Abstract
Aim
To compare the diagnostic accuracy of fine-needle aspiration cytology (FNAC) and core needle biopsy (CNB) in patients with US-detected breast lesions.
Patients and methods
Between September 2011 and May 2013, 3469 consecutive breast US examinations were performed. 400 breast nodules were detected in 398 patients. 210 FNACs and 190 CNBs were performed. 183 out of 400 (46%) lesions were surgically removed within 30 days form diagnosis; in the remaining cases, a six month follow up US examination was performed. Sensitivity, specificity, diagnostic accuracy, positive predictive (PPV) and negative predictive (NPV) values were calculated for FNAC and CNB.
Results
174 out of 400 (43%) malignant lesions were found while the remaining 226 resulted to be benign lesions. 166 out of 210 (79%) FNACs and 154 out of 190 (81%) CNBs provided diagnostic specimens. Sensitivity, specificity, diagnostic accuracy, PPV and NPV of 97%, 94%, 95%, 91% and 98% were found for FNAC, and values of 92%, 82%, 89%, 92% and 82% were obtained for CNB. Sensitivity, specificity, diagnostic accuracy, PPV and NPV of 97%, 96%, 96%, 97% and 96% were found for FNAC, and values of 97%, 96%, 96%, 97% and 96% were obtained for CNB.
Conclusion
FNAC and CNB provide similar values of diagnostic accuracy.
Keywords: Breast, Fine-needle aspiration cytology, Core needle biopsy, FNAC, CNB
Introduction
Breast cancer still represents the leading tumor among women and the incidence of the disease is rising all over the world (1, 2). The risk of developing breast cancer is related to a number of factors including the events of reproductive life and lifestyle factors that modify endogenous levels of sex hormones. Diet has been also found to play an important role in the etiology of breast cancer.
Mammography represents the most used modality for breast cancer screening, with mortality reduction of 30–40% in screened population (3, 4). However, its sensitivity is decreased in young women with radiologically dense breast (5). Another limitation of planar mammography is represented by the two-dimensional visualization of a three-dimensional volumetric structure such as the breast, with a consequent superimposition of tissue. These limitations are partially solved by the full-field digital mammography (FFDM), with its improved dynamic range, tissue contrast and post-processing, and by the digital breast tomosynthesis (DBT), which partially addresses the two-dimensional breast representation of planar mammography (4). Dual-energy contrast enhanced digital mammography represents a novel technique and has a reported sensitivity of 93% versus 78% of conventional mammography alone (6).
With the recent advances in technology, US and MRI allow to delineate occult malignancy in women with dense breast tissue, especially in case of high risk patients (5, 7, 8).
Recently, nuclear medicine imaging technology has been introduced in the field of breast cancer with the development of positron emission tomography (PET), PET-CT and, ultimately, positron emission mammography (PEM) (9).
Despite the described imaging techniques, pathological characterization still plays an essential role for differential diagnosis and for avoiding surgical over-treatment in case of breast lesions with suspicious features (10).
Fine-needle aspiration cytology (FNAC), core needle biopsy (CNB) and vacuum assisted breast biopsy (VABB) represent the current methods of choice for pathological diagnosis, both with their specific advantages and limitations.
In case of US detected breast nodules, FNAC is a well-established method for the diagnosis of breast lesions. It has the advantages of being highly accurate in experienced hands, cost effective, and useful for small lesions not eligible for CNB (11). Its limitations are represented by the lack of experienced cytologists in many institutions, the inability to reliably distinguish invasive from in situ carcinoma and the difficulty in precisely evaluating cytologic and morphologic features in breast aspirates with the histological classification system used as the “gold-standard”, particularly in benign lesions (12).
CNB has been reported to achieve better sensitivity and specificity especially in non palpable lesions that appear as not definitively benign or malignant. US-guided CNB is currently recognized as a reliable alternative to surgical biopsy for the histological diagnosis of breast lesions.
The purpose of this study is to compare the diagnostic accuracy of FNAC and CNB in patients with US-detected breast lesions.
Patients and methods
Between September 2011 and May 2013, 3469 consecutive breast US examinations were performed. A total of 400 breast nodules with a mean diameter of 1.3 cm (range 0.6–2.3 cm) were detected in 398 patients, including 2 multi-focal and 2 multi-centric lesions. 398 patients were women and 2 men, with mean age of 49.7 years (range 19–83 years). 239 were asymptomatic patients who underwent breast US for cancer screening, while the remaining 161 underwent breast examination for breast lump. In all cases clinical examination was previously performed. In 219 cases, mammography and US were performed, while in the remaining patients only US was carried out. US breast lesion were classified according to the Echographic BIRADS (Breast Imaging Reporting and Data System) Lexicon (10, 13, 14). In particular, five categories were identified. Category U1 corresponded to negative examination; category U2 corresponded to benign finding; category U3 to probably benign finding; category U4 to suggestive abnormality; category U5 to highly suggestive of malignancy.
All detected breast nodules were classified as mass-lesions basing on US features.
210 FNACs and 190CNBs were performed by the same radiologist with 5 years’ experience in the field of breast imaging in all cases and were examined by the same pathologist with more than ten years’ experience in the field of breast cytology and histology.
Indications for FNAC were represented by nodules with benign US features detected for the first time or with a maximum diameter of more than 2 cm or by suspicious lesions with a maximum diameter of less than 1 cm, retroareolar or deep, adjacent to chest wall lesions or in the case of patients with contraindications to CNB related to coagulation disease.
Indications for CNB were represented by suspicious lesions with a maximum diameter of more than 1 cm or in case of inadequate CNB results.
FNACs were performed free hand, under US guidance, using a 21G needle. The sampled material was treated with spray fixative solution.
In case of CNB, an anesthetic drug (carbocaine) was locally injected before the procedure; than, a 14 G needle was used for the sampling and 5 to 7 fragments were sampled in all case (15, 16) and fixed in formalin solution. The US guidance was performed by using a 13 MHz probe (Sonosite, Bothell, WA, US).
FNAC and CNB results were classified according to the F.O.N.C.A.M. guidelines from C1 to C5 and B1 to B5 for FNAC and CNB, respectively. In particular, five categories were identified. C1/B1 corresponded to insufficient sample; C2/B2 to benign lesion; C3/B3 to probably benign lesion; C4/B4 to probably malignant lesion; C5/B5 to malignant lesion (17).
183 out of 400 (46%) lesions were surgically removed within 30 days from diagnosis; in the remaining cases, a six month follow up US examination was performed.
Sensitivity, specificity, diagnostic accuracy, positive predictive (PPV) and negative predictive (NPV) values were calculated for both FNAC and CNB and represented by ROC analysis, having the histological post-operative control (n=183) and the six month follow-up US examination (n=217) as the reference standard. For both FNACs and CNBs, 95% confidence intervals (CIs) were also calculated for diagnostic accuracy proportion.
Results
174 out of 400 (43%) malignant lesions were found in the examined series (144 invasive ductal carcinomas and 30 invasive lobular carcinomas). 226 out of 400 (56%) results to be benign lesions (190 fibroadenomas, 23 flogosed cysts, 2 fibrotic areas, 2 phyllodes, 2 diabetic mastopathy, 4 sclerosing adenosis, 2 zonal mastitis, 1 papilloma) (Table 1).
Table 1.
CORRELATION BETWEEN FNAC-CNB RESULTS AND HISTOLOGICAL FINDINGS.
| HISTOLOGY | C1 | B1 | C2 | B2 | C3 | C4 | B4 | C5 | B5 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Invasive ductal carcinoma | 8 | 12 | 2 | 3 | 49 | 70 | n=144 | |||
| Invasive lobular carcinoma | 4 | 12 | 14 | n=30 | ||||||
| Fibroadenoma | 30 | 20 | 80 | 60 | n=190 | |||||
| Flogosed cyst | 6 | 17 | n=23 | |||||||
| Fibrosis | 2 | n=2 | ||||||||
| Phyllodes | 1 | 1 | n=2 | |||||||
| Diabetic mastopathy | 2 | n=2 | ||||||||
| Sclerosing adenosis | 2 | 2 | n=4 | |||||||
| Zonal mastitis | 2 | n=2 | ||||||||
| Papilloma | 1 | n=1 | ||||||||
|
| ||||||||||
| N | 44 | 36 | 99 | 67 | 3 | 3 | 3 | 61 | 84 | 400 |
FNAC
166 out of 210 (79%) FNACs provided diagnostic specimens, while 44 were inadequate for the diagnosis (C1). Among the remaining 166 examined nodules, FNAC resulted to be diagnostic with 61 C5, 99 C2, 3 C3, 3 C4 lesions. The 61 C5 nodules resulted to be 49 invasive ductal and 12 invasive lobular carcinomas at the histological control; the 99 C2 nodules resulted to be 80 fibroadenomas and 17 flogosed cysts; 2 C2 were surgically removed because of the high suspicious US features (U4) and resulted to be invasive ductal carcinomas at the definitive histological examination. The 3 C3 resulted to be fibrotic areas (n=2), phyllodes tumor (n=1) while the 3 C4 fibrotic area related to diabetes in two cases and phyllodes tumor in the other one.
Therefore, among diagnostic specimens, 61 true positives, 97 true negatives, 6 false positives and 2 false negatives were found.
Among non-diagnostic specimens, 36 out of 44 (81%) resulted to be benign lesions (30 fibroadenomas, 6 flogosed cysts) and 8 (19%) resulted to be malignant (invasive ductal carcinomas).
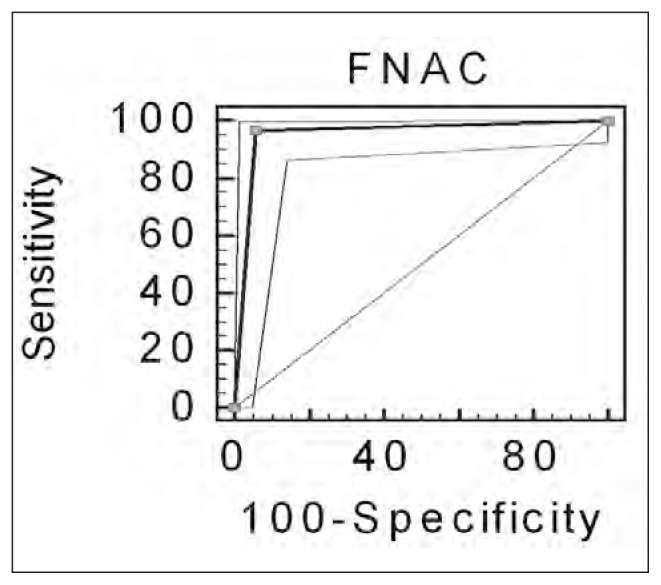
Overall values of 97% (95% CIs of 0.99 and 0.89), 94% (95% CIs of 0.98 and 0.88), 95% (95% CIs of 0.95 and 0.81), 91% and 98% were obtained respectively for sensitivity, specificity, diagnostic accuracy, PPV and NPV (Figure 1).
Fig. 1.
ROC curves as obtained by comparing FNAC results with the gold standard.
CNB
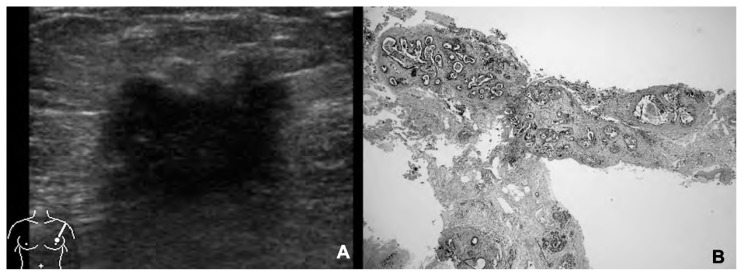
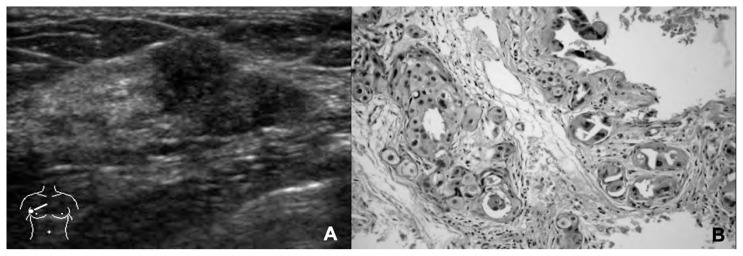
154 out of 190 (81%) CNBs provided diagnostic specimens, while 36 were inadequate for the diagnosis (B1). Among the remaining 154 examined nodules, CNB resulted to be diagnostic with 84 B5, 67 B2, 3 B4. The 84 B5 resulted to be 70 invasive ductal (Figure 2) and 14 invasive lobular carcinomas; the 67 B2 were 60 fibroadenomas, 2 sclerosing adenosis, 2 zonal mastitis; 3 B2 were surgically removed because of the highly suspicious US features (U4) and resulted to be 3 invasive ductal carcinomas (Figure 3). The 3 B4 resulted to be 2 sclerosing adenosis and 1 papilloma.
Fig. 2.
Invasive ductal carcinoma. A) Breast nodule with inhomogeneous US features and irregular margins. Ultrasound class 5. B) CNB using 14 G needle. Class B5 (hematoxylin eosin stain, ×100 magnification).
Fig. 3.
Invasive ductal carcinoma. A) Breast nodule with inhomogeneous US features and lobulated margins. Ultrasound class 4 resulted to be class B2 at the CNB using 14 G needle. B) The definitive histopathological specimen confirmed the diagnosis of a malignant breast lesion (hematoxylin eosin stain, ×100 magnification).
Therefore, among diagnostic specimens, 84 true positives, 64 true negatives, 3 false positives and 3 false negatives were found.
Among non-diagnostic specimens, 20 out of 36 (56%) resulted to be benign lesions (fibroadenomas) and 16 (44%) resulted to be malignant (12 invasive ductal carcinoma, 4 invasive lobular carcinoma).
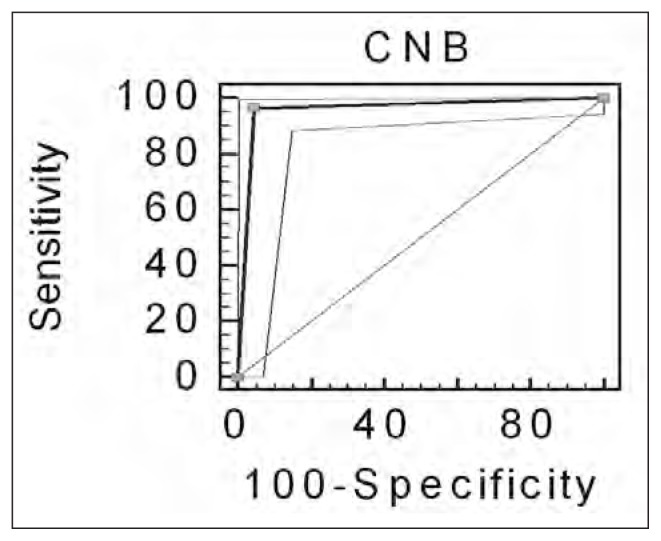
Overall values of 97% (95% CIs of 0.90 and 0.99), 96% (95% CIs of 0.99 and 0.88), 96% (95% CIs of 0.97 and 0.81), 97% and 96% were obtained respectively for sensitivity, specificity, diagnostic accuracy, PPV and NPV (Figure 4).
Fig. 4.
ROC curves as obtained by comparing CNB results with the gold standard.
Discussion
FNAC and CNB represent the most widely used methods for pathological diagnosis of breast nodules, both with their specific advantages and limitations. The overall sensitivity and specificity of FNAC and CNB in the classification of breast lesions depend on the radiological and histological features and on specific variables intrinsic to the technique. In most cases, CNB has both higher sensitivity and specificity than FNAC in diagnosing benign and malignant lesions (18–21). However, as reported by Willems et al, the studies which reported high sensitivity (97.1%), specificity (99.1%), PPV (99.3%) and NPV (96.2%) included only definitive benign and malignant lesions and excluded the atypical and suspicious categories (21, 22). In fact, Westenend et al. reported that the PPV of FNAC for malignancy was comparable with CNB, but decreased for suspicious lesions and in case of atypia (23).
Besides, CNB allows the discrimination between in situ and invasive lesions and is a more accurate method to distinguish between invasive lobular and invasive ductal carcinoma, based on histological and immuno-histochemical features. This preoperative distinction can be relevant for planning the extent of the surgical approach, for the choice of an adequate chemotherapy and for the increased risk of contra-lateral disease in the case of invasive lobular carcinoma (21, 24).
As regards to technical aspects, FNAC is more suitable for lesions close to the chest wall, vessels and implant, for very small or deep and difficult to reach lesions and for patients on anticoagulants. As a general feature of cytology, good quality FNAC depends on the competence of the aspirator, and its interpretation is primarily determined by the experience of the pathologist (21, 25, 26).
Besides, the success rate of FNAC for obtaining a definite diagnosis also depends both on the palpability and size of the lesion. FNAC has average success rates of 75–90% for palpable and 34–58% for non-palpable breast lesions, whereas success rates reported for CNB are 97% and 94%, respectively (18, 21, 27). Another important criterion is represented by the lesion size. FNAC has a success rate of only 50% for lesions less than 10 mm, while CNB is successful in over 90% of such lesions. Therefore, the success rate of FNAC seems to be especially low for non-palpable lesions and for those smaller than 10 mm. Moreover, FNAC accuracy rates are also decreased for large tumors and for calcified lesions because of an higher rate of insufficient sampling than masses (21, 28).
The main advantages of FNAC are minimal invasiveness, reduced cost, pathological assessment of small lesions, which are not amenable to CNB. Moreover, it allows same day diagnosis of breast cancer and the identification and management, on the same day, of those patients with benign disease.
Therefore, FNAC should be considered as the first method to evaluate breast lesions, recognized by means of imaging techniques; CNB should be performed for unanswered diagnostic cases (C1–C3) and when it is necessary to have such information as invasiveness or histological type of breast lesion.
Besides, Capalbo et al. recently reported that the presence of the pathologist on site could allow to obtain high rate of adequate samples and to reach a diagnostic concordance between FNAC and histology of 98.1% (10).
In our experience, comparable results for FNAC and CNB were obtained in terms of sensitivity (97% vs 97%), specificity (94% vs 96%), diagnostic accuracy (95% vs 96%) and NPV (98 vs 96). As for any diagnostic procedure, a higher NPV is important to minimize under-treatment and it was achieved by CNB.
On the other side, the main difference between the two methods was represented by PPV, which resulted 91% for FNAC and 97% for CNB; therefore, basing on our results, the risk of over-treatment could tend to be higher for FNAC as compared with CNB. In fact, the number of false positives was higher for FNAC and they were mainly represented by fibrotic areas or phyllodes tumors.
On the contrary, despite advances in biopsy devices and techniques, false-negative diagnoses still remain unavoidable and may delay the diagnosis and treatment of breast cancer. The most common reasons for false-negative diagnosis are represented by technical or sampling errors, failure to recognize or act on radiologic-histological discordance, and the lack of imaging follow-up after a benign biopsy result. Technical difficulties (poor lesion or needle visualization, especially after the injection of local anesthetic drug, deeply located lesions, dense fibrotic tissue) cause inaccurate sampling but can be reduced by using modified standard techniques.
Optimization of technique, radiologic-histological correlation, and post-biopsy follow-up protocols are recommended in order to reduce the occurrence of false-negative diagnosis at US-guided CNB performed by radiologists (11, 12, 29). In our experience, the number of false negatives resulted to be similar for both FNAC and CNB (2 vs 3, respectively) and the definitive histological control was mandatory in case of highly suspicious radiological features (invasive ductal carcinomas in all cases).
Our study has some important limitations, mainly represented by the small number of enrolled patients, the potential bias for patient recruitment basing on the selection criteria of the study, the impossibility of evaluating the reproducibility of each technique and the inter-observer variability, the lack of a direct confrontation between FNAC and CNB for each lesion, the minimal inhomogeneous sample size for FNAC and CNB and the lack of a confrontation with mammographic lesions.
In conclusion, FNAC and CNB represent accurate methods for the characterization of US-detected breast nodules, with similar values of diagnostic accuracy, sensitivity, specificity and NPV. In experienced hands, FNAC could be still considered the first method to evaluate breast lesions being less invasive. CNB has a higher PPV and should be performed for uncertain diagnostic cases and when the evaluation of the invasiveness or histological type of breast lesion is mandatory.
Footnotes
Conflict of interest
The Authors declare that they have no conflict of interest to the publication of this article.
References
- 1.Vestito A, Mangieri FF, Gatta G, Moschetta M, Turi B, Ancona A. Breast carcinoma in elderly women. Our experience. G Chir. 2011;32(10):411–6. [PubMed] [Google Scholar]
- 2.Brenner RJ, Parisky Y. Alternative breast-imaging approaches. Radiol Clin North Am. 2007;45:907–23. doi: 10.1016/j.rcl.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Kerlikowske K, Carney PA, Geller B, Mandelson MT, Taplin SH, Malvin K, et al. Performance of screening mammography among women with and without a first-degree relative with breast cancer. Ann Intern Med. 2000;133:855–863. doi: 10.7326/0003-4819-133-11-200012050-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lindfors KK, Boone JM, Nelson TR, Yang K, Kwan AL, Miller DF. Dedicated breast CT: initial clinical experience. Radiology. 2008;246:725–733. doi: 10.1148/radiol.2463070410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan SW, Cheung PS, Chan S, Lau SS, Wong TT, Ma M, et al. Benefit of ultrasonography in the detection of clinically and mammographically occult breast cancer. World J Surg. 2008;32:2593–8. doi: 10.1007/s00268-007-9273-2. [DOI] [PubMed] [Google Scholar]
- 6.Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C, Moskowitz CS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology. 2013;266:743–51. doi: 10.1148/radiol.12121084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinstein S, Rosen M. Breast MR imaging: current indications and advanced imaging techniques. Radiol Clin North Am. 2010;48:1013–42. doi: 10.1016/j.rcl.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Moschetta M, Telegrafo M, Capuano G, Rella L, Scardapane A, Angelelli G, et al. Intra-prosthetic breast MR virtual navigation: A preliminary study for a new evaluation of silicone breast implants. Magn Reson Imaging. 2013;31:1292–7. doi: 10.1016/j.mri.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Kalles V, Zografos GC, Provatopoulou X, Koulocheri D, Gounaris A. The current status of positron emission mammography in breast cancer diagnosis. Breast Cancer. 2013;20:123–30. doi: 10.1007/s12282-012-0433-3. [DOI] [PubMed] [Google Scholar]
- 10.Capalbo E, Sajadidehkordi F, Colombi C, Ticha V, Moretti A, Peli M, et al. Revaluation of breast cytology with pathologist on-site of lesions with suspicious sonographic features. Eur J Radiol. 2013;82:1410–5. doi: 10.1016/j.ejrad.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Smith MJ, Heffron CC, Rothwell JR, Loftus BM, Jeffers M, Geraghty JG. Fine needle aspiration cytology in symptomatic breast lesions: still an important diagnostic modality? Breast J. 2012;18(2):103–10. doi: 10.1111/j.1524-4741.2012.01223.x. [DOI] [PubMed] [Google Scholar]
- 12.Berner A, Davidson B, Sigstad E, Risberg B. Fine-needle aspiration cytology vs. core biopsy in the diagnosis of breast lesions. Diagn Cytopathol. 2003;29(6):344–8. doi: 10.1002/dc.10372. [DOI] [PubMed] [Google Scholar]
- 13.Levy L, Suissa M, Chiche JF, Teman G, Martin B. BIRADS ultrasonography. Eur J Radiol. 2007;61(2):202–11. doi: 10.1016/j.ejrad.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Costantini M, Belli P, Lombardi R, Franceschini G, Mulè A, Bonomo L. Characterization of solid breast masses: use of the sonographic breast imaging reporting and data system lexicon. J Ultrasound Med. 2006;25(5):649–59. doi: 10.7863/jum.2006.25.5.649. [DOI] [PubMed] [Google Scholar]
- 15.Kirshenbaum K, Keppke A, Hou K, Dickerson M, Gajjar M, Kirshenbaum G. Reassessing specimen number and diagnostic yield of ultrasound guided breast core biopsy. Breast J. 2012;18(5):464–9. doi: 10.1111/j.1524-4741.2012.01269.x. [DOI] [PubMed] [Google Scholar]
- 16.Fishman JE, Milikowski C, Ramsinghani R, Velasquez MV, Aviram G. US-guided core-needle biopsy of the breast: how many specimens are necessary? Radiology. 2003;226(3):779–82. doi: 10.1148/radiol.2263011622. [DOI] [PubMed] [Google Scholar]
- 17.Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS lexicon for US and mammography: interobserver variability and positive predictive value. Radiology. 2006;239(2):385–91. doi: 10.1148/radiol.2392042127. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim AE, Bateman AC, Theaker JM, Low JL, Addis B, Tidbury P, et al. The role and histological classification of needle core biopsy in comparison with fine needle aspiration cytology in the preoperative assessment of impalpable breast lesions. J Clin Pathol. 2001;54(2):121–5. doi: 10.1136/jcp.54.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatada T, Ishii H, Ichii S, Okada K, Fujiwara Y, Yamamura T. Diagnostic value of ultrasound-guided fine-needle aspiration biopsy, core-needle biopsy, and evaluation of combined use in the diagnosis of breast lesions. J Am Coll Surg. 2000;190(3):299–303. doi: 10.1016/s1072-7515(99)00300-2. [DOI] [PubMed] [Google Scholar]
- 20.de Barra AA, de Gobbi HL, Rezende CA, Gouvêa AP, de Lucena CE, Reis JH, et al. A comparision of aspiration cytology and core needle biopsy according to tumor size of suspicious breast lesions. Diagn Cytopathol. 2008;36(1):26–31. doi: 10.1002/dc.20748. [DOI] [PubMed] [Google Scholar]
- 21.Willems SM, van Deurzen CH, van Diest PJ. Diagnosis of breast lesions: fine-needle aspiration cytology or core needle biopsy? A review. J Clin Pathol. 2012;65(4):287–92. doi: 10.1136/jclinpath-2011-200410. [DOI] [PubMed] [Google Scholar]
- 22.Boerner S, Fornage BD, Singletary E, Sneige N. Ultrasound-guided fine-needle aspiration (FNA) of nonpalpable breast lesions: a review of 1885 FNA cases using the National Cancer Institute-supported recommendations on the uniform approach to breast FNA. Cancer. 1999;25;87(1):19–24. doi: 10.1002/(sici)1097-0142(19990225)87:1<19::aid-cncr4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 23.Westenend PJ, Sever AR, Beekman-De Volder HJ, Liem SJ. A comparison of aspiration cytology and core needle biopsy in the evaluation of breast lesions. Cancer. 2001;93(2):146–50. doi: 10.1002/cncr.9021. [DOI] [PubMed] [Google Scholar]
- 24.Provenzano E, Pinder SE. Pre-operative diagnosis of breast cancer in screening: problems and pitfalls. Pathology. 2009;41(1):3–17. doi: 10.1080/00313020802563478. [DOI] [PubMed] [Google Scholar]
- 25.Parker SH, Burbank F, Jackman RJ, Aucreman CJ, Cardenosa G, Cink TM, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology. 1994;193(2):359–64. doi: 10.1148/radiology.193.2.7972743. [DOI] [PubMed] [Google Scholar]
- 26.He Q, Fan X, Yuan T, Kong L, Du X, Zhuang D, et al. Eleven years of experience reveals that fine-needle aspiration cytology is still a useful method for preoperative diagnosis of breast carcinoma. Breast. 2007;16(3):303–6. doi: 10.1016/j.breast.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Hukkinen K, Kivisaari L, Heikkilä PS, Von Smitten K, Leidenius M. Unsuccessful preoperative biopsies, fine needle aspiration cytology or core needle biopsy, lead to increased costs in the diagnostic workup in breast cancer. Acta Oncol. 2008;47(6):1037–45. doi: 10.1080/02841860802001442. [DOI] [PubMed] [Google Scholar]
- 28.Manfrin E, Falsirollo F, Remo A, Reghellin D, Mariotto R, Dalfior D, et al. Cancer size, histotype, and cellular grade may limit the success of fine-needle aspiration cytology for screen-detected breast carcinoma. Cancer. 2009;117(6):491–9. doi: 10.1002/cncy.20053. [DOI] [PubMed] [Google Scholar]
- 29.Youk JH, Kim EK, Kim MJ, Lee JY, Oh KK. Missed breast cancers at US-guided core needle biopsy: how to reduce them. Radiographics. 2007;27(1):79–94. doi: 10.1148/rg.271065029. [DOI] [PubMed] [Google Scholar]