Abstract
As a topical hemostatic agent, thrombin has wide application for many surgical treatments. However, native thrombin always suffers from its physical and chemical instabilities. In this work, a nanocomplexation strategy was developed for modifying the stability and hemostatic efficacy of thrombin, in which a water-soluble cationic amylose derivative containing poly(l-lysine) dendrons was prepared by a click reaction and then used to complex thrombin in an aqueous system. For resultant thrombin nanocomplexes, their morphology and particle size distribution were investigated. Their stabilities were studied in terms of activity retention percentages under different storage time, pH values, and illumination time. In addition, their ability to achieve in vitro fibrinogen and blood coagulation were evaluated. Via a rat hepatic hemorrhage model and a rat iliac artery hemorrhage model, these thrombin nanocomplexes were confirmed to have good tissue biocompatibility and in vivo hemostatic effectiveness.
Keywords: thrombin, nanoparticles, amylose derivative, complexation, stability, hemostatic activity
Introduction
Hemorrhage during surgical procedures is common and is potentially life-threatening for patients.1–4 In the case of cardiac surgery, for example, bleeding may result from several aspects inherent to cardiac procedures, including the placement of cardiac suture lines in great vessels or chambers of the heart, as well as the creation of high-pressure anastomoses.5 Therefore, effective and rapid hemostasis is critical to optimize surgical outcomes. For this reason, various topical hemostatic agents such as thrombin, porcine gelatin, bovine gelatin, bovine collagen, regenerated oxidized cellulose, and their combination products frequently have been used when hemorrhage is not controlled by conventional hemostatic methods.6–9 Among them, thrombin is of increasing interest and can be applied to the bleeding site in a dry form or after reconstitution with sterile isotonic saline.9 It is known that thrombin is a naturally derived enzyme that has had a prominent role in hemostasis for decades.10,11 It can initiate the conversion of fibrinogen to fibrin without a foreign body or inflammatory reactions, and thus has been regarded as an ideal hemostatic agent.10,11 However, such a hemostatic agent always suffers from physical and chemical instabilities, which create some difficulties in its formulation and use. In addition, thrombin may rapidly denature and easily lose its biological activity in the environments, with the exception of its physiological one.12
In this work, we developed a new route for the stability improvement and bioactivity retention of thrombin. In our strategy, a water-soluble cationic amylose derivative with good biocompatibility was prepared by a click reaction between azidized amylose and propargyl focal point poly(l-lysine) dendron, and was then used to complex thrombin in an aqueous system. As a result, thrombin nanocomplexes with good stability and bioactivity retention were obtained. In particular, their hemostatic efficacy was confirmed by fibrinogen binding assay, fresh blood coagulation analysis, rat hepatic hemorrhage model, and rat iliac artery hemorrhage model.
Materials and methods
Materials
Azidized amylose (Amy-N3) was prepared according to a similar method reported by Azzam et al.13 Propargyl focal point poly(l-lysine) dendron (PLLD-G3; generation, 3) was synthesized by divergent and convergent approaches, as reported in a recent publication.14 Copper sulfate (CuSO4·5H2O) and sodium ascorbate (99%) were purchased from Alfa Aesar. Thrombin and chloral hydrate were obtained from Sigma. Sprague-Dawley rats were purchased from the Animal Center of Sun Yat-sen University (Guangzhou, People’s Republic of China). Other chemical reagents of analytical grade were obtained commercially and used directly.
Preparation of cationic amylose derivative
For the preparation of water-soluble cationic amylose derivative (CAD) containing poly(l-lysine) dendrons, 10 mL dimethylsulfoxide, 0.515 g PLLD-G3 (0.29 mmol), and 0.05 g Amy-N3 were added successively under magnetic stirring. After a clear solution was obtained, 18 mg CuSO4·5H2O and 65 mg sodium ascorbate were then added successively under nitrogen atmosphere. The resultant reaction mixture was heated to 40°C for 48 hours. After the reaction, the product was dialyzed in distilled water for 3 days (molecular weight cut-off, 14,000) and lyophilized to obtain CAD with a yield of 65%. Fourier transform infrared (FTIR) measurements were performed to confirm the preparation of CAD by a Perkin-Elmer Paragon 1,000 spectrometer at frequencies ranging from 500 to 4,000 cm−1. Each sample was thoroughly mixed with KBr and pressed into a pellet form. The content of poly(l-lysine) dendrons in CAD was determined by a Vario EL cube elemental analyzer.
Complexation of CAD with thrombin
For the complexation of CAD with thrombin, 20 mg CAD was first added into a three-necked flask and dissolved in 500 mL phosphate-buffered saline solution (pH 7.4) at room temperature with gentle stirring. The thrombin stock solution was prepared by dissolving 3,000 U thrombin in 100 mL phosphate-buffered saline solution (pH 7.4) at room temperature and then was slowly dropped into an aqueous solution of CAD at room temperature, followed by gentle agitation for 30 min. For resultant CAD/thrombin complexes, the morphology was observed by a JEM2010 transmission electron microscopy (Japan), and the particle size distribution was investigated by a ZetaPALS analyzer (Brookhaven Instruments Corporation) at 25°C with a 90° scattering angle.
Stability assays
For native thrombin and its nanocomplexes with CAD, their stabilities were evaluated in terms of activity retention under different conditions, including storage time (0, 1, 2, 3, 4, 5, 10, 20, 30, 40, 50, and 60 days), pH values (6.5–6.8, 7.0–7.4, and 7.6–8.0), and illumination time (0, 1, 2, 3, 4, 5, and 10 days). To obtain the activity retention, native thrombin or the thrombin nanocomplexes were mixed with the thrombin chromogenic substrate S-2238 (H-D-Phe-Pip-Arg-p-nitroanilide, 150 μM; Chromogenix). After 1 min, the generation of p-nitroaniline was monitored by measuring OD at 405 nm. The activity of thrombin was determined as the rate of hydrolysis of S-2238 from the linear range of absorbance at 405 nm with time.
Fibrinogen coagulation tests
For the fibrinogen coagulation tests, 1 mL of 3% fibrinogen solution and 5 U native thrombin or the thrombin nanocomplexes were added into a test tube (1.5×5 cm), respectively. The time for the formation of coagulum was recorded to evaluate in vitro hemostatic efficacy of native thrombin or the thrombin nanocomplexes.
Clotting time tests
For the clotting time tests, 5 U native thrombin or the thrombin nanocomplexes were added, respectively, into a test tube. Then 1 mL fresh blood was poured into each test tube. The time for the blood coagulation was recorded to evaluate in vitro hemostatic efficacy of native thrombin or the thrombin nanocomplexes.
Tissue biocompatibility evaluation
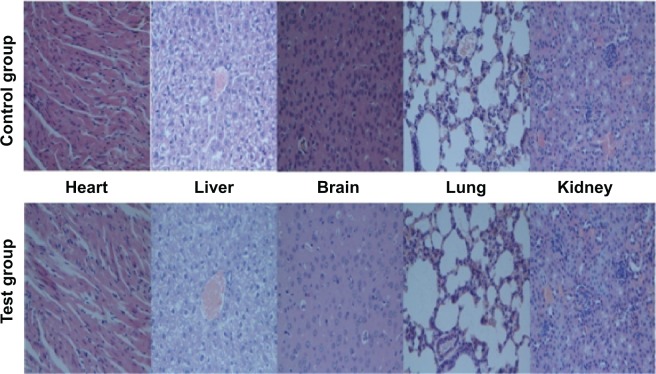
For the tissue biocompatibility evaluation, the thrombin nanocomplexes (1 kU/kg mouse) were dispersed homogeneously in distilled water by ultrasonic shaking and then injected into six female BALB/c mice (4 weeks old, 18–20 g) through the tail vein. For a comparison, physiological saline was used as a control reagent. After 7 days, all animals were killed. The heart, liver, brain, lung, and kidney were separated, washed twice with phosphate-buffered saline solution, and fixed in 4% formaldehyde for histologic examination. The histologic analyses for these tissues were performed to investigate whether the thrombin nanocomplexes induced tissue damage, inflammation, or systemic thrombosis.
Hemostatic efficacy evaluation in rat hepatic hemorrhage model
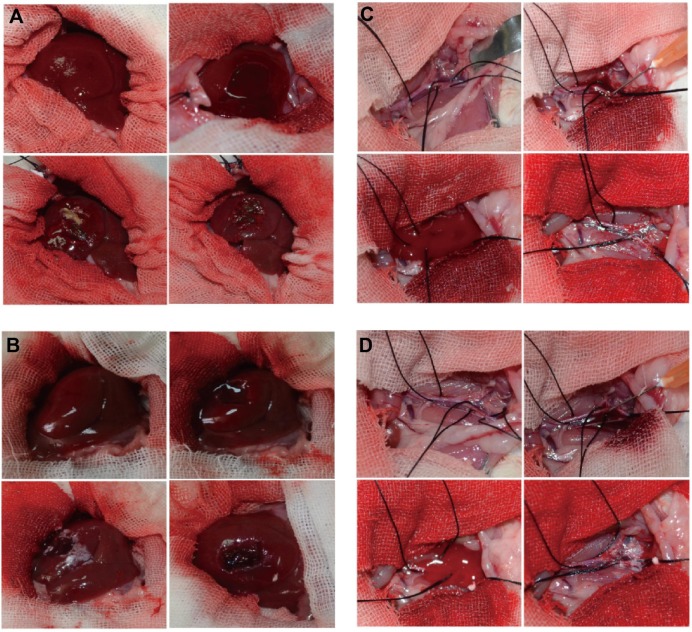
For the hemostatic efficacy evaluation in rat hepatic hemorrhage model, twelve female Sprague–Dawley rats (300–350 g) obtained from the Animal Center of Sun Yat-sen University were randomly assigned to one of two groups and were housed in an air conditioned room (humidity, 72%–75%; temperature, 23°C–25°C; lighting, light–dark cycle every 12 hours). Rats were anesthetized with intraperitoneal injection of 10% (w/v) chloral hydrate (0.3 mL/100 g), shaved on the abdomen, and fixed on their backs to the operating table. The operative area of skin was sterilized with 75% alcohol, and the abdomen was opened with surgical scissors and forceps, layer by layer, longitudinally. The right lobe of the liver was incised for 1×1 cm, and the surface tissue was removed to establish a hepatic hemorrhage model, treated, respectively, with native thrombin and the thrombin nanocomplexes.
Hemostatic efficacy evaluation in a rat iliac artery hemorrhage model
For the hemostatic efficacy evaluation in the rat iliac artery hemorrhage model, twelve female Sprague–Dawley rats (300–350 g) obtained from the Animal Center of Sun Yat-sen University were randomly assigned to one of two groups and were housed in an air conditioned room (humidity, 72%–75%; temperature, 23°C–25°C; lighting, light–dark cycle every 12 hours). Rats were anesthetized with intraperitoneal injection of 10% (w/v) chloral hydrate (0.3 mL/100 g), shaved on the abdomen, and fixed on their backs to the operating table. The operative area of skin was sterilized with 75% alcohol, and the abdomen was opened with surgical scissors and forceps, layer by layer, longitudinally. The right iliac artery was separated from the aorta abdominalis, and the right iliac artery was then punctured with a 1 mL syringe to establish the right iliac artery hemorrhage model, treated, respectively, with native thrombin and the thrombin nanocomplexes.
Statistical analyses
The results were expressed as means ± standard deviations. Differences between means were analyzed for statistical significance by the paired Student’s t-test and by using SPSS 13.0 statistical analysis software. A value of P<0.05 was considered statistically significant.
Results and discussion
Characterization of CAD
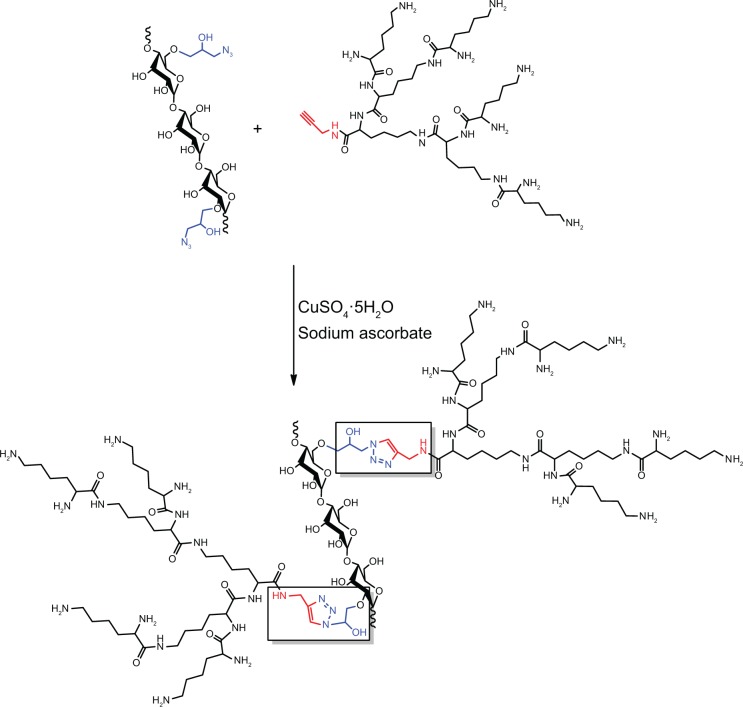
The water-soluble CAD containing poly(l-lysine) dendrons was prepared for the first time by a click conjugation reaction between azidized amylose (Amy-N3) and propargyl focal point poly(l-lysine) dendron of third-generation (PLLD-G3), as shown in Figure 1. To confirm the preparation of CAD, FTIR analyses were performed. Figure 2 gives the FTIR spectra of Amy-N3, PLLD-G3, and CAD. As seen, the characteristic absorption bands of Amy-N3 appeared at 3,460 cm−1 (νO-H, pyranose), 2,106 cm−1 (azido groups), and 1,022 cm−1 (νC-O, pyranose). The characteristic absorption bands of PLLD-G3 appeared at 2,917 cm−1, 2,842 cm−1 (νC-H), 1,604 cm−1 (νC=O), and 1,396 cm−1 (νCO-NH). After the click conjugation, the spectrum of CAD did not show the characteristic absorption bands of the azido group (2,106 cm−1) but did exhibit the main characteristic bands of Amy-N3 and PLLD-G3. These results indicate the success of the click conjugation between Amy-N3 and PLLD-G3. On the basis of elemental analysis, the degree of substitution of PLLD-G3 on the amylose, which is defined as the number of PLLD-G3 per 100 anhydroglucose units of amylose, was determined to be 9.35.
Figure 1.
Schematic illustration for the preparation of cationic amylose derivative (CAD).
Figure 2.
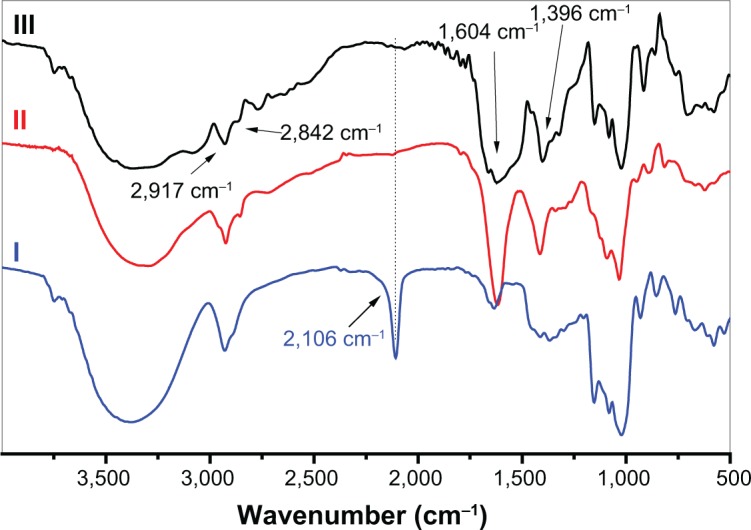
Fourier transform infrared spectra of azidized amylose, propargyl focal point poly(l-lysine) dendron of the third generation and their click conjugate (CAD III).
Formation of thrombin nanocomplexes
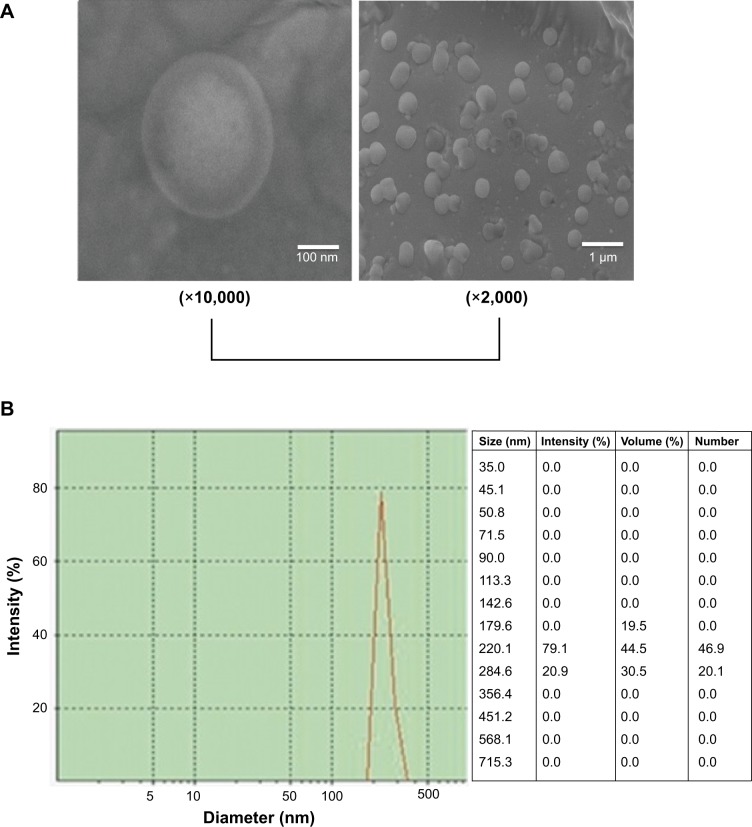
Because of the conjugation of cationic poly(l-lysine) dendrons with a high density of primary amino groups, CAD could electrostatically interact with thrombin in an aqueous system, resulting in the formation of CAD/thrombin complexes. To confirm this, we carried out transmission electron microscopy observation and particle size distribution measurements for resultant CAD/thrombin complexes. As seen from Figure 3, these complexes showed a roughly spherical morphology and had a nanoscale size distribution. Such a similar strategy has been successfully used to obtain colloidally stable nanocomplexes of dendronized cyclodextrin derivatives with plasmid DNA or small interfering RNA for the delivery of nucleic acids.14,15
Figure 3.
The morphology (A) and particle size distribution (B) of cationic amylose derivative/thrombin complexes.
Improved stability of thrombin nanocomplexes
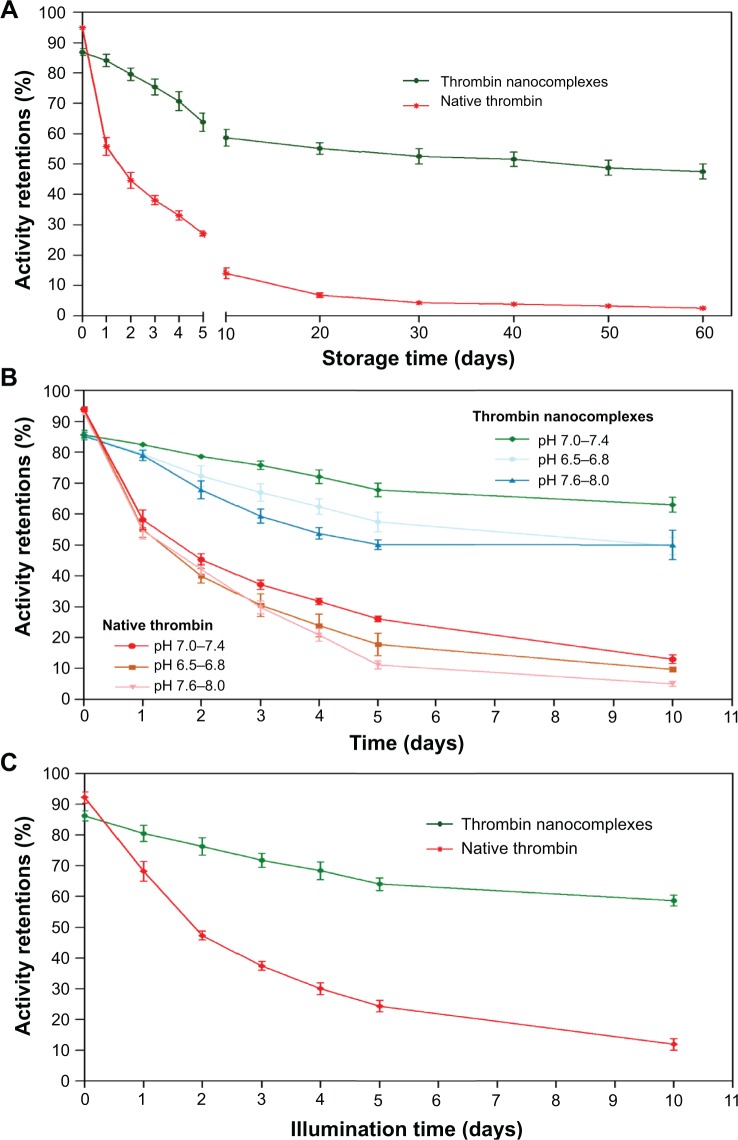
For the thrombin nanocomplexes with CAD, their stabilities under different conditions were investigated in terms of activity retention when compared with native thrombin, as shown in Figure 4. To investigate the effect of storage time, the storage time varied from 0 to 60 days, and the storage temperature was kept to 25°C. From Figure 4A, it was found that the thrombin nanocomplexes could retain a high activity level during a long storage period. In contrast, native thrombin was easy to lose its activity. For example, the activity retention percentage after 5 days was observed to be about 64% for the thrombin nanocomplexes and about 27% for native thrombin, respectively. After 60 days, the thrombin nanocomplexes still showed an activity retention percentage higher than 50%, whereas native thrombin only had an activity retention percentage lower than 5%. Similar phenomena were also observed when we studied the effect of pH value (Figure 4B) or illumination time (Figure 4C) on the activity retentions of native thrombin and the thrombin nanocomplexes. These results demonstrate that the complexation of CAD with thrombin greatly improves the physical and chemical stabilities of thrombin, which will be favorable for its use as a topical hemostatic agent.
Figure 4.
Effects of storage time (A), pH value (B), and illumination time (C) on the activity retentions of native thrombin and the thrombin nanocomplexes.
In vitro hemostatic efficacy of thrombin nanocomplexes
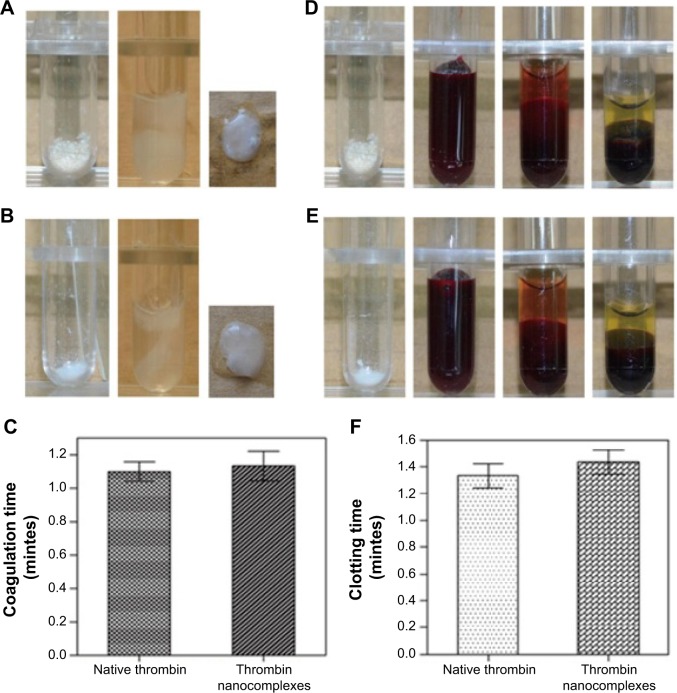
Further investigation dealt with the in vitro hemostatic efficacy of thrombin nanocomplexes by fibrinogen and blood coagulation tests. For a comparison study, native thrombin was also investigated in the same way. As shown in Figure 5, the thrombin nanocomplexes could rapidly induce the fibrinogen and blood coagulations, similar to native thrombin. There are no obvious differences between the fibrinogen coagulation/clotting time of thrombin nanocomplexes and the fibrinogen coagulation/clotting time of native thrombin. The thrombin nanocomplexes still have a strong fibrinogen and blood coagulation ability, similar to native thrombin. These results demonstrate that the nanocomplexation of thrombin with the CAD did not affect significantly the biofunctionality of thrombin.
Figure 5.
(A) Photographs for the fibrinogen coagulation test in the case of the thrombin nanocomplexes. (B) Photographs for the fibrinogen coagulation test in the case of native thrombin. (C) The fibrinogen coagulation time of native thrombin and the thrombin nanocomplexes. (D) Photographs for the blood coagulation test in the case of the thrombin nanocomplexes. (E) Photographs for the blood coagulation test in the case of native thrombin. (F) The clotting time of native thrombin and the thrombin nanocomplexes.
In vivo tissue biocompatibility and hemostatic efficacy of thrombin nanocomplexes
To explore the possibility of the thrombin nanocomplexes as an ideal topical hemostatic agent, the in vivo tissue biocompatibility and hemostatic efficacy of thrombin nanocomplexes were investigated. Figure 6 gives the representative histological images of the mice treated, respectively, with physiological saline and aqueous suspension of the thrombin nanocomplexes after 7 days. As seen, the thrombin nanocomplexes did not result in acute inflammation response, thrombus formation, or other adverse effects for the treated heart, liver, brain, lungs, and kidney tissues. Therefore, the thrombin nanocomplexes have a good biocompatibility, which will become an advantage when they are used as a topical hemostatic agent. Figure 7 shows the hemostatic efficacies of thrombin nanocomplexes and native thrombin in rat hepatic hemorrhage model and in rat iliac artery hemorrhage model. As observed, the thrombin nanocomplexes have a good hemostatic property similar to native thrombin, showing its potential application as a topical hemostatic agent.
Figure 6.
Representative histologic images of the mice treated, respectively, with physiologic saline (control group) and aqueous suspension of the thrombin nanocomplexes (test group) after 7 days.
Figure 7.
(A) Hemostatic efficacy of thrombin nanocomplexes in rat hepatic hemorrhage model. (B) Hemostatic efficacy of native thrombin in rat hepatic hemorrhage model. (C) Hemostatic efficacy of thrombin nanocomplexes in rat iliac artery hemorrhage model. (D) Hemostatic efficacy of native thrombin in rat iliac artery hemorrhage model.
Conclusion
With an attempt to improve the stability and hemostatic properties of thrombin, we developed a nanocomplexation strategy by using a novel cationic amylose derivative containing poly(l-lysine) dendrons with a high density of primary amino groups. The obtained thrombin nanocomplexes were found to have much higher activity retention percentages under different storage times, pH values, and illumination times when compared with native thrombin. Moreover, they were confirmed to show in vitro and in vivo hemostatic effectiveness, as well as good biocompatibility.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51273216, 21074152, J1103305), Doctoral Research Program of Education Ministry in China (20090171110023 and 20130171120095), Integration of Production and Research projects of Guangdong province (20110908), Key Project of Scientific and Technical Innovation for Universities in Guangdong Province (cxzd1102), and Natural Science Foundation of Guangdong Province (S2013010012549 and S2013040014401).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Champion HR, Bellamy RF, Roberts CP, Leppaniemi A. A profile of combat injury. J Trauma. 2003;54((5)(suppl)):S13–S19. doi: 10.1097/01.TA.0000057151.02906.27. [DOI] [PubMed] [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60((6)(suppl)):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 3.Pusateri AE, McCarthy SJ, Gregory KW, et al. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Trauma. 2003;54(1):177–182. doi: 10.1097/00005373-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ari Z, Cardin F, McCormick AP, Wannamethee G, Burroughs AK. A predictive model for failure to control bleeding during acute variceal haemorrhage. J Hepatol. 1999;31(3):443–450. doi: 10.1016/s0168-8278(99)80035-x. [DOI] [PubMed] [Google Scholar]
- 5.Bruckner BA, Blau LN, Rodriguez L, et al. Microporous polysaccharide hemosphere absorbable hemostat use in cardiothoracic surgical procedures. J Cardiothorac Surg. 2014;9(1):134. doi: 10.1186/s13019-014-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu R, Sun W, Zhou H, et al. The performance of a fly-larva shell-derived chitosan sponge as an absorbable surgical hemostatic agent. Biomaterials. 2010;31(6):1270–1277. doi: 10.1016/j.biomaterials.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Balmert SC, Little SR. Biomimetic delivery with micro- and nanoparticles. Adv Mater. 2012;24(28):3757–3778. doi: 10.1002/adma.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watters JM, Van PY, Hamilton GJ, Sambasivan C, Differding JA, Schreiber MA. Advanced hemostatic dressings are not superior to gauze for care under fire scenarios. J Trauma. 2011;70(6):1413–1419. doi: 10.1097/TA.0b013e318216b796. [DOI] [PubMed] [Google Scholar]
- 9.Gabay M. Absorbable hemostatic agents. Am J Health Syst Pharm. 2006;63(13):1244–1253. doi: 10.2146/ajhp060003. [DOI] [PubMed] [Google Scholar]
- 10.Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251(2):217–228. doi: 10.1097/SLA.0b013e3181c3bcca. [DOI] [PubMed] [Google Scholar]
- 11.Ofosu FA, Crean S, Reynolds MW. A safety review of topical bovine thrombin-induced generation of antibodies to bovine proteins. Clin Ther. 2009;31(4):679–691. doi: 10.1016/j.clinthera.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Parrilla JJ, Aznar J, Estelles A. Letter: Fibrinolytic activity in the endometrial adenocarcinoma. Thromb Diath Haemorrh. 1975;34(3):864–866. [PubMed] [Google Scholar]
- 13.Azzam T, Raskin A, Makovitzki A, et al. Cationic polysaccharides for gene delivery. Macromolecules. 2002;35(27):9947–9953. [Google Scholar]
- 14.Ma D, Zhang HB, Chen YY, Lin JT, Zhang LM. New cyclodextrin derivative containing poly(l-lysine) dendrons for gene and drug co-delivery. J Colloid Interface Sci. 2013;405:305–311. doi: 10.1016/j.jcis.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Li N, Luo HC, Yang C, et al. Cationic star-shaped polymer as an siRNA carrier for reducing MMP-9 expression in skin fibroblast cells and promoting wound healing in diabetic rats. Int J Nanomedicine. 2014;9:3377–3387. doi: 10.2147/IJN.S66368. [DOI] [PMC free article] [PubMed] [Google Scholar]