
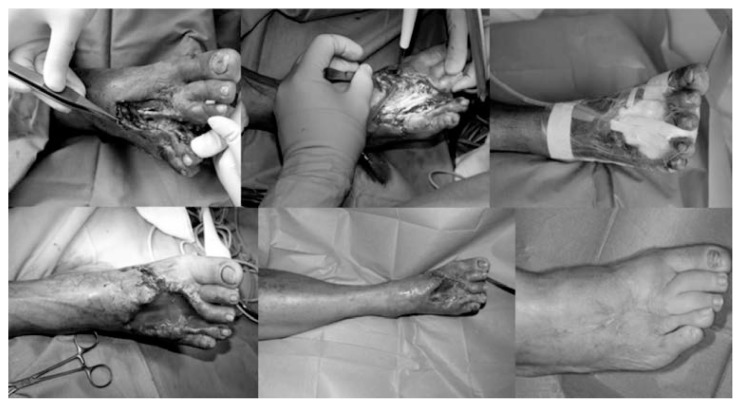
Figure 3 shows diabetic foot reconstructed by the dermal substitute Integra® plus STSG.
Fig. 3.
Severe diabetic foot infection (first sequence); urgent surgical debridement with tendon exposure (second sequence); negative pressure therapy (third sequence); graft with Integra® (fourth sequence); Integra® at 3 week after application (fifth sequence). Wound healing after skin graft. At 24 months TcPO2 54 mm Hg (sixth sequence).
Discussion and conclusions
The usage of skin substitutes has had a major diffusion over the last few years and they so far represent some of the most used materials by many specialists: their spread has gone hand in hand with the publication of many reports in the specialized literature. Yet only few authors have objectively evaluated the actual result of the reconstruction achieved with the skin substitute (13, 14). Given the shortcomings over the objective assessments of the substitute in the literature we have decided to evaluate the peripheral pressure of tissue oxygen in the diabetic foot reconstruction: in such pathology the oxygen level is particularly important for the healing process. The results we have achieved (even though with a low number of monitored patients) show that the reconstruction with the skin substitute allows reaching an oxygenation value which is on average higher by around 10 mm Hg. Our study estimated in an objective way, by TcPO2 value measurements, the oxygenation of the wound bed in diabetic feet after reconstruction by STSG only and after adding dermal substitute Integra® to the wound bed before final STSG coverage. During the first month after reconstruction no statistically significant differences were found. After 3 months TcPO2 studies revealed statistically significant higher oxygen tissue pressure in diabetic feet covered by Integra® plus STSG. These findings endorse in an objective way the clinical findings already reported while using the dermal substitute in terms of better vascularization. From the curve’s inclination we can observe that the TcPO2 in the graft increases up to a value of around 40 mm Hg: on the other hand, the TcPO2 in Integra® keeps on gradually augmenting until it reaches on average a value of 55 mm Hg in one-year period. This behaviour probably reflects a process of maturation and remoulding which seems to be slower in these wounds. The increase of TcPO2 over time can be explained by various factors: the regeneration of the capillary layer, the reduction of the interstitial edema and the decreased inflammatory response. These processes occur within a tridimensional and thicker structure: as a result they are slower, they require more time but could lead to the formation of a higher number of capillaries. In one patient with a postraumatic loss of tissue localized on the anterior surface of the right thigh and knee, Martini (15) et al. used the videocapillaroscopy to assess the microcirculatory pattern of the autologous skin grafted on Integra® in comparison with autologous skin grafted on granulation tissue. The results were compared with an area of undamaged skin of the contralateral knee. The results showed that the capillary density was similar between the regions of the lesion treated with dermal substitute, autologous skin graft and the undamaged contralateral knee skin. The fact that the usage of the skin substitute brings an increase of 10 mm Hg on average constitutes for us a pivotal feature. As already mentioned a higher level of TcPO2 is associated to a better healing process and this is important in the diabetic foot and in the reconstruction of the inferior limbs, especially in a patient with peripheral vascular disease affecting the inferior limbs. We deem that the reconstruction using the skin substitute Integra® leads to a better mechanical resistance thanks to the presence of a derma which can be placed on another derma of healthy dermis: as showed by Papa (10) et al. through the histology this persists after five years since the transplant. On the other hand, as we proved through this study the reconstruction via the skin substitute leads to an increased peripheral tissue oxygenation if compared to the reconstruction performer with the sole skin graft and thus this could allow reconstructions and attachment of skin transplants even at values which are lower by 40 mm Hg (and up to 20 mm Hg).
Footnotes
Best Communication Award at the XXV National Congress of the “Società Polispecialistica Italiana dei Giovani Chirurghi”, Bari, 13–15 June 2013
Conflict of interest statement
The Authors have no conflict of interest nor any financial and personal relationships with other people or organisations that could inappropriately influence (bias) their work.
References
- 1.International Consensus on the Diabetic Foot & Practical Guidelines on the Management and Prevention of the Diabetic Foot. 2011 [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG TASC II Working Group. Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E, III, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Arsenault KA, McDonald J, Devereaux PJ, Thorlund K, Tittley JG, Whitlock RP. The use of transcutaneous oximetry to predict complications of chronic wound healing: A systematic review and meta-analysis. Wound Rep Reg. 2011;19:657–663. doi: 10.1111/j.1524-475X.2011.00731.x. [DOI] [PubMed] [Google Scholar]
- 4.Fife CE, Smart DR, Sheffield PJ, Hopf HW, Hawkins G, Clarke D. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence. Undersea Hyperb Med. 2009;36:43–53. [PubMed] [Google Scholar]
- 5.Silverstein G. Dermal Regeneration Template in the Surgical Management of Diabetic Foot Ulcers: A Series of Five Cases. Journal of Foot&Ankle Surgery. 2006;45(1):28–33. doi: 10.1053/j.jfas.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003 Jun;26(6):1701–5. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 7.Clerici G, Caminiti M, Curci V, Quarantiello A, Faglia E. The use of dermal substitute to preserve maximal foot length in diabetic foot wounds with tendon and bone exposure following urgent surgical debridement for acute infection. Int Wound J. 2010 Jun;7(3):176–183. doi: 10.1111/j.1742-481X.2010.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen DQA, Dickson WA. A review of the use of a dermal skin substitute in burns care. Journal of Wound Care. 2006;15(8):373–6. doi: 10.12968/jowc.2006.15.8.26944. [DOI] [PubMed] [Google Scholar]
- 9.Heimbach D, Luterman A, Burke J, Cram A, Herndon D, Hunt J, et al. Artificial dermis for major burns. A multi-center randomised clinical trial. Annals of Surgery. 1988;208:313–9. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papa G, Pangos M, Renzi N, Ramella V, Panizzo N, Arnez ZM. Five years of experience using a dermal substitute: indications, histologic studies, and first results using a new single-layer tool. Dermatol Surg. 2011 Nov;37(11):1631–7. doi: 10.1111/j.1524-4725.2011.02156.x. [DOI] [PubMed] [Google Scholar]
- 11.Fang P, Engrav LH, Gibran NS, Honari S, Kiriluk DB, Cole JK, Fleckman P, Heimbach DM, Bauer GJ, Matsumura H, Warner P. Dermatome Setting for Autografts to Cover INTEGRA®. J Burn Care Rehabil. 2002;23:327–332. doi: 10.1097/00004630-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Greenhalgh DG, Warden GD. Transcutaneous Oxygen and Carbon Dioxide Measurements for Determination of Skin Graft “Take”. J Burn Care Rehabil. 1992;13:334–9. doi: 10.1097/00004630-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Meier TO, Guggenheim V, Vetter ST, Husmann M, Haile SR, Amann-Vesti BR. Microvascular regeneration in meshed skin transplants after severe burns. Burns. 2011;37:1010–1014. doi: 10.1016/j.burns.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Dai QA. Nguyen An objective long-term evaluation of Integra (a dermal skin substitute) and split thickness skin grafts, in acute burns and reconstructive surgery. Burns. 2010;36:23–2. doi: 10.1016/j.burns.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Martini R, Andreozzi GM, Tiengo C, Azzena B, Mazzoleni F. Videocapillaroscopy study of post traumatic lower limb loss of tissue treated with and without acellular dermal substitute. Clinical Hemorheology and Microcirculation. 2012;51:149–157. doi: 10.3233/CH-2011-1516. [DOI] [PubMed] [Google Scholar]