Abstract
Pneumonia is a common cause of pediatric hospitalization and almost 50% of children hospitalized for pneumonia develops meta pneumonic pleural effusion, most of which resolve spontaneously (1). The meta pneumonic effusion remains a major source of morbidity and mortality in the pediatric population and is a complication on the rise in both the U.S. (2) and Europe (3–6).
There is no uniformity of treatment of the meta pneumonic effusion in its early stages and are still questioning some aspects of proper management, remains uncertain and not always shared the operative timing (7). The treatment options are represented, in combination with antibiotic therapy, the thoracentesis (8), the positioning of one or more pleural drainage (9), fibrinolytic therapy (10), the toilet of the pleural cavity by means of video-assisted thoracoscopic surgery (VATS) (11) or “open” with thoracotomy (12) or traditional mini thoracotomy. We report our experience concerning the processing of meta pneumonic effusion, suggesting how the video thoracoscopy may be the treatment of choice in the early stages of the disease.
Keywords: VATS, Meta pneumonic effusion, Empyema
Introduction
According to the definition of the American Thoracic Society, the pathological manifestations of the meta pneumonic effusion are divided into three phases (13):
- Exudative phase, which lasts more than 3–4 days and is rapidly evolving into a collection of low viscosity fluids and cellular content (pleural fluid clear, sterile; leukocytes < 10.000/l; LDH < 1.000U/l; glucose > 40mg/dl; pH > 7.2);
- Phase fibrin-purulent (empyema), which marks the appearance of pus and fibrin and during which the harvest of fluids and starts compressing the lung, characterized by bacterial invasion of the pleural space, progressive inflammation, activation of coagulation factors and suppression of fibrinolysis, invasion of polymorphonuclear cells, fibrin deposition with or without “loculation” (glucose < 40mg/dl; pH: 7–7.2; LDH > 1000 U/l; protein > 2.5g/dl).
- The stage of organization, characterized the activity of fibroblasts both pleural surfaces that induces the formation of fibrous membranes inelastic (rigid rind), with pleural thickening, loculation of pleural cavity; fibrous tissue traps the lung parenchyma reducing the movements of the chest.
The stage of effusion influence the response to therapy, which must ensure the restoration of lung function, thus obviating the onset of a restrictive lung disease and ensuring rapid healing with good functional recovery. Treatment options may be medical or surgical (14). The type of antibiotic used depends on the age, medical condition of the patient and by the agent suspected to be responsible. The type of antibiotics should be targeted and then corrected according to the crop carried out. When medical treatment fails is mandatory the surgery approach (thoracentesis, placement of drainage tube, fibrinolytic therapy). In the past, thoracotomy and debridement were used in the later stages of empyema. With the increased use of endoscopic techniques, thoracoscopy seems to be an excellent alternative to surgery “open“ especially if performed in the early stages of the disease (15). This allows, for debridement with minimal morbidity and quicker recovery (16).
Patients and methods
Over the past four years, 8 children with a diagnosis of meta pneumonic pleural effusion were admitted to our unit (M:F = 3:1; aged 2.4 to 6.10 years). Of these, three have come to our observation with a meta pneumonic effusion stage I (two with localization to the left and 1 right) (Figure 1), 4 with meta pneumonic effusion stage II (3 left and 1 right) and 1 with bilateral empyema. Three children with effusion in exudative phase were subjected to thoracentesis with the positioning of the drainage tube: two of them stayed in hospital for about 12 days but in the third case the effusion has evolved toward the phase-fibrin and purulent and therefore it has been necessary to subject the patient to a surgical debridement by thoracotomy (with an hospitalization of 18 days). Two children diagnosed with the input of effusion during fibrin-purulent were subjected to thoracentesis and placement of drainage tube: one was discharged after 14 days whilst the second one (with bilateral location) was subsequently subjected to thoracotomy. With a small deposit which is being organized underwent surgery segmentectomy by thoracotomy with debridement. The two cases referred in the first instance to thoracoscopy (meta pneumonic effusion to the second stage) were hospitalized for 6 days (Figures 2, 3).
Fig. 1.
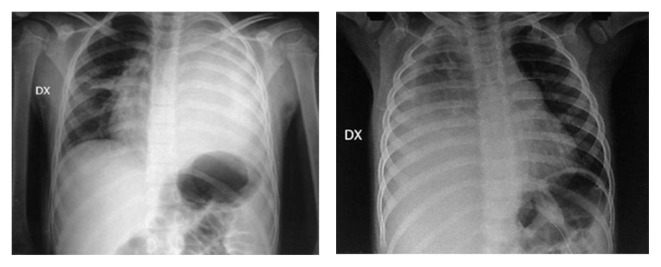
Two cases of metapneumonic effusion.
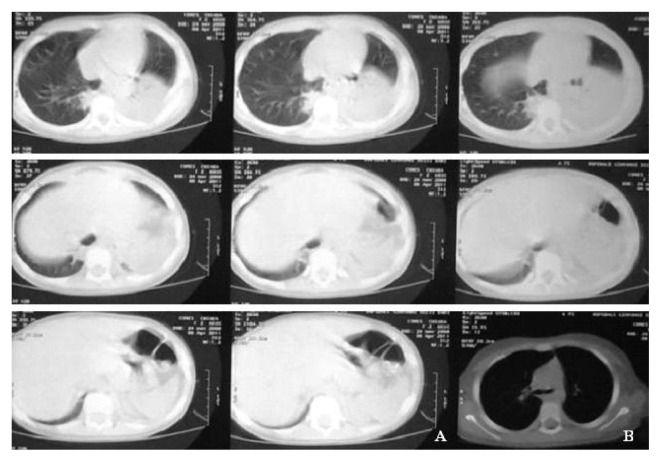
Fig. 2.
CT before (A) and after (B) VATS in a left meta pneumonic effusion at the second stage.
Fig. 3.
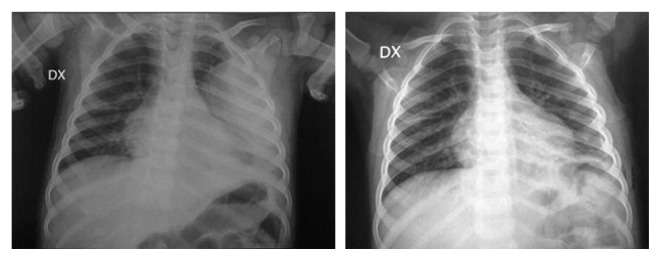
Rx before and after VATS in a left meta pneumonic effusion at the second stage.
Discussion
Traditionally a patient’s joint with fever, cough and dyspnoea is subjected to chest radiography, and then, in the presence of meta pneumonic effusion, for ultrasonography or CT scan to detect the possible presence of loculations (17) (Figure 4). The antibiotics doses with or without more invasive maneuvers (thoracentesis or placement of drainage tube) remains the algorithm treatment of choice for many centers and only when the payment progresses to a stage fibrin-purulent or organization, we proceed to thoracotomy surgery or thoracoscopy. In our brief experience we have found benefits with the primary VATS, the results are in fact giving lower time of hospitalization, stay drains, X-ray exposure, antibiotic treatment. The two patients who underwent VATS were subject to less number of procedures therefore with benefits also in economic terms (18).
Fig. 4.
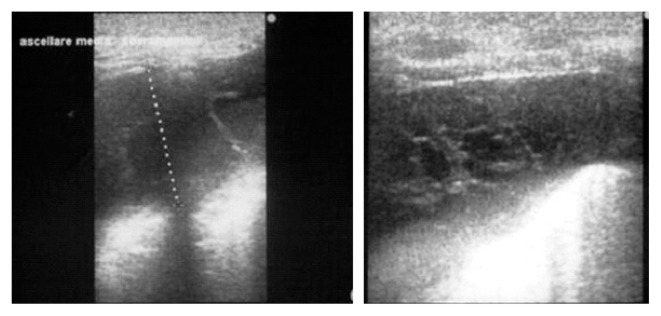
U.S. demonstrating effusion and loculations of costo-diaphragmatic sinus.
Conclusions
On the basis of the data we collected in our experience the primary thoracoscopic approach is associated with a shorter hospitalization, with a reduction in surgical procedures, reduction of antibiotic and analgesic. The benefits of an early and primary use of video-assisted thoracoscopic surgery includes the ability to identify the stage and effusion type (inspecting a more accurate cost-phrenic spaces and the apex of the lung and performing bacterial culture and physical-chemical liquid with white blood cell count), a quick etiological diagnosis, a direct and more accurately drainage pipe thus to provide decortications (19, 20). VATS, therefore, has a minimal morbidity and allows to achieve all the purposes of the surgical treatment. Our experience confirms, therefore, that the minimally invasive thoracoscopic treatment in the early stages is simple, safe and decisive for thoracic pathology of meta pneumonic effusions.
Footnotes
Best Communication Award at the XXV National Congress of the “Società Polispecialistica Italiana dei Giovani Chirurghi” Bari, 13–15 June 2013
References
- 1.Lichtenstein R, Suggs A, Campbell J. Pediatric pneumonia. Emer Clin N Am. 2003 May;21(2):437–51. doi: 10.1016/s0733-8627(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 2.Byington C, Spencer LY, Johnson TA, Pavia AT, Allen D, Mason EO, Kaplan S, Carroll KC, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infects Dis. 2002;34(4):434–40. doi: 10.1086/338460. [DOI] [PubMed] [Google Scholar]
- 3.Gupta R, Crowley S. Increasing pediatric empyema admissions. Thorax. 2006;61(2):179–81. doi: 10.1136/thx.2005.049510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer D, Iqbal S, Hasan A, Hamilton L. Empyema thoracic is still increasing in UK children. BMJ. 2006 Jun 3;332(7553):1333. doi: 10.1136/bmj.332.7553.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bueno Campana M, Agundez Reigosa B, Jimeno Ruiz S, Echàvarri Olavarrìa F, Martinez Granero MA. Is the incidence of parapneumonic pleural effusion increasing? An Pediatr. 2008 Feb;68(2):92–8. doi: 10.1157/13116221. [DOI] [PubMed] [Google Scholar]
- 6.Ampofo K, Herbener A, Blaschke AJ, Heyrend C, Poritz M, Korgenski K, Rolfs R, et al. Association of 2009 pandemic influenza A (H1N1) infectione and increased hospitalization with parapneumonic empyema in children in UTAH. Pediatr Infect Dis J. 2010 Oct;29(10):905–9. doi: 10.1097/INF.0b013e3181df2c70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaffé A, Balfour-Lynn IM. Management of empyema in children. Pediatr Pulmonol. 2005;40(2):148–56. doi: 10.1002/ppul.20251. [DOI] [PubMed] [Google Scholar]
- 8.Shoseyov D, Bibi H, Shatzberg G, Klar A, Akerman J, Hurvitz H, Maayan C. Short-term course and outcome of treatments of pleural empyema in pediatric patients: repeated ultrasound-guided needle thoracocentesis vs chest tube drainage. Chest. 2002 Mar;121(3):836–40. doi: 10.1378/chest.121.3.836. [DOI] [PubMed] [Google Scholar]
- 9.Satish B, Bunker M, Seddon P. Management of thoracic empyema in childhood: does the pleural thickening matter? Arch Dis Child. 2003;88:918–21. doi: 10.1136/adc.88.10.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao CT, Wu JM, Liu CC, Wu MH, Chuang HY, Wang JN. Treatment of complicated parapneumonic pleural effusion with intrapleural streptokinase in children. Chest. 2004 Feb;125(2):566–71. doi: 10.1378/chest.125.2.566. [DOI] [PubMed] [Google Scholar]
- 11.Cohen G, Hjortdal V, Ricci M, Jaffe A, Wallis C, Dinwiddie R, Elliott MJ, de Leval MR. Primary thoracoscopic treatment of empyema in children. J Thorac Cardiovasc Surg. 2003 Jan;125(1):79–83. doi: 10.1067/mtc.2003.88. [DOI] [PubMed] [Google Scholar]
- 12.Alexiou C, Goyal A, Firmin RK, Hickey MS. Is open thoracotomy still a good treatment option for the management of empyema in children? Ann Thorac Surg. 2003 Dec;76(6):1854–8. doi: 10.1016/s0003-4975(03)01076-2. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society. Management of non tuberculous empyema. Am Rev Respir Dis. 1962;85:935–6. [Google Scholar]
- 14.Balfour-Lynn IM, Abrahamson E, Cohen G, Hartley J, King S, Parikh D, Spencer D, et al. BTS Guidelines for the management of pleural infection in children. Thorax. 2005 Feb;60:11–21. doi: 10.1136/thx.2004.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah SS, DiCristina CM, Bell LM, Ten Have T, Metlay JP. Primary early thoracoscopy and reduction in length of hospital stay and additional procedures among children with complicated pneumonia. Arch Pediatr Adolesc Med. 2008;162(7):675–81. doi: 10.1001/archpedi.162.7.675. [DOI] [PubMed] [Google Scholar]
- 16.Kercher KW, Attorri RJ, Hoover JD, Morton D. Thoracoscopic decortications as first-line therapy for pediatric parapneumonic empyema. Chest. 2000 Jul;118(1):24–7. doi: 10.1378/chest.118.1.24. [DOI] [PubMed] [Google Scholar]
- 17.Knudtson J, Grewal H. Pediatric empyema-an algorithm for early thoracoscopic intervention. JSLS. 2004 Jan-Mar;8(1):31–4. [PMC free article] [PubMed] [Google Scholar]
- 18.Shah SS, Ten Have T, Metlay JP. Costs of treating children with complicated pneumonia: a comparison of primary video-assisted thoracoscopic surgery and chest tube placement. Pediatr Pulmonol. 2010 Jan;45(1):71–7. doi: 10.1002/ppul.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li ST, Gates RL. Primary operative management for pediatric empyema. Arch Pediatr Adolesc. 2008 Jan;162(1):44–8. doi: 10.1001/archpediatrics.2007.10. [DOI] [PubMed] [Google Scholar]
- 20.Avansino JR, Goldman B, Sawin RS, Flum DR. Primary operative versus nonperative therapy for pediatric empyema: a meta-analysis. Pediatrics. 2005;115(6):1652–9. doi: 10.1542/peds.2004-1405. [DOI] [PubMed] [Google Scholar]