Summary
Tomato fruits were treated by exogenous melatonin. The effect of melatonin on ethylene biosynthesis, ethylene perception, and ethylene signalling may contribute to fruit ripening and quality improvement in tomato.
Key words: Ethylene, gene expression, melatonin, postharvest, ripening, tomato.
Abstract
In this study, the effect of melatonin on the postharvest ripening and quality improvement of tomato fruit was carried out. The tomatoes were immersed in exogenous melatonin for 2h, and then the related physiological indicators and the expression of genes during post-harvest life were evaluated. Compared with control check (CK), the 50 µM melatonin treatment significantly increased lycopene levels by 5.8-fold. Meanwhile, the key genes involved in fruit colour development, including phytoene synthase1 (PSY1) and carotenoid isomerase (CRTISO), showed a 2-fold increase in expression levels. The rate of water loss from tomato fruit also increased 8.3%, and the expression of aquaporin genes, such as SlPIP12Q, SlPIPQ, SlPIP21Q, and SlPIP22, was up-regulated 2- to 3-fold under 50 µM melatonin treatment. In addition, 50 µM melatonin treatment enhanced fruit softening, increased water-soluble pectin by 22.5%, and decreased protopectin by 19.5%. The expression of the cell wall modifying proteins polygalacturonase (PG), pectin esterase1 (PE1), β-galactosidase (TBG4), and expansin1 (Exp1) was up-regulated under 50 µM melatonin treatment. Melatonin increased ethylene production by 27.1%, accelerated the climacteric phase, and influenced the ethylene signalling pathway. Alteration of ethylene production correlated with altered 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS4) expression. The expression of ethylene signal transduction-related genes such as NR, SlETR4, SlEIL1, SlEIL3, and SlERF2, was enhanced by 50 µM melatonin. The effect of melatonin on ethylene biosynthesis, ethylene perception, and ethylene signalling may contribute to fruit ripening and quality improvement in tomato. This research may promote the application of melatonin on postharvest ripening and quality improvement of tomato fruit as well as other horticultural productions in the future.
Introduction
Fruit ripening is a highly coordinated, genetically programmed, irreversible phenomenon involving a series of physiological, biochemical, and organoleptic changes that lead to changes in colour, texture, flavour, aroma, and nutritional status (Prasanna et al., 2007; Rugkong et al., 2011). Tomato fruit ripening is accompanied by changes in colour from green to red, softening, and increased levels of compounds that contribute to flavour and aroma, such as organic acids, sugars and volatiles. The colour change is due to the unmasking of previously present pigments by degradation of chlorophyll coupled with the synthesis of different types of anthocyanins and the accumulation of carotenoids such as β-carotene, xanthophyll esters, xanthophylls, and lycopene. Indeed, lycopene is considered a major carotenoid in tomato that provides red colour (Ronen et al., 1999). Softening is thought to be the result of cell wall disassembly, decreased cell adhesion (Vicente et al., 2007; Lunn et al., 2013), and the cumulative effects of reducing cellular turgor pressure (Shackel et al., 1991). The changes in cell wall structure are accompanied by a solubilization of pectins and depolymerization of hemicellulosic polysaccharides.
In addition to the structural matrices of the cell wall, another important contributor to texture and fruit firmness is cellular turgor. Cellular turgor is governed by the water status within fruit and the relative water distribution within the cell and in the cell wall (Seymour et al., 2013). Water transport across biological membranes is facilitated by water channel proteins called aquaporins (Chrispeels and Maurel, 1994). Aquaporin proteins during ripening regulate the passage of water from the symplast to the apoplast, which would presumably also affect water movement out of the cell (Seymour et al., 2013).
Flavour is defined as the combination of taste and odour. Aroma of the ripe fruit is attributed to the production of a complex mixture of volatile compounds such as hexanal, myrcene, and ocimene, and degradation of bitter principles, tannins, flavonoids, and related compounds (Prasanna et al., 2007). The majority of plant volatiles on a quantitative and qualitative basis originate from saturated and unsaturated fatty acids (Schwab et al., 2008). Hexanal is formed by lipid oxidation of unsaturated fatty acids on the maceration of fruit, which is critical to ripe aroma and tomato-like flavour (Baldwin et al., 1998; Alexander and Grierson, 2002). The taste development is due to a general increase in sweetness, which is the consequence of increased gluconeogenesis, hydrolysis of polysaccharides, especially starch, decreased acidity, and accumulation of sugars and organic acids resulting in an excellent sugar/acid blend (Prasanna et al., 2007).
Tomato (Solanum lycopersicum L.) is one of the most important horticultural crops. It has long served as a model system for studying fleshy fruit development and ripening owing to its relatively small genome, ease of genetic manipulation, well‐characterized developmental mutants, and relatively short life cycle. Tomato belongs to the climacteric class of fruits, which includes banana, apple, and pear. Most climacteric fruits show increased ethylene production at or just before the onset of ripening and require ethylene to complete the process (Alexander and Grierson, 2002). Therefore, ethylene synthesis, perception, and signalling are very important events for fruit ripening. The pathway of ethylene biosynthesis has been determined (Adams and Yang, 1979). The key enzyme is 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS), which regulates the production of ACC from S-adenosylmethionine (SAM) (Alexander and Grierson, 2002; Prasanna et al., 2007). ACC oxidase (ACO) is an important enzyme for the control of ethylene production. Ethylene perception is by the target cells through receptors (ETRs), which act as negative regulators of the ethylene response pathway. The signal transduction cascade involves both positive and negative regulators (CTR, EIN2, EIN3 etc.) and regulation of target gene expression by transcription factors such as ethylene response factors (ERFs) (Olmedo et al., 2006; Bapat et al., 2010).
Melatonin, or N-acetyl-5-methoxytryptamine, is a hormone found in animals, plants, and microbes (Manchester et al., 2000; Burkhardt et al., 2001; Paredes et al., 2009; Posmyk and Janas, 2009). Besides its function as synchronizer of the biological clock, melatonin is also a powerful free-radical scavenger and wide-spectrum antioxidant (Tan et al., 1993). Melatonin was initially discovered in plants by two groups in 1995 (Dubbels et al., 1995; Hattori et al., 1995). Since then, it has been detected in the roots, leaves, flowers, fruits, and seeds of a considerable variety of plant species (Cao et al., 2006; Okazaki and Ezura, 2009; Park, 2011; Lei et al., 2013; Zhao et al., 2013). Melatonin concentration varies among plant organs depending on the given physiological and environmental conditions. Its highest level was found in flowers and seeds, which may be related to their high sensitivity to environmental stresses, e.g. UV irradiation (Posmyk and Janas, 2009). However, little is known about its physiological function in plants. Nocturnal increases of melatonin were detected in Chenopodium rubrum, suggesting that melatonin in plants may have functions analogous to those in animals (Kolar et al., 2002). Melatonin also showed antioxidative activities in plants as observed in animals (Rodriguez et al., 2004). Melatonin plays regulatory roles in plant metabolism (Wang et al., 2014), acts as a growth-regulatory signal similar to auxin, delays flower induction (Kolar et al., 2003), slows root formation (Hernandez-Ruiz et al., 2004), and promotes adventitious and lateral root regeneration (Zhang et al., 2013, 2014).
As a healthy ingredient contained in the diet, many fruits, including apple, cherry, banana, strawberry, pineapple, grape, and tomato, provide natural melatonin (Reiter et al., 2005; Sae-Teaw et al., 2013). Melatonin was first detected in wild tomato species Solanum pimpinellifolium (currant tomato) in 1995. It was also reported that melatonin accumulates in mature tomato fruits and seeds, and the melatonin content in the pericarp of tomato fruit increased from mature green stage to red stage (Okazaki and Ezura, 2009; Feng et al., 2014). However, the role of melatonin in tomato fruit ripening is not well understood.
In this study, we focused on the effect of melatonin on the postharvest ripening of tomato fruit, and revealed the mechanism of melatonin on ethylene biosynthesis, lycopene synthesis, cell wall structure, and water loss. This research may promote the application of melatonin on postharvest ripening and quality improvement of tomato fruit as well as other horticultural productions in the future.
Materials and methods
Regents
All chemicals used in experiments were of analytical grade. Melatonin (N-acetyl-5-methoxytryptamine) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd, China.
Plant material
The following experiments were conducted at China Agricultural University, Beijing (39.9_N 116.3_E). The tomato plant used in this study, variety “Bmei”, is a cherry tomato. Fruits were collected at the green stage of maturity with homogeneous size and randomly grouped into three lots (100 fruits per lot) for treatment in triplicate.
Fruit treatment
The tomato fruits were rinsed briefly in water before treatment to avoid soil contamination. After that, the tomatoes were immersed in solution for 2h. The five solutions made for treatments were: control (distilled water), M1 (1 µM melatonin), M50 (50 µM melatonin), M100 (100 µM melatonin), and M500 (500 µM melatonin). Following immersion, the fruits were dried for 15min at room temperature (RT). The tomato fruits were then stored for 25 days at 15 °C and 80% relative humidity. Three independent trials were carried out. At each sampling date, fruit were used for measurements of ethylene production, colour, and firmness, and then the pericarp tissues were frozen with liquid nitrogen, powdered, and stored at –80 °C until further use.
Melatonin extraction and analysis
A total of 4g tomatoes were homogenized with 10ml methanol, then ultra-sonicated (80 Hz) for 35min at 45 °C. After centrifugation at 10 000g at 4 °C for 15min twice, the supernatants were collected and dried under nitrogen gas. Samples were dissolved in 2ml 5% methanol and transferred to a C18 solid phase extraction (SPE) cartridge (ProElutTM, DIKMA, China) for the purification of melatonin. Melatonin was extracted and analysed as described previously (Zhao et al., 2013). The mobile phase was a mixture of acetonitrile: 50 mmol l–1 Na2HPO4/H3PO4 buffer pH 4.5 (15:85), which was delivered at a flow rate of 1.0ml min–1. The injection volume of the extract was 10 µl. Melatonin was detected using excitation and emission wavelengths at 280 and 348nm, respectively. Samples were determined in triplicate.
Determination of ethylene production
Ethylene production of the tomato fruit was measured by enclosing three fruits in 5 L air-tight containers for 2h at 20 °C, withdrawing 1ml of the headspace gas with a syringe, and injecting it into a gas chromatograph (model Agilent, 6890N, USA) fitted with a flame ionization detector and an activated alumina column.
Fruit firmness and water loss measurement
Fruit firmness was measured at the furthest two points apart on the equator of each fruit (i.e. equidistant between the top and bottom of each fruit) with a Texturometer (Model GY-B, Xingke, Jilin, China) fitted with a 2mm plunger.
The percent weight loss of tomato fruit samples was calculated by taking the difference between initial weight and final weight divided by initial weight. Data was recorded for twenty biological replicates each in three independent sets.
Soluble sugar measurement
To measure the content of soluble sugars, 0.5g of tomato tissue was homogenized with 5ml of 95% ethanol. One hundred µl of alcoholic extract was mixed with 3ml anthrone (150mg anthrone, 50ml of 72% sulphuric acid, W/W) 50. The samples were boiled for 10min. The light absorption of the samples was measured at 630nm using a 2800UV/VIS model spectrophotometer. Contents of soluble sugar were determined using sucrose standard.
Pectin measurement
Tomato tissue (0.5g) derived from 4 fruits, free of placental tissue and seeds, was homogenized with 25ml 95% ethanol, and boiled for exactly 30min. After cooling at RT, the reaction was centrifuged at 8000g for 15min, the supernatant was removed, and the pellet suspended in 25ml 95% ethanol and boiled for 30min. After repeating 3–5 times, the pellet was suspended in 20ml distilled water, and incubated in a 50 °C water bath for 30min. The mixture was then centrifuged at 8000g for 15min, and the supernatant containing the water-soluble pectin was transferred to a 100ml volumetric flask. The pellet was dissolved with 25ml 0.5mol l–1 H2SO4 and boiled for 1h. Following centrifugation at 8000g for 15min, the supernatant containing the proto-pectin was transferred to a 100ml volumetric flask.
The pectin content was determined by mixing 1ml of collected pectin with 6ml of concentrated hydrochloric acid. The reaction was boiled for 20min, cooled in tap water, followed by the addition of 0.2ml 1.5g l–1 carbazole and a 30min incubation in the dark at RT. The absorbance at 530nm was measured against reagent blanks and pectin content was calculated based on a galacturonic acid standard curve.
Lycopene content measurement
Lycopene content was measured at 17 d after melatonin treatment as described in Fish et al. (Fish et al., 2002) with modifications. Partially thawed tomato pericarp tissue (5g) derived from 4 fruits, free of placental tissue and seeds, and 50ml of hexane–acetone–ethanol (2:1:1, v/v) were homogenized for 1min wrapped in aluminum foil. After homogenization, 15ml of water was added and the samples vortexed for 10 s. Following phase separation on ice, lycopene concentration was determined by measuring the absorbance of the organic phase (hexane) at 503nm. All the procedures were performed under dim light. Lycopene content was calculated using the molar extinction coefficient of 17.2 l mol−1m−1 and expressed on a fresh weight basis as mg kg−1. Three independent samples derived from four fruits at each measurement interval were used for lycopene measurement.
Extraction of volatiles and gas chromatography-mass spectrometry analysis
The aroma components were analysed by SPME/ GC-MS according to Liu et al. (Liu et al., 2011). Pericarp tissue (20g) was obtained from tomato fruits, immediately frozen in liquid nitrogen, and stored at –80 °C until extraction. The MS was operated using an EI ion source, with a temperature of 170 °C, electron energy 70eV, and photomultiplier tube voltage of 350V. A 2-µl aliquot of the pooled organic phases (extracted as described above), was directly injected into the gas chromatograph-mass spectrometer for volatile analysis; at least two extractions for each sample were performed.
The ion source and the transfer line were set to 200 °C and 260 °C, respectively. Volatile compounds were separated on a column (30 m×0.25mm inside diameter, 0.25 µm film). The column temperatures were programmed as follows: 60 °C for 2min, raised to 220 °C at 8 °C min−1, then held for 20min. The injector temperature was 250 °C. Helium was the carrier gas at 1ml min−1 in the splitless mode. Electron impact mass spectra were recorded in the 30–550 amu range with a scanning speed of 0.5 scan s−1.
RNA Extraction, reverse transcription-PCR and real-time quantitative PCR assay
Total RNA was extracted from the fruits using TRIzol reagent according to the manufacturer’s protocol (Invitrogen, Burlington, ON, Canada). The quality and quantity of all RNA samples were assessed by agarose gel electrophoresis and spectrophotometry (Thermo NanoDropTM 2000c, USA). First-strand cDNA was reverse transcribed from 2 µg of total RNA using the PrimeScriptTM RT reagent kit (Takara, Japan) according to the manufacturer’s instructions.
Primers used for real-time PCR, designed using Primer 5 software, are listed in Table S1. Specificity of each primer to its corresponding gene was checked using the BLASTN program of the NCBI. Real-time PCR was performed using the SYBR Premix Ex Taq™ kit (TaKaRa, Japan). Reactions contained 1 µl of primer mix, 2 µl cDNA template, 10 µl SYBR Premix Ex Taq™ (2×) mix, and 7 µl water for a total volume of 20 µl. Reactions were carried out under the following conditions: 95 °C/30 s (1 cycle); 95 °C/10 s, 60 °C/34 s (40 cycles), using ABI Prism 7500 Sequence Detection System and Software (PE Applied Biosystems, USA). To normalize sample variance, Tomato Sl-Actin-51 (accession No. Q96483) gene was used as endogenous control. To determine relative fold differences for each sample, the Ct value of genes was calculated relative to a calibrator using the formula 2–ΔΔCt. At least two to three independent RNA isolations were used for cDNA synthesis and each cDNA sample was subjected to real-time PCR analysis in triplicate.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA). Means were compared by the Fisher’s LSD test at a significance level of 0.05.
Results
Melatonin enhanced pigment accumulation of tomato fruit
Lycopene, one of the main carotenoids in tomato, is mainly responsible for the red colour and varies with different ripening stages. To test the effect of melatonin on tomato fruit ripening during postharvest, preliminary experiments were carried out to determine the appropriate concentrations of melatonin. In this study, we found that lycopene accumulated significantly in melatonin-treated fruits (Fig. 1C) at 17 d after melatonin treatment (DAT), compared with the control (CK). A 5.1-fold increase was observed with 50 µM melatonin treatment (36.7mg per g FW), and there were no significant changes among 50 µM, 100 µM, and 500 µM melatonin (Fig. 1A).
Fig. 1.
Melatonin’s effect on tomato pigment accumulation.CK: fruits pre-treated with water; M50: fruits pre-treated with 50 µmol l–1 melatonin; M50: fruits pre-treated with 50 µmol l–1 melatonin. (A) Lycopene content at 17 d after treatment. (B) Real-time PCR analysis of PSY1 and CRTISO expression levels in fruit at 17d after treatment. Vertical bars at each time point represent the least significant difference (LSD) test when significant at P=0.05. (C) The colour of tomato at 17 d after treatment. The scale bar indicates 1cm.
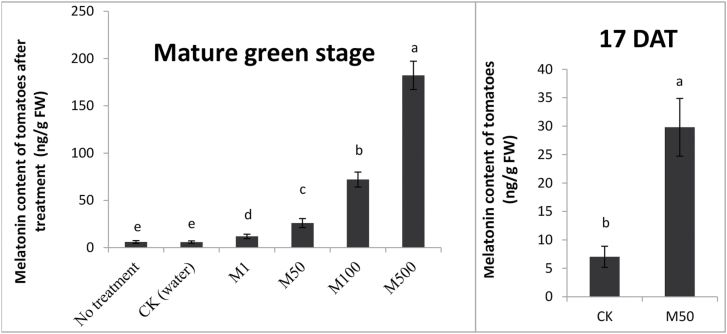
To know how much melatonin was absorbed by the tomatoes, we measured melatonin contents of the fruits. Compared with CK (water treatment), the melatonin contents of melatonin-treated mature green tomatoes were increased significantly. The highest melatonin contents were 182.2ng per g FW in M500, and gradually decreased as exogenous melatonin concentration declined. The MT contents were 72.0, 25.9, 11.8, and 5.9ng per g FW in MT100, MT50, MT1, and CK, respectively (Fig. 2A). Previous studies reported the occurrence of melatonin in various tomatoes; the melatonin contents ranged from 0.6–114.5ng per g FW (Sturtz et al., 2011; Feng et al., 2014). Thus, melatonin application at 50 µM seemed to be the most effective as it produced the highest level of lycopene and the lowest residue level of melatonin. Therefore, 50 µM melatonin was used in this study.
Fig. 2.
Melatonin content in tomato fruits after treatment. Tomatoes were treated without (water) or with melatonin in concentrations: 1, 50, 100, and 500 µM. The melatonin content of tomatoes after treatment at (A): mature green stage and (B): 17 DAT. Vertical bars at each time point represent the LSD when significant at P=0.05.
To better understand the effects of exogenously applied melatonin on melatonin contents in tomato fruits, we also measured melatonin concentrations of the fruits at 17 DAT (day after melatonin treatment) (Fig. 2B). The melatonin contents at 17 DAT in both CK and M50 were higher than those of the corresponding mature green fruits; the melatonin concentrations were 7.0 and 29.8ng per g FW in CK and M50, respectively.
To further understand the effect of melatonin on the lycopene accumulation in tomato fruit, we analysed the expression of SlPSY1 and SlCRTISO genes, which are involved in lycopene biosynthesis. In general, both of these genes were up-regulated about 2-fold in the melatonin-treated fruits (Fig. 1B). The enhanced expression of lycopene synthesis genes following melatonin treatment was consistent with increased lycopene accumulation and colour change observed in M50-fruits. The result suggests that melatonin may have a role in mediating lycopene biosynthesis-related genes to alter lycopene concentration in tomato fruit, and finally affect the colour change of tomato fruit.
Melatonin softened and changed cell wall structure
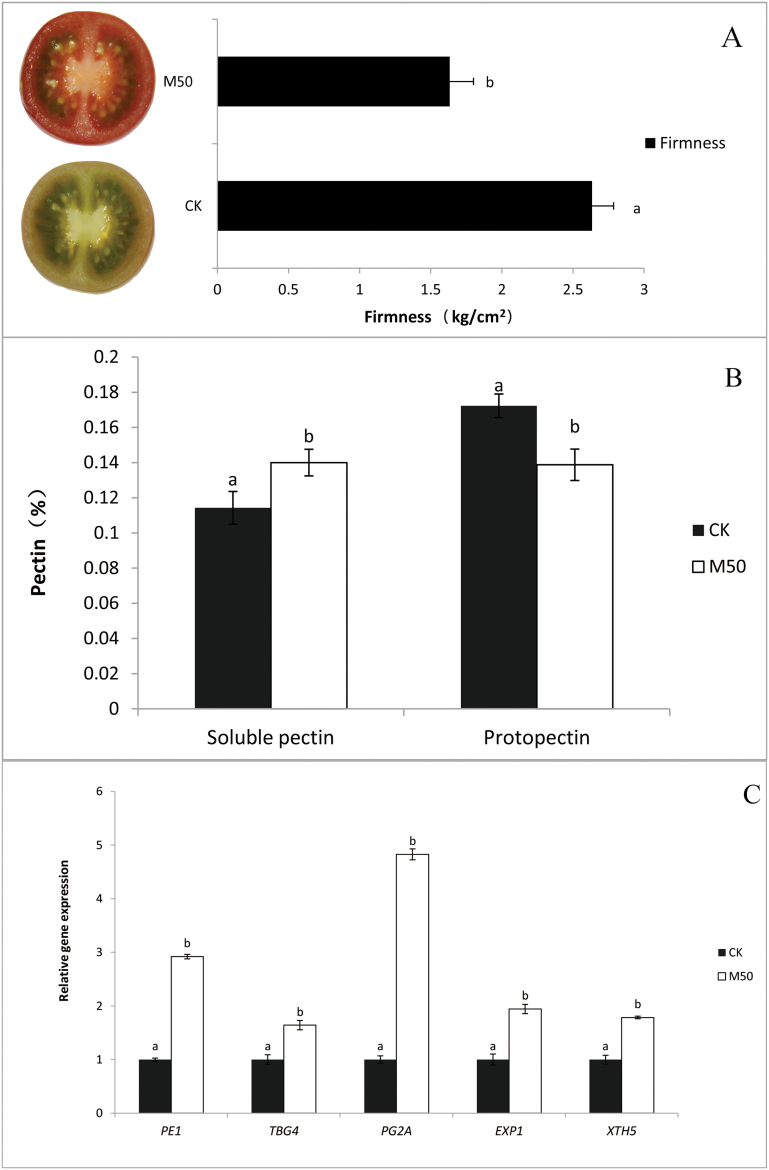
Fruit softening was measured using a Texturometer to test fruit pericarp firmness. The data showed that control tomatoes were substantially firmer than melatonin-treated fruits at 17 DAT. The firmness decreased by 38.0% in M50-fruits compared with CK. (Fig. 3A)
Fig. 3.
Melatonin affects softening and firmness of tomato fruit. CK: fruits pre-treated with water; M50: fruits pre-treated with 50 µmol l–1 melatonin. (A) Firmness. (B) Pectin level. (C) Real-time PCR analysis of cell wall structure-related gene expression in tomato fruits at 17 d after treatment. Vertical bars at each time point represent the LSD when significant at P=0.05. (This figure is available in colour at JXB online.)
To reveal whether the cell wall structure changed, we also measured the content of pectin, which is a structural heteropolysaccharide contained in the primary cell walls of plants. We found that the content of proto-pectin was reduced by 19.5%, whereas the soluble pectin content increased 22.5% in M50-fruits (Fig. 3B).
To fully understand how melatonin changes cell wall structure in tomato fruit, five cell wall structure-related genes were investigated. The expression of cell wall structure-related genes (SlPE1, SlTBG4, SlPG2a, SlExp1 and SlXTH5) following 50 µM melatonin treatment was generally higher than untreated controls. The expression of the SlPG2a gene, which is involved in the hydrolysis of pectin, was sharply increased by almost 5-fold in M50-fruits when compared with CK-fruits (Fig. 3C). The result that SlPG2a expression was up-regulated in the melatonin-treated fruits was consistent with reduced pectin levels in these tomato fruits.
At 17 DAT, 50 µM melatonin-treated fruits were getting softer than control fruit (Fig. 3A), and the expression of cell wall structure-related genes (SlPE1, SlTBG4, SlPG2a, SlExp1, and SlXTH5) was up-regulated by 50 µM melatonin treatment (Fig. 3C) was consistent with reduced pectin level in the M50 fruit. It indicates that melatonin may lead to fruit softening by up-regulating cell wall structure-related genes, especially the SlPG2a gene, which is responsible for much of the depolymerization of de-esterified pectic homogalacturonans (Hadfield and Bennett, 1998; Brummell and Harpster, 2001).
Texture of fruit not only affects consumer preference, but also has a significant impact on shelf life and transportability. Shelf life is the recommended maximum time for which products can be stored, during which the defined quality of a specified proportion of the goods remains acceptable under expected (or specified) conditions of distribution, storage, and display. High quality tomato has a firm, turgid appearance, uniform and shiny colour, without signs of mechanical injuries, shrivelling or decay. Mature-green tomatoes have a storage life of 1–3 weeks in normal atmosphere storage (NA) at 13–21 °C (Burg, 2004). In the present study, both melatonin-treated and control groups had a similar shelf life and a good merchantability at 21 DAT.
Melatonin treatments increased rate of water loss and aquaporin expression
Initially, no significant changes in fruits weight loss (%) were observed in control fruits and melatonin-treated fruits. However, as ripening proceeded, the weight loss significantly (P<0.5) increased by 8.3% and 9.7% at 17 DAT and 24 DAT, respectively, in melatonin-treated fruits (Fig. 4A).
Fig. 4.
Melatonin has an effect on water loss in tomato fruit. CK: sample pre-treated with water; M50: sample pre-treated with 50 µmol l–1 melatonin. (A) The rate of water loss of tomato during postharvest storage. (B) Real-time PCR analysis of aquaporin genes expression in tomato fruits at 17 d after treatment. Vertical bars at each time point represent the LSD when significant at P=0.05.
Furthermore, we analysed the effect of melatonin treatment on the expression of five aquaporin genes, which are considered to be factors that influence water loss, in tomato fruits at 17 DAT. Our results indicate that, compared with CK, SlPIP12, SlPIP21 and SlPIP22 expression levels had a 2-fold increase, and SlPIPQ had a 3-fold increase in M50-fruits, whereas Sl4SQ did not change significantly (Fig. 4B). These results suggest that melatonin may affect water loss in fruit by altering the expression of some aquaporin genes.
Melatonin increased ethylene production and influenced on ethylene signalling pathway
Ethylene production was determined during fruit storage. As shown in Fig. 4A, ethylene production was highest in melatonin-treated fruits at 17 DAT, whereas the climacteric peak of CK appeared at 19 DAT. The climacteric peak of 50 µM melatonin-treated fruit was higher, and the production of ethylene was increased by 27.1% in the peak when compared with CK. Our data indicate that melatonin enhanced ethylene production, and affected the timing of the climacteric peak.
To determine whether the expression of ethylene biosynthesis-related genes was consistent with the above results, we analysed the gene expression of SlACS2, SlACS4, and SlACO1, which are responsible for autocatalytic ethylene production during fruit ripening (Klee and Giovannoni, 2011). Compared with CK, SlACS4 was up-regulated 3-fold in M50, whereas SlACS2 and SlACO1 showed no significant changes in response to melatonin treatment (Fig. 5B). These results revealed that melatonin might increase ethylene production by enhancing SlACS4 expression.
Fig. 5.
Melatonin has an effect on ethylene production, signalling pathway, and relative gene expression. CK: sample pre-treated with water; M50: sample pre-treated with 50 µmol l–1 melatonin. (A) Ethylene production of tomato fruits during postharvest storage. (B) Real-time PCR analysis of ethylene biosynthesis-related genes expression in tomato fruits at 17 d after treatment. (C) Real-time PCR analysis of ethylene signalling-related gene expression in tomato fruits at 17 d after treatment. Vertical bars at each time point represent the LSD when significant at P=0.05.
To better understand the role of melatonin in mediating ethylene perception and signalling in tomato fruit, we analysed the expression of two ethylene receptor genes and five ethylene signalling-related genes. Both ethylene receptor genes, SlNR and SlETR4 were up-regulated in M50-fruit by 2.3-fold (Fig. 5C). The CTR and EIN gene are involved directly in transducing the ethylene receptor signal (Seymour et al., 2013). SlEIN/EIL1 and three genes were up-regulated by exogenous melatonin, whereas the expression of SlCTR1 and SlEIN/EIL2 was almost unresponsive to melatonin treatment (Fig. 5C). Ethylene response factor (ERF) is a well-known transcriptional regulator involved in controlling ethylene responses (Seymour et al., 2013). In our study, SlERF2 was highly up-regulated by 2.5-fold under melatonin treatment (Fig. 5C).
In this study, we observed that the climacteric peak of melatonin-treated samples occurred earlier, and ethylene production was increased compared with CK (Fig. 5A). Furthermore, at the climacteric peak time under 50 µM melatonin treatment, the expression of ethylene biosynthesis-related gene (SlACS4), ethylene receptor genes (SlNR and SlETR4), and ethylene signalling-related genes (SlEIL1, SlEIL3, and SlERF2) were up-regulated to varying degrees. Taken together, these results indicate that melatonin may affect the ethylene production by control of gene expression on ethylene biosynthesis and signal transduction pathway.
Melatonin contributed to tomato aroma and flavour
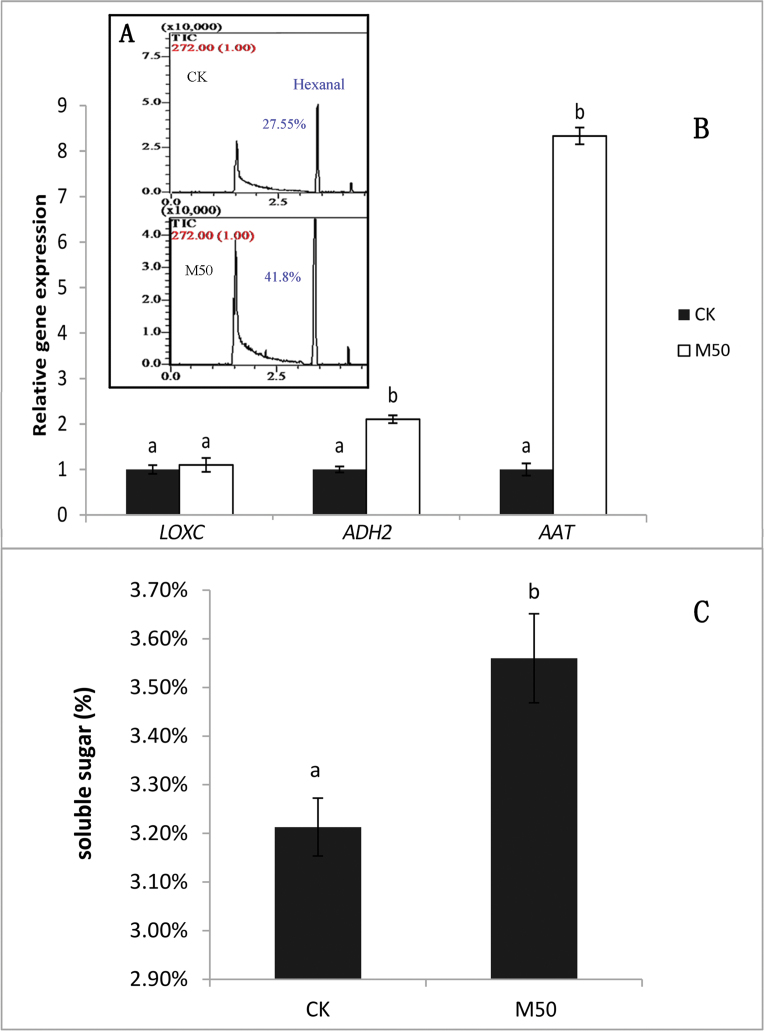
To understand the effect of melatonin on aroma in tomato fruit, we used the GC-MS technique to detect the volatiles of tomato fruits. Hexanal, a compound related to ripe aroma and tomato-like flavour (Baldwin et al., 1998; Maul et al., 2000), increased by 51.8% in melatonin-treated fruits (Fig. 6A).
Fig. 6.
Melatonin has an effect on tomato flavour components. CK: samples pre-treated with water; M50: samples pre-treated with 50 μmol/L melatonin. (A) The volatiles content of hexanal, and (B) related gene expression. (C) Water-soluble sugar of tomato fruit at 17 d after treatment. Vertical bars at each time point represent the LSD when significant at P=0.05. (This figure is available in colour at JXB online.)
Furthermore, we analysed the expression of SlLoxC, SlADH2, and SlAAT, which are involved in volatile compound (such as hexanal) metabolism. Our data showed that SlADH2 and SlAAT were both up-regulated by melatonin treatment, whereas SlLoxC did not show a significant response (Fig. 6B). The expression level of SlAAT markedly increased by 8-fold under 50 µM melatonin treatment when compared with CK.
To better understand the role of melatonin in fruit ripening, we also measured soluble sugar in melatonin-treated and control fruits. Significantly higher levels of soluble sugar (by 10.9%) were detected in M50-fruit compared with control sample at 17 DAT (Fig. 6C).
Both the sugars and volatiles are critical to good flavour in tomato fruit (Klee and Giovannoni, 2011). Volatiles determine the unique flavour of tomato, whereas the sugars are essential for good taste. We inferred that the biosynthetic pathway of volatiles may have been affected by melatonin treatment. Moreover, the level of soluble sugar in M50-fruit was higher than that of CK, indicating that melatonin may have a critical role in tomato flavour.
Discussion
Melatonin was detected in tomato in 1995 (Dubbels et al., 1995), and its level in the pericarp of tomato fruit increases from mature green stage to red stage (Van Tassel et al., 2001; Okazaki and Ezura, 2009). Our study found that exogenous melatonin could accelerate the process of tomato fruit postharvest ripening, as indicated by accelerated red colour development and increased fruit softening, water loss, pigment accumulation, and flavour formation. Moreover, the expression of associated genes was up-regulated by melatonin. The increment of ethylene production in melatonin-treated fruit is a consequence of up-regulated SlACS4 expression. The expression of genes involved in ethylene signal transduction pathway such as SlNR, SlETR4, SlEIL1, and SlEIL3 was altered by melatonin, suggesting that ethylene perception and sensitivity was affected by melatonin.
During ripening, tomato fruit colour changes from green to red as the result of carotenoid accumulation and chlorophyll degradation. Lycopene, the most abundant carotenoid found in tomatoes, is also important in human nutrition and health, as a precursor of Vitamin A and a potent antioxidant (DellaPenna and Pogson, 2006; Klee and Giovannoni, 2011). Lycopene biosynthesis in plants occurs via the central isoprenoid pathway beginning with the formation of phytoene from geranylgeranyl diphosphate (GGPP) and four desaturation steps (Li and Yuan, 2013). The first step of carotenoid biosynthesis is catalysed by PSY1, a fruit-specific phytoene synthase. CRTISO encodes carotenoid isomerase, an enzyme that isomerizes poly-cis-lycopene to all-trans-lycopene (Isaacson et al., 2004). Many hormones can impact lycopene biosynthesis. Recently, advances have been made in the regulation mechanism of ethylene on lycopene biosynthesis in tomato fruit. The Nr mutant (a single amino acid change in Nr ethylene receptor) exhibits reduced ethylene sensitivity and accumulates low amounts of lycopene in ripened fruits (Lanahan et al., 1994). Moreover, PSY is under strong positive ethylene control during ripening (Klee and Giovannoni, 2011). In our research, melatonin treatment enhanced lycopene accumulation (Fig. 1A) and ethylene production (Fig. 5A), implying that melatonin may increase the content of lycopene by impacting ethylene biosynthesis and signalling.
During fruit development and ripening, fruit softening, primarily owing to cell wall degradation and reduction of intercellular adhesion, is a prerequisite for a desirable eating quality of a fruit. Fruit cell walls are a complex network of polysaccharides and proteins, wherein the primary cell wall contains, on average, about 35% pectin, 25% cellulose, 20% hemicellulose, and 10% structural protein (Li et al., 2010). Cell wall modifications lead to progressive degradation of cell wall polymers and loss of integrity of the middle lamella, which is rich in pectins that control cell-to-cell adhesion, thus influencing fruit texture (Brummell and Harpster, 2001). In general, pectins are the first cell wall components to be modified during fruit ripening. PE (pectin esterase) is the main pectin de-esterification enzyme, and PG (polygalacturonase) is the main enzyme promoting pectin solubilization. PEs may act synergistically with PGs, with PE action resulting in the generation of homogalacturonan (HG) substrates that are more susceptible to PG-mediated hydrolysis (Seymour et al., 2013). Depolymerization of hemicelluloses is also a common feature of ripening fruit; endo-transglycosylase hydrolase (XTH) plays an important role in this process by modifying xyloglucan, the predominant hemicellulose of cell walls (Brummell, 2006). In tomato, both ethylene biosynthesis and signal transduction play important roles in regulating fruit ripening-related textural changes (Li et al., 2010). Gene expression analysis has revealed that ethylene directly regulates the transcription of both a softening-related PpPG gene and an expansin (PpExp3), thus suggesting that the rapid loss of firmness in ripening fruit is caused by cell wall metabolism, and is controlled by ethylene at the transcriptional level (Hayama et al., 2006). Moreover, PpPG2 and PpPME2 are reported to be involved in ethylene-regulated solubilization of polyuronides in peach fruit (Murayama et al., 2009). In this study, we found that ethylene production and signalling was affected by melatonin, and M50-fruit was softer than CK-fruit (Fig. 3A). Our findings suggest that melatonin may influence ethylene to regulate fruit textural changes.
Water movement has been considered a possible contribution to fruit firmness, and aquaporin proteins may influence water loss during ripening (Seymour et al., 2013). Aquaporins (AQPs) are water channel proteins that allow rapid and selective transport of water across membranes, which would presumably also affect water movement out of the cell. Fruit softening could be associated not only with cell wall disassembly, but also to loss of cell turgor mediated by water flow through aquaporins (Alleva et al., 2010). Aquaporins are also considered to be regulated by ethylene during fruit ripening; for example, the stimulation of AQP2 by ethylene in grape skin tissues reached 300% (Chervin et al., 2008). Some aquaporins investigated in our study were up-regulated in melatonin-treated fruit (Fig. 4B). This result was consistent with the advancement of ethylene production, implying that melatonin may have an effect on the ethylene pathway to enhance the expression of aquaporin genes, leading to increased water loss in melatonin-treated fruit.
At present, consumers are concerned about the quality of fruit, including nutritive value, aroma, colour, and shelf life. Colour and aroma volatiles are two major quality attributes of tomato fruits. AAT is capable of combining various alcohols and acyl CoAs, resulting in the synthesis of a wide range of esters. Aliphatic esters contribute to aroma of nearly all fruit and are emitted by vegetative tissues (Schwab et al., 2008). ADH genes that are suspected to participate in the production of aromas are expressed in a developmentally regulated manner, particularly during fruit ripening (Manriquez et al., 2006). Both AAT and ADH2 are involved in the biosynthetic pathway leading to esters (such as hexanal) formation. Synthesis of the volatiles that are up-regulated during ripening is dependent upon ethylene, as there are no ripening-associated increases in the ethylene-insensitive Nr mutant (Zhu et al., 2005; Klee and Giovannoni, 2011). Ethylene also affects the essential enzymes involved in the volatile biosynthetic pathways to influence the level of volatiles, such as lipoxygenase (LOX), alcohol dehydrogenase (ADH), and alcohol acyltransferase (AAT) (Zhu et al., 2005). Melatonin treatment up-regulated ADH2 and AAT gene expression (Fig. 6B) and influenced ethylene biosynthesis, ethylene perception, and ethylene signalling. Therefore, melatonin may have a role in ethylene biosynthesis and signalling to indirectly influence the level of volatiles.
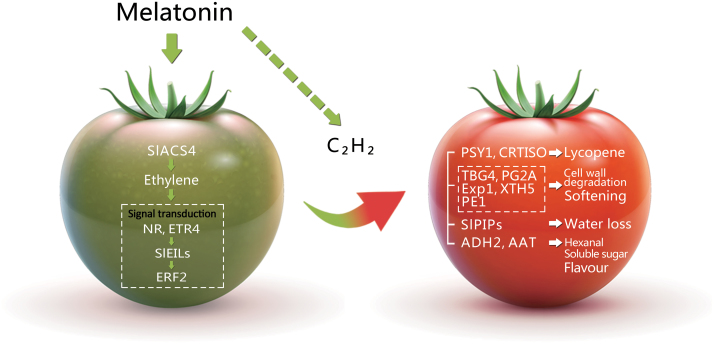
Ethylene plays a key regulatory role during ripening of many fruits by regulating carotenoid lycopene synthesis, conversion of starch to sugars, and enhancing cell wall-degrading enzyme activity. Exogenous melatonin could promote tomato fruit ripening during postharvest life, thus positively impacting ethylene production and signalling. We can infer that melatonin-induced fruit ripening events, such as lycopene accumulation, cell wall degradation and volatiles biosynthesis, are dependent on ethylene biosynthesis and signal transduction (Fig. 7). The findings expanded our understanding of melatonin function in ripening tomato fruits, providing evidence that melatonin is involved in fruit maturation.
Fig. 7.
A model of the role of melatonin in postharvest ripening tomato fruits. Melatonin may promote tomato fruit ripening by affecting ethylene production and signalling. Arrows represent positive regulation, the dotted lines represent regulatory steps in which a direct physical link between upstream and downstream components has yet to be demonstrated.
Supplementary data
Supplementary data are available at JXB online.
Table S1. The primers used for qRT-PCR.
Author contributions
Q. Sun, N. Zhang, B. Zhao and Y.-D. Guo designed the research; Q. Sun, N. Zhang, J. Wang, H. Zhang, D. Li, J. Shi, R. Li performed the research; Q. Sun, N. Zhang, S. Weeda, S. Ren, and Y.-D. Guo analysed data; Q. Sun,S. Weeda, S. Ren, and Y.-D. Guo wrote the paper.
Supplementary Material
Acknowledgements
This work was partly supported by a grant to Y.-D. Guo (2012CB113900) and the Beijing Key Laboratory of Growth and Developmental Regulation for Protected Vegetable Crops.
References
- Adams DO, Yang SF. 1979. Ethylene biosynthesis—identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proceedings of the National Academy of Sciences, USA 76, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L, Grierson D. 2002. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. Journal of Experimental Botany 53, 2039–2055. [DOI] [PubMed] [Google Scholar]
- Alleva K, Marquez M, Villarreal N, Mut P, Bustamante C, Bellati J, Martinez G, Civello M, Amodeo G. 2010. Cloning, functional characterization, and co-expression studies of a novel aquaporin (FaPIP2;1) of strawberry fruit. Journal of Experimental Botany 61, 3935–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin EA, Scott JW, Einstein MA, Malundo TMM, Carr BT, Shewfelt RL, Tandon KS. 1998. Relationship between sensory and instrumental analysis for tomato flavor. Journal of the American Society for Horticultural Science 123, 906–915. [Google Scholar]
- Bapat VA, Trivedi PK, Ghosh A, Sane VA, Ganapathi TR, Nath P. 2010. Ripening of fleshy fruit: Molecular insight and the role of ethylene. Biotechnology Advances 28, 94–107. [DOI] [PubMed] [Google Scholar]
- Brummell DA. 2006. Cell wall disassembly in ripening fruit. Functional Plant Biology 33, 103–119. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH. 2001. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47, 311–340. [PubMed] [Google Scholar]
- Burg SP. 2004. Preface, Horticultural commodity requirements. In: Burg SP, ed. Postharvest physiology and hypobaric storage of fresh produce. Oxford: CABI Publishing, 397–399. [Google Scholar]
- Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ. 2001. Detection and quantification of the antioxidant melatonin in montmorency and balaton tart cherries (Prunus cerasus). Journal of Agricultural and Food Chemistry 49, 4898–4902. [DOI] [PubMed] [Google Scholar]
- Cao J, Cole IB, Murch SJ. 2006. Neurotransmitters, neuroregulators and neurotoxins in the life of plants. Canadian Journal of Plant Science 86, 1183–1188. [Google Scholar]
- Chervin C, Tira-umphon A, Terrier N, Zouine M, Severac D, Roustan J-P. 2008. Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiologia Plantarum 134, 534–546. [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Maurel C. 1994. Aquaporins—the molecular-basis of facilitated water-movement through living plant-cells. Plant Physiology 105, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DellaPenna D, Pogson BJ. 2006. Vitamin synthesis in plants: Tocopherols and carotenoids. Annual Review of Plant Biology 57, 711–738. [DOI] [PubMed] [Google Scholar]
- Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W. 1995. Melatonin in edible plants identified by radioimmunoassay and by high-performance liquid chromatography-mass spectrometry. Journal of Pineal Research 18, 28–31. [DOI] [PubMed] [Google Scholar]
- Feng X, Wang M, Zhao Y, Han P, Dai Y. 2014. Melatonin from different fruit sources, functional roles, and analytical methods. Trends in Food Science & Technology 37, 21–31. [Google Scholar]
- Fish WW, Perkins-Veazie P, Collins JK. 2002. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. Journal of Food Composition and Analysis 15, 309–317. [Google Scholar]
- Hadfield KA, Bennett AB. 1998. Polygalacturonases: many genes in search of a function. Plant Physiol 117, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtanikaneko R, Hara M, Suzuki T, Reiter RJ. 1995. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochemistry and Molecular Biology International 35, 627–634. [PubMed] [Google Scholar]
- Hayama H, Tatsuki M, Ito A, Kashimura Y. 2006. Ethylene and fruit softening in the stony hard mutation in peach. Postharvest Biology and Technology 41, 16–21. [Google Scholar]
- Hernandez-Ruiz J, Cano A, Arnao MB. 2004. Melatonin: a growth-stimulating compound present in lupin tissues. Planta 220, 140–144. [DOI] [PubMed] [Google Scholar]
- Isaacson T, Ohad I, Beyer P, Hirschberg J. 2004. Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiology 136, 4246–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ. 2011. Genetics and control of tomato fruit ripening and quality attributes. Annual Review Genetics 45, 41–59. [DOI] [PubMed] [Google Scholar]
- Kolar J, Johnson CH, Machackova I. 2002. Presence and possible role of melatonin in a short-day flowering plant, Chenopodium rubrum . Advances in Experimental Medicine and Biology 460, 391–393. [DOI] [PubMed] [Google Scholar]
- Kolar J, Johnson CH, Machackova I. 2003. Exogenously applied melatonin (N-acetyl-5-methoxytryptamine) affects flowering of the short-day plant Chenopodium rubrum . Physiologia Plantarum 118, 605–612. [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. 1994. The never ripe mutation blocks ethylene perception in tomato. Plant Cell 6, 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Wang L, Tan D-X, Zhao Y, Zheng X-D, Chen H, Li Q-T, Zuo B-X, Kong J. 2013. Identification of genes for melatonin synthetic enzymes in ‘Red Fuji’ apple (Malus domestica Borkh. cv. Red) and their expression and melatonin production during fruit development. Journal of Pineal Research 55, 443–451. [DOI] [PubMed] [Google Scholar]
- Li L, Yuan H. 2013. Chromoplast biogenesis and carotenoid accumulation. Archives of Biochemistry and Biophysics 539, 102–109. [DOI] [PubMed] [Google Scholar]
- Li X, Xu C, Korban SS, Chen K. 2010. Regulatory mechanisms of textural changes in ripening fruits. Critical Reviews in Plant Sciences 29, 222–243. [Google Scholar]
- Liu C, Liu Y, Yi G, Li W, Zhang G. 2011. A comparison of aroma components of pineapple fruits ripened in different seasons. African Journal of Agricultural Research 6, 1771–1778. [Google Scholar]
- Lunn D, Phan TD, Tucker GA, Lycett GW. 2013. Cell wall composition of tomato fruit changes during development and inhibition of vesicle trafficking is associated with reduced pectin levels and reduced softening. Plant Physiology and Biochemistry 66, 91–97. [DOI] [PubMed] [Google Scholar]
- Manchester LC, Tan DX, Reiter RJ, Park W, Monis K, Qi WB. 2000. High levels of melatonin in the seeds of edible plants—Possible function in germ tissue protection. Life Sciences 67, 3023–3029. [DOI] [PubMed] [Google Scholar]
- Manriquez D, El-Sharkawy I, Flores FB, El-Yahyaoui F, Regad F, Bouzayen M, Latche A, Pech J-C. 2006. Two highly divergent alcohol dehydrogenases of melon exhibit fruit ripening-specific expression and distinct biochemical characteristics. Plant Molecular Biology 61, 675–685. [DOI] [PubMed] [Google Scholar]
- Maul F, Sargent SA, Sims CA, Baldwin EA, Balaban MO, Huber DJ. 2000. Tomato flavor and aroma quality as affected by storage temperature. Journal of Food Science 65, 1228–1237. [Google Scholar]
- Murayama H, Arikawa M, Sasaki Y, Dal Cin V, Mitsuhashi W, Toyomasu T. 2009. Effect of ethylene treatment on expression of polyuronide-modifying genes and solubilization of polyuronides during ripening in two peach cultivars having different softening characteristics. Postharvest Biology and Technology 52, 196–201. [Google Scholar]
- Okazaki M, Ezura H. 2009. Profiling of melatonin in the model tomato (Solanum lycopersicum L.) cultivar Micro-Tom. Journal of Pineal Research 46, 338–343. [DOI] [PubMed] [Google Scholar]
- Olmedo G, Guo HW, Gregory BD, Nourizadeh SD, Aguilar-Henonin L, Li HJ, An FY, Guzman P, Ecker JR. 2006. ETHYLENE-INSENSITIVE5 encodes a 5’→3’ exoribonuclease required for regulation of the EIN3-targeting F-box proteins EBF1/2. Proceedings of the National Academy of Sciences, USA 103, 13286–13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ. 2009. Phytomelatonin: a review. Journal of Experimental Botany 60, 57–69. [DOI] [PubMed] [Google Scholar]
- Park WJ. 2011. Melatonin as an endogenous plant regulatory signal: debates and perspectives. Journal of Plant Biology 54, 143–149. [Google Scholar]
- Posmyk MM, Janas KM. 2009. Melatonin in plants. Acta Physiologiae Plantarum 31, 1–11. [Google Scholar]
- Prasanna V, Prabha TN, Tharanathan RN. 2007. Fruit ripening phenomena—An overview. Critical Reviews in Food Science and Nutrition 47, 1–19. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Manchester LC, Tan DX. 2005. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 21, 920–924. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ. 2004. Regulation of antioxidant enzymes: a significant role for melatonin. Journal of Pineal Research 36, 1–9. [DOI] [PubMed] [Google Scholar]
- Ronen G, Cohen M, Zamir D, Hirschberg J. 1999. Regulation of carotenoid biosynthesis during tomato fruit development: Expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant Journal 17, 341–351. [DOI] [PubMed] [Google Scholar]
- Rugkong A, McQuinn R, Giovannoni JJ, Rose JKC, Watkins CB. 2011. Expression of ripening-related genes in cold-stored tomato fruit. Postharvest Biology and Technology 61, 1–14. [Google Scholar]
- Sae-Teaw M, Johns J, Johns NP, Subongkot S. 2013. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. Journal of Pineal Research 55, 58–64. [DOI] [PubMed] [Google Scholar]
- Schwab W, Davidovich-Rikanati R, Lewinsohn E. 2008. Biosynthesis of plant-derived flavor compounds. Plant Journal 54, 712–732. [DOI] [PubMed] [Google Scholar]
- Seymour GB, Chapman NH, Chew BL, Rose JKC. 2013. Regulation of ripening and opportunities for control in tomato and other fruits. Plant Biotechnology Journal 11, 269–278. [DOI] [PubMed] [Google Scholar]
- Shackel KA, Greve C, Labavitch JM, Ahmadi H. 1991. Cell turgor changes associated with ripening in tomato pericarp tissue. Plant Physiology 97, 814–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtz M, Cerezo AB, Cantos-Villar E, Garcia-Parrilla MC. 2011. Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chemistry 127, 1329–1334. [DOI] [PubMed] [Google Scholar]
- Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. 1993. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocrine Journal 1, 57–60. [Google Scholar]
- Van Tassel DL, Roberts N, Lewy A, O’Neill SD. 2001. Melatonin in plant organs. Journal of Pineal Research 31, 8–15. [DOI] [PubMed] [Google Scholar]
- Vicente AR, Saladie M, Rose JKC, Labavitch JM. 2007. The linkage between cell wall metabolism and fruit softening: looking to the future. Journal of the Science of Food and Agriculture 87, 1435–1448. [Google Scholar]
- Wang L, Zhao Y, Reiter RJ, He C, Liu G, Lei Q, Zuo B, Zheng XD, Li Q, Kong J. 2014. Changes in melatonin levels in transgenic ‘Micro-Tom’ tomato overexpressing ovine AANAT and ovine HIOMT genes. Journal of Pineal Research 56, 134–142. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhang HJ, Zhao B, et al. 2014. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. Journal of Pineal Research 56, 39–50. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhao B, Zhang HJ, Weeda S, Yang C, Yang ZC, Ren SX, Guo YD. 2013. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). Journal of Pineal Research 54, 15–23. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tan D, Lei Q, et al. 2013. Melatonin and its potential biological functions in the fruits of sweet cherry. Journal of Pineal Research 55, 79–88. [DOI] [PubMed] [Google Scholar]
- Zhu HL, Zhu BZ, Fu DQ, Xie YH, Hao YL, Luo YB. 2005. Role of ethylene in the biosynthetic pathways of aroma volatiles in ripening fruit. Russian Journal of Plant Physiology 52, 691–695. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.