Abstract
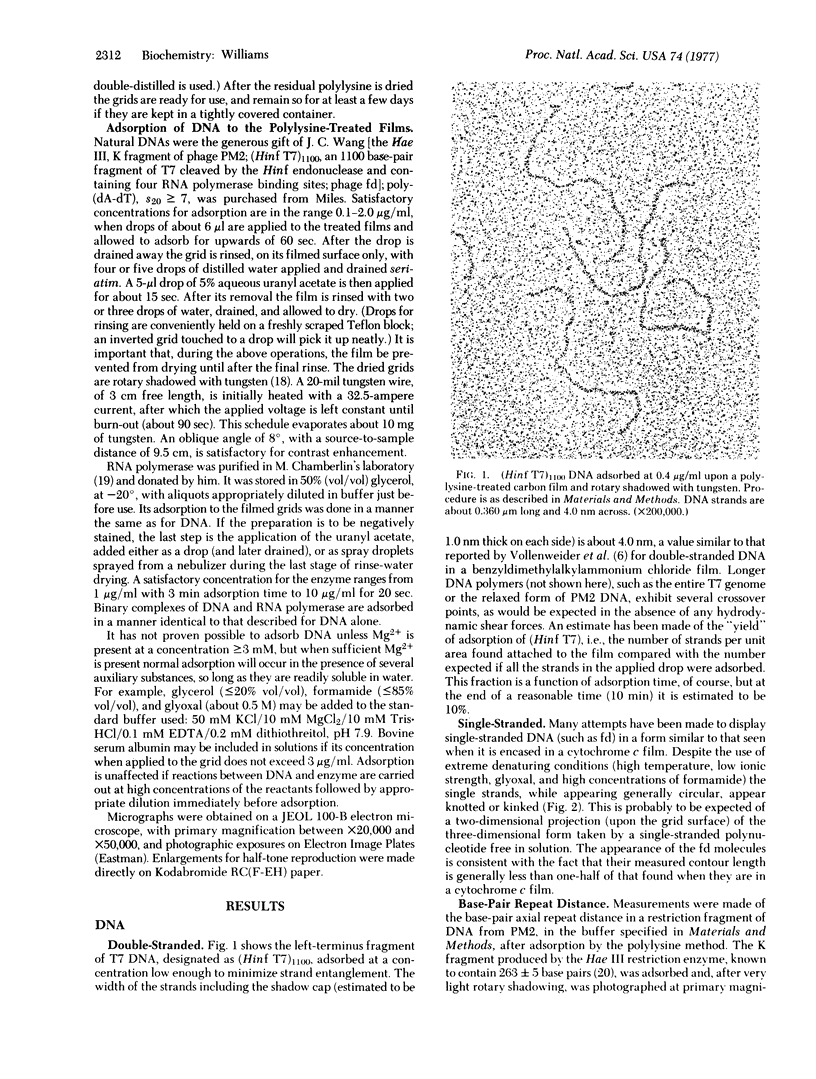
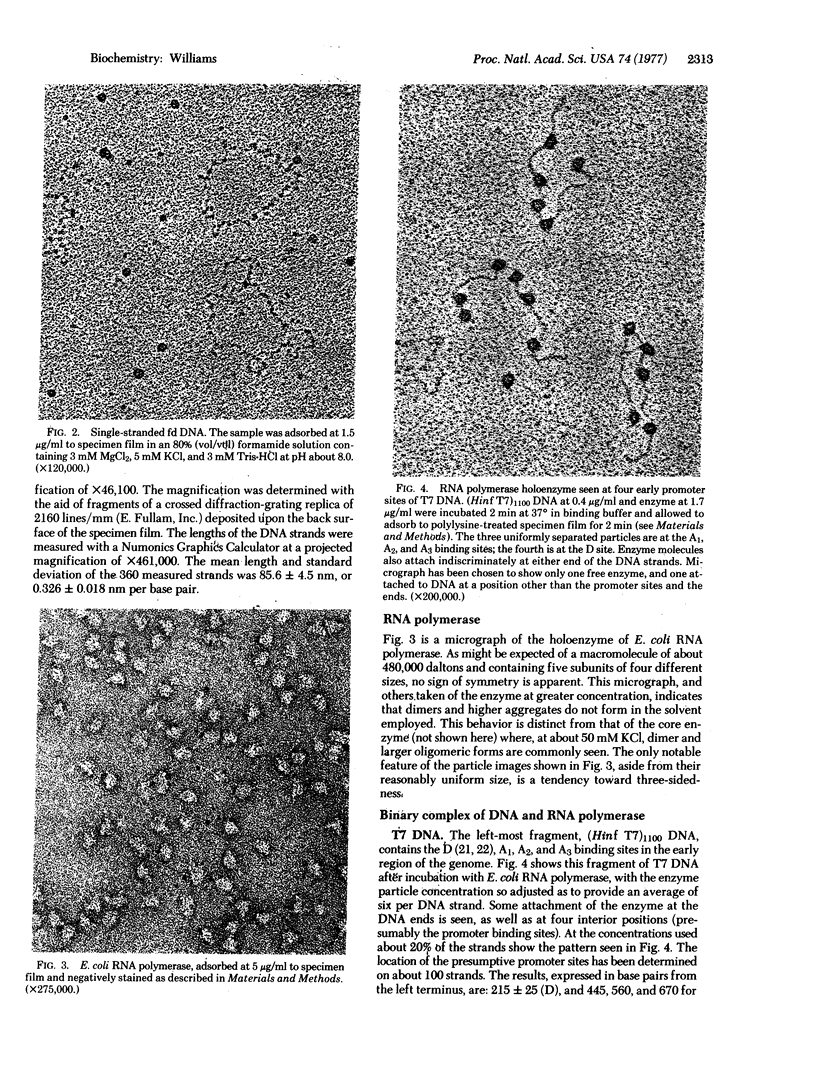
Enzymes and nucleic acids, both free and as bound in binary complexes, adsorb to electron microscope specimen films in well-distributed fashion if a dilute solution of polylysine is previously applied to the films. Electron micrographs are exhibited that demonstrate the usefulness of the technique in visualizing double- and single-stranded DNA, Escherichia coli RNA polymerase (nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) in negative stain, and polymerase complexed to poly(dA-dT) and to an 1100 base-pair restriction fragment of bacteriophage T7 DNA containing the early promoters. The base-pair spacing of DNA prepared for electron microscopy by the polylysine method was found to be 0.326 nm. Four promoter sites on the T7 fragment were located at 215, 440, 560, and 670 base-pair distances from the left terminus. When poly(dA-dT) was incubated with a 20-to-1 weight ratio of polymerase the bound enzyme particles were found to be about two-thirds as closely packed as is sterically permissible.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C., Dubochet J. Electron microscopic localization of the binding sites of Escherichia coli RNA polymerase in the early promoter region of T7 DNA. Eur J Biochem. 1974 May 15;44(2):617–624. doi: 10.1111/j.1432-1033.1974.tb03519.x. [DOI] [PubMed] [Google Scholar]
- Brack C., Delain E. Electron-microscopic mapping of AT-rich regions and of E. coli RNA polymerase-binding sites on the circular kinetoplast DNA of Trypanosoma cruzi. J Cell Sci. 1975 Mar;17(3):287–306. doi: 10.1242/jcs.17.3.287. [DOI] [PubMed] [Google Scholar]
- Colvill A. J., Van Bruggen E. F., Fernández-Morán H. Physical properties of a DNA-dependent RNA polymerase from Escherichia coli. J Mol Biol. 1966 May;17(1):302–304. doi: 10.1016/s0022-2836(66)80113-4. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Dausse J. P. Localization of Escherichia coli RNA polymerase initiation sites in T7 DNA early promoter region. FEBS Lett. 1975 Feb 1;50(2):214–218. doi: 10.1016/0014-5793(75)80491-1. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- FUCHS E., ZILLIG W., HOFSCHNEIDER P. H., PREUSS A. PREPARATION AND PROPERTIES OF RNA-POLYMERASE PARTICLES. J Mol Biol. 1964 Dec;10:546–550. doi: 10.1016/s0022-2836(64)80076-0. [DOI] [PubMed] [Google Scholar]
- Griffith J., Huberman J. A., Kornberg A. Electron microscopy of DNA polymerase bound to DNA. J Mol Biol. 1971 Jan 28;55(2):209–214. doi: 10.1016/0022-2836(71)90192-6. [DOI] [PubMed] [Google Scholar]
- HART R. G. The nucleic acid fiber of the tobacco mosaic virus particle. Biochim Biophys Acta. 1958 Jun;28(3):457–464. doi: 10.1016/0006-3002(58)90506-7. [DOI] [PubMed] [Google Scholar]
- HIGHTON P. J., BEER M. An electromicroscopic study of extended single polynucleotide chains. J Mol Biol. 1963 Jul;7:70–77. doi: 10.1016/s0022-2836(63)80019-4. [DOI] [PubMed] [Google Scholar]
- Heyden B., Nüsslein C., Schaller H. Initiation of transcription within an RNA-polymerase binding site. Eur J Biochem. 1975 Jun 16;55(1):147–155. doi: 10.1111/j.1432-1033.1975.tb02147.x. [DOI] [PubMed] [Google Scholar]
- Highton P. J., Whitfield M. The control of the configuration of nucleic acid molecules deposited for electron microscopy, by ionic bombardment of carbon films. J Microsc. 1974 Apr;100(3):299–306. doi: 10.1111/j.1365-2818.1974.tb03941.x. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Schleif R. High resolution electron microscopic studies of genetic regulation. J Mol Biol. 1976 Dec;108(2):471–490. doi: 10.1016/s0022-2836(76)80131-3. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Wang J. C. Physicochomecial studies on interactions between DNA and RNA polymerase. Isolation and mapping of a T7 DNA fragment containing the early promoters for Escherichia coli RNA polymerase. Biochemistry. 1976 Dec 28;15(26):5776–5783. doi: 10.1021/bi00671a014. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- Koller T., Sogo J. M., Bujard H. An electron microscopic method for studying nucleic acid-protein complexes. Visualization of RNA polymerase bound to the DNA of bacteriophages T7 and T3. Biopolymers. 1974 May;13(5):995–1009. doi: 10.1002/bip.1974.360130514. [DOI] [PubMed] [Google Scholar]
- Kovacic R. T., van Holde K. E. Sedimentation of homogeneous double-strand DNA molecules. Biochemistry. 1977 Apr 5;16(7):1490–1498. doi: 10.1021/bi00626a038. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Characterization of DNA condensates induced by poly(ethylene oxide) and polylysine. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4288–4292. doi: 10.1073/pnas.72.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Talaer J. Y., Kermici M., Jeanteur P. Isolation of Escherichia coli RNA polymerase binding sites on T5 and T7 DNA: further evidence for sigma-dependent recognition of A-T-rich DNA sequences. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2911–2915. doi: 10.1073/pnas.70.10.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure B., Oudet P., Chambon P., Yaniv M. Transcription of polyoma virus DNA in vitro. Localization of Escherichia coli RNA polymerase initiation sites. J Mol Biol. 1976 Nov;108(1):83–97. doi: 10.1016/s0022-2836(76)80096-4. [DOI] [PubMed] [Google Scholar]
- Lubin M. Observations on the structure of RNA polymerase and its attachmnt to DNA. J Mol Biol. 1969 Jan 14;39(1):219–233. doi: 10.1016/0022-2836(69)90343-x. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Nanninga N., Meyer M., Sloof P., Reijnders L. Electron microscopy of Escherichia coli ribosomal RNA: spreading without a basic protein film. J Mol Biol. 1972 Dec 30;72(3):807–810. doi: 10.1016/0022-2836(72)90193-3. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Sugiura M., Takanami M. RNA polymerase binding sites of phage fd replicative form DNA. Nat New Biol. 1972 May 24;237(73):108–109. doi: 10.1038/newbio237108a0. [DOI] [PubMed] [Google Scholar]
- Portmann R., Sogo J. M., Koller T., Zillig W. Binding sites of E. coli RNA polymerase on T7 DNA as determined by electron microscopy. FEBS Lett. 1974 Sep 1;45(1):64–67. doi: 10.1016/0014-5793(74)80811-2. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Sogo J. M., Koller T. A routine method for protein-free spreading of double- and single-stranded nucleic acid molecules. Proc Natl Acad Sci U S A. 1975 Jan;72(1):83–87. doi: 10.1073/pnas.72.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]