Abstract
Lemongrass oil (LGO) is a volatile oil extracted from the leaves of Cymbopogon citratus that has become one of the most important natural oils in the pharmaceutical industry because of its diverse pharmacologic and clinical effects. However, LGO suffers from low aqueous solubility, which could lead to a reduced effect. Moreover, the instability of its major active constituent, citral, could lead to volatilization, reaction with other formulation ingredients, and consequently, skin irritation. To surmount these problems, this research aims to formulate lemongrass-loaded ethyl cellulose nanosponges with a topical hydrogel with an enhanced antifungal effect and decreased irritation. The minimal inhibitory concentration and minimal fungicidal concentration of LGO against Candida albicans strain ATC 100231, determined using the broth macrodilution method, were found to be 2 and 8 μL/mL, respectively. The emulsion solvent evaporation technique was used for the preparation of the nanosponges. The nanosponge dispersions were then integrated into carbopol hydrogels (0.4%). Nine formulations were prepared based on a 32 full factorial design employing the ethyl cellulose:polyvinyl alcohol ratio and stirring rate as independent variables. The prepared formulations were evaluated for particle size, citral content, and in vitro release. Results revealed that all the nanosponge dispersions were nanosized, with satisfactory citral content and sustained release profiles. Statistical analysis revealed that both ethyl cellulose:polyvinyl alcohol ratio and stirring rate have significant effects on particle size and percentage released after 6 hours; however, the effect of the stirring rate was more prominent on both responses. The selected hydrogel formulation, F9, was subjected to surface morphological investigations, using scanning and transmission electron microscopy, where results showed that the nanosponges possess a spherical uniform shape with a spongy structure, the integrity of which was not affected by integration into the hydrogel. Furthermore, the selected formulation, F9, was tested for skin irritation and antifungal activity against C. albicans, where results confirmed the nonirritancy and the effective antifungal activity of the prepared hydrogel.
Keywords: Cymbopogon Citratus, Citral, Volatile oil, factorial design, ethyl cellulose, Candida albicans, gel
Introduction
Lemongrass (Cymbopogon citratus) is a tall, perennial grass that is planted in almost all tropical and subtropical countries.1,2 The volatile oil obtained from the fresh leaves of the plant is one of the most important essential oils in the food, cosmetics, and pharmaceutical industries. The importance of lemongrass oil (LGO) in the pharmaceutical industry is a result of its various pharmacologic and clinical effects. Among the most prominent effects of LGO are its reported broad-spectrum antibacterial, antifungal, and insect repellant activities when applied topically.3–7 In addition, it is known to have several effects when administered orally, including sedative, antioxidant, and anti-inflammatory activities.7–10 The antimicrobial effect of LGO is attributed to its citral content, which is the major constituent of the oil (more than 75% by weight).11 Citral is a natural mixture of isomeric acyclic monoterpene aldehydes geranial (transcitral) and neral (ciscitral).12 However, both geranial and neral suffer from instabilities resulting from temperature, oxygen, and light that can lead to volatilization, oxidation, or reaction with other formulation ingredients, causing skin irritation.13,14 Moreover, LGO is characterized by low aqueous solubility.14 These drawbacks limit the practical use of LGO in spite of its various beneficial effects and potent antimicrobial activity.
Nanosponges represent a novel class of encapsulating nanoparticles that have emerged recently as a promising delivery system in both the pharmaceutical and the cosmoceutical fields.15 They are sponge-like, virus-sized nanoparticles with an average diameter below 1 μm that possess a noncollapsible structure and a porous surface.15–17 The increased interest in this emerging technology is attributed to the merged advantages of both the microsponges and the nanosize-mediated delivery systems. Because of their porous nature, they are capable of encapsulating a wide variety of active ingredients within their cavities and releasing them in a controlled manner. In addition, their nanometric size increases their ability to enhance the solubility of poorly soluble drugs.18–20 Nanosponges can also be incorporated in topical hydrogels, offering the advantage of reduced irritation, decreased adverse effects, and improved retention onto the skin compared with conventional topical delivery systems.17,21 Therefore, nanosponges can be considered a promising futuristic platform for the delivery of oral and topical pharmaceuticals with the potential to improve the solubility of lipophilic drugs, enhance the stability of readily degradable compounds, and deliver actives in a controlled manner.15,21
Ethyl cellulose is a cellulose derivative in which some of the hydroxyl groups on the glucose units are converted into ethyl ether groups. It is a hydrophobic, nonswellable polymer that is practically insoluble in water but soluble in several organic solvents, including alcohol and methylene chloride. It has been extensively used in microencapsulation.22 Ethyl cellulose has been used successfully by several researchers for the development of topically applied microsponges and nanosponges, with enhanced performance.17,23–25
This present research aimed to develop lemongrass-loaded ethyl cellulose nanosponges with topical hydrogel with an enhanced antifungal effect and decreased irritation, using the emulsion solvent evaporation technique. A 32 full factorial design was applied to investigate the effect of formulation factors on the in vitro release of lemongrass and the particle size of the prepared nanosponges. In addition, the selected formula was tested for skin irritation and in vivo antifungal activity in male albino rats.
Materials and methods
Materials
LGO and ethyl cellulose (EC) were purchased from Sigma Aldrich (Darmstadt, Germany). Polyvinyl alcohol (PVA), Mowiol® 40–88, was purchased from E.I. du-Pont de Nemours Co (Wilmington, DE, USA). Carbopol 940 was purchased from Goodrich Chemical Co (Akron, OH, USA). High-performance liquid chromatography (HPLC)-grade methanol and acetonitrile were obtained from Merck (Darmstadt, Germany). All other reagents and chemicals were of analytical grade.
Determination of minimum inhibitory concentration and minimum fungicidal concentration of LGO
Minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) of LGO were determined using the broth macrodilution method, based on the reference document M27-A3 for yeasts, with modifications.26 Serial twofold dilutions of the LGO in 10% aqueous dimethyl sulfoxide containing 0.5% Tween 80 were prepared to yield concentrations in the range of 512–0.5 μL/mL. Sabourad dextrose agar tubes were inoculated with Candida albicans ATC 100231 suspensions at a concentration of 2.5×103 colony-forming units/mL (CFU/mL). After the addition of the LGO dilutions, the agar tubes were incubated aerobically at 35°C for 48 hours. The MIC, corresponding to the fungistatic activity, was determined to be the lowest concentration of oil that results in 100% inhibition of visible growth of C. albicans. To determine the MFC, 10 μL of the content of each negative tube and the first tube showing visible growth were subcultured on Sabourad agar tubes and reincubated for an additional 48 hours at 35°C. The MFC, corresponding to the fungicidal activity, was determined as the lowest concentration of oil that results in fungal death. Each experiment was performed in duplicate and repeated three times.27–29
Design and preparation of topical hydrogels integrating LGO-loaded nanosponges
LGO-loaded nanosponges were prepared using the emulsion solvent evaporation method.17 Next, 200 μL LGO was dispersed in 10 mL dichloromethane containing a specified amount of EC. The prepared disperse phase was dropped slowly onto a 15 mL aqueous solution containing a specified amount of PVA, with stirring at a constant rate for 30 minutes, using an overhead stirrer (Model RW 20 digital, IKA, Germany). A specified amount of carbopol 940, which was allowed to swell overnight in 5 mL double-distilled water, was then added to the formed nanosponge dispersion with continued stirring at the same rate for an additional 10 minutes to allow the formation of carbopol hydrogels (0.4% w/v) integrating LGO nanosponges. The formed hydrogels were allowed to stand for 15 minutes without stirring to expel the trapped air and were then stored in tightly closed screw-capped glass bottles at 5°C for further investigation.
A 32 full factorial design was applied to investigate the effect of the EC:PVA ratio (X1) and the stirring rate (X2), as independent variables, on the in vitro characteristics of the prepared formulations. EC:PVA ratios of 1:1, 1:2, and 1:3 (w/w) and stirring rates of 6,000, 10,000, and 14,000 rpm were investigated (Table 1). Combinations of the three levels of each variable resulted in the preparation of nine formulations (detailed formulations are outlined in Table 2). Particle size of the nanosponges (Y1) and percentage LGO released after 6 hours from the carbopol hydrogels (Y2) were studied as dependent variables.
Table 1.
Levels and factors used in the applied 32 full factorial experimental design
| Factors | Levels
|
||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1 (ethyl cellulose:polyvinyl alcohol ratio, w:w) | 1:1 | 1:2 | 1:3 |
| X2: stirring rate, rpm | 6,000 | 10,000 | 14,000 |
Abbreviation: w, weight.
Table 2.
Formulations of topical hydrogels integrating lemongrass oil-loaded nanosponges
| Ingredients | Lemongrass oil, μL | Ethyl cellulose:polyvinyl alcohol (w/w) | Carbopol 940, % (w/v) | Dichloromethane, mL | Stirring rate, rpm | Curing time, minutes | Double-distilled water, mL |
|---|---|---|---|---|---|---|---|
| F1 | 200 | 1:1 | 0.4 | 10 | 6,000 | 30 | 20 |
| F2 | 200 | 1:2 | 0.4 | 10 | 6,000 | 30 | 20 |
| F3 | 200 | 1:3 | 0.4 | 10 | 6,000 | 30 | 20 |
| F4 | 200 | 1:1 | 0.4 | 10 | 10,000 | 30 | 20 |
| F5 | 200 | 1:2 | 0.4 | 10 | 10,000 | 30 | 20 |
| F6 | 200 | 1:3 | 0.4 | 10 | 10,000 | 30 | 20 |
| F7 | 200 | 1:1 | 0.4 | 10 | 14,000 | 30 | 20 |
| F8 | 200 | 1:2 | 0.4 | 10 | 14,000 | 30 | 20 |
| F9 | 200 | 1:3 | 0.4 | 10 | 14,000 | 30 | 20 |
Abbreviations: f, formulation; v, volume; w, weight.
Particle size measurements
The mean particle size of the nanosponge dispersion was determined by a dynamic light scattering technique, using Zetatrac (Microtrac, Inc., Montgomeryville, PA, USA), after 20-fold dilution with double-distilled water. Each measurement was performed five times.
Surface morphological studies
The surface structure of the prepared nanosponges was investigated, using a scanning electron microscope (Philips XL30, Eindhoven, the Netherlands). The nanosponge dispersions before the addition of carbopol were centrifuged at 20,000 rpm and 4°C for 30 minutes (Model Z 32 HK, Hermle Labortechnik, Germany), and the excess solvent was decanted. The separated nanosponges were dried in a thermostatically controlled oven at 40°C and coated with gold palladium before investigation.
In addition, the selected formulation F9 comprising nanosponges integrated in carbopol 940 hydrogel was photographed using a transmission electron microscope (Jeol, 100 CX-TEM), after staining with 2% uranyl acid to assess the effect of the integration of the nanosponges into the hydrogel on their structure.
Citral content in the hydrogels incorporating LGO-loaded nanosponges
HPLC quantitative analysis of citral in LGO was performed according to the modified method of Weisheimer et al30 using an HPLC apparatus consisting of an Isocratic pump (model LC-10 AD; Shimadzu, Kyoto, Japan) and a photodiode array detector (SPD-M20A; Shimadzu) connected to a system controller (model CBM-20A; Shimadzu). The analytical column was a reversed phase column (RP18) that was 250 mm in length × 4 mm in internal diameter and had a particle size of 5 μm (Nova-Pak, Waters Associates, USA). The mobile phase consisted of acetonitrile, water, and methanol (50:35:15, v/v). The wavelength was set at 240 nm, and the flow rate was maintained at 1.2 mL/minute. Concentrations of citral in samples were calculated with reference to the developed calibration curve of total peak areas against the nominal citral concentration. The HPLC method was validated in terms of linearity, precision, accuracy, and percentage recovery.
The percentage of oil in the gel was determined by citral content: 250 mg gel was dispersed in 10 mL methanol. The dispersion was sonicated in a water bath sonicator (Ultrasonic Bath Model QS3, Ultrawave Ltd, UK) for 1 hour and then centrifuged at 4,500 rpm and 4°C for 30 minutes (Model Z 32 HK, Hermle Labortechnik, Germany). The supernatant was then filtered through a membrane filter (0.22 μm, Millipore) and analyzed by HPLC quantitative method of assay. The percentage citral incorporated into the gel was calculated as follows:
where CE is the content of citral determined by HPLC analysis (sum of isomers neral and geranial contents) and CTh is the theoretical content of citral.30
In vitro release studies
In vitro release studies of LGO from hydrogels were carried out using a cellulose dialysis bag (molecular weight cutoff=12,000 to 14,000 Da; Sigma-Aldrich Corporation, St Louis, MO, USA). One gram of the prepared hydrogel integrating LGO-loaded nanosponges was placed directly in the dialysis bag, which was then placed in a beaker containing 100 mL phosphate buffer (pH 6.8). The beaker was continuously shaken at 100 strokes/minute in an incubator at 37°C±0.5°C. Aliquots of 2 mL were withdrawn at specified time intervals and replaced by fresh release medium. The oil concentration of the samples was determined using the previously described HPLC quantitative method of assay. All experiments were carried out in triplicate.31
Statistical analysis
The effect of studied variables (EC:PVA ratio, X1; stirring rate, X2) on the response variables (particle size, Y1; percentage LGO released after 6 hours, Y2) was statistically analyzed using Design-Expert® Software Version 7.0.0 (Stat-Ease Inc, Minneapolis, MN, USA). An analysis of variance test was applied to evaluate the level of significance of the independent variables on the response variables, as well as the interaction between them. The relation between the independent variables and the response ones were described using polynomial equations. The design was evaluated by either a linear, two-factor interaction or a quadratic sequential model, and the equation was then determined according to the model selected:
for the linear model,
for two-factor interaction, and
for the quadratic model, where Y is the response variable and X1 and X2 (main effects) are the coded levels of the independent variables that represent the average result of changing one factor at a time from its low to its higher levels.
The interaction term (X1X2) expresses how the response changes on the interaction of the two factors studied. The polynomial terms ( and ) are included to evaluate nonlinearity. b1 is the arithmetic mean response of the nine formulations. The values of b1 and b2 are the estimated coefficients for factors X1 and X2, respectively, and are related to the magnitude of the effect of these variables on the response. A synergistic effect on the response is indicated by a positive sign of the coefficient, whereas an antagonistic effect is indicated by a negative sign.32 The level of statistical significance was set at a 5% confidence level.
Skin irritation test
The irritation effect of the chosen gel formulation, F9, was evaluated by carrying out the Draize patch test on six albino rats.33 The animals’ backs were shaved 24 hours before the application of the formulation, and then 0.5 g gel was spread uniformly on the hair-free skin within an area of 4 cm2. The skin was observed for any visible changes after 24, 48, and 72 hours from the application of the formulation. The mean erythemal scores were graded starting from 0 to 4. According to the degree of erythema, the grades were recorded as follows: no erythema = 0; slight (light pink discoloration of skin) = 1; moderate (dark pink) = 2; more severe (light red) = 3; and most severe (dark red) = 4.34 Formalin and placebo hydrogel formulation were used as positive and negative controls, respectively.
Antifungal activity
Male albino rats weighing 150–180 g were housed in individual cages and allowed to receive food and water ad libitum. Animals were subjected to an intraperitoneal injection of cyclophosphamide (100 mg/kg, body weight) for 3 days before the fungal infection to suppress their immunity, with the aim of achieving a heavy cutaneous infection. A working culture of C. albicans (C. albicans, ATCC 10231) grown for 48 hours at 30°C on Sabouraud dextrose agar was used to induce the infection. The grown cells were collected, washed, and resuspended in sterile saline to a final concentration of 107 CFU/mL. Animals were divided into two groups, each comprising twelve rats. An area of 3 cm2 was marked on each animal’s shaved back and gently rubbed with the candida suspension, using a sterile swab to cause infection. An occlusive dressing was applied to the infected area, fixed in place by a sterile adhesive bandage, and kept covered for 48 hours to allow for the flaring of infection. Animals in Group I did not receive any treatment (control group). Animals in Group II received treatment by applying the selected hydrogel formulation, F9, topically for 3 consecutive days (treated group). Six animals from each group were killed 48 hours after the last treatment, and the remaining six animals from each group were killed 96 hours after the last treatment. The infected skin was excised, and samples were washed, placed into Sabourad dextrose agar culture media, and incubated for 48 hours at 37°C±1°C. The CFU values were then recorded. All the results were expressed as mean ± standard deviation. The treated group was compared with the control group by analysis of variance at P<0.05.34
Results and discussion
MIC and MFC of LGO
LGO showed significant antifungal activity against C. albicans strain ATC 100231. The MFC was markedly higher than the MIC. The values of the MIC and the MFC were 2 and 8 μL/mL, respectively. The determined values of the MIC and MFC were lower than the previously reported ones for LGO.35 The difference probably could be a result of using different sources of the oil. The fungistatic and fungicidal activity of LGO is attributed to its citral content (geranial and neral isomers).6,11
Particle size
The mean particle size of the prepared nanosponges is presented in Table 3. The mean particle size ranged from 293±0.044 to 906±0.056 nm. All the nanosponge formulations showed particle sizes in the nano range (<1 μm). Statistical analysis revealed that the sequential model was suggested to be linear, indicating that the interaction between the two independent variables on the nanosponge particle size was insignificant. The R-squared statistic indicates that the model, as fitted, explains 94.53% of the variability in the responses. The response surface plot of the effects of the EC:PVA ratio (X1) and the stirring rate (X2) on the particle size (Y1) is illustrated in Figure 1. The linear equation relating the particle size (Y1) to the EC:PVA ratio (X1) and the stirring rate (X2) in terms of coded values is presented by:
| (1) |
Table 3.
In vitro evaluation of lemongrass oil-loaded nanosponges topical hydrogels
| Formulation | Particle size, nm* | Citral content, %† | Percentage lemongrass oil released after 6 hours, %† |
|---|---|---|---|
| F1 | 660±0.059 | 95.39±2.83 | 97.51±0.06 |
| F2 | 832±0.059 | 94.34±4.74 | 88.58±0.57 |
| F3 | 906±0.056 | 95.85±2.10 | 89.12±1.84 |
| F4 | 513±0.019 | 94.14±3.22 | 97.12±0.06 |
| F5 | 525±0.110 | 95.32±5.69 | 79.99±0.03 |
| F6 | 539±0.190 | 95.66±5.25 | 36.12±0.03 |
| F7 | 293±0.044 | 94.73±4.35 | 54.14±1.52 |
| F8 | 353±0.041 | 96.22±2.99 | 39.32±1.28 |
| F9 | 410±0.080 | 98.40±3.00 | 26.25±0.39 |
Notes:
Values are expressed as mean ± SD (n=5).
Values are expressed as mean ± SD (n=3).
Abbreviation: f, formulation.
Figure 1.
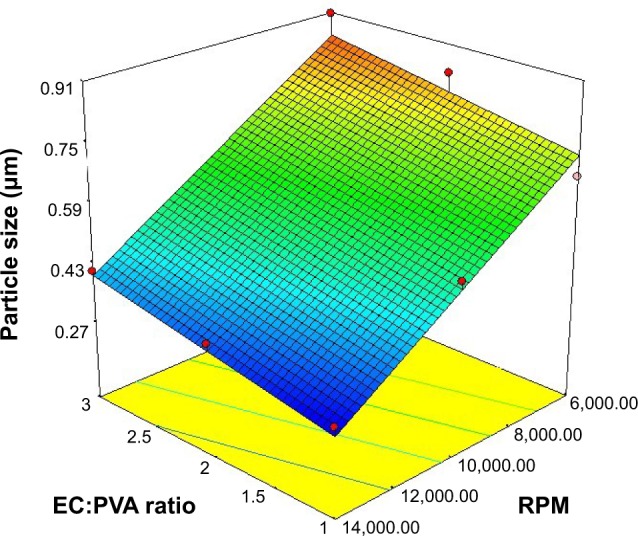
Response surface plot of the effect of ethyl cellulose (EC):polyvinyl alcohol (PVA) ratio (X1) and stirring rate (X2) on the particle size of the prepared nanosponges (Y1).
The observed increase in the particle size of the nanosponges with the increase in the EC:PVA ratio, evidenced by the positive coefficient of the term X1, was found to be statistically significant (P=0.0301). Increasing the surfactant concentration resulted in foaming that could lead to formation of aggregates.36
In contrast, the increase in the stirring rate was found to have a statistically significant negative effect on the particle size of the nanosponges (P<0.0001); that is, increasing the stirring rate (X2) was accompanied by a significant reduction in the particle size. The reduced particle size at higher stirring rates could be ascribed to the increased mechanical shear that could lead to the formation of finer emulsion globules and, as a consequence, smaller nanosponges. Conversely, at lower stirring rates, the tendency of the formed emulsion globules to coalescence and aggregate is increased, leading to increased particle size.24,37 The statistical analysis revealed that the stirring rate had a more pronounced effect on the particle size, as evidenced by its lower P-value and the relatively larger coefficient of the term X2 in Equation 1.
Surface morphology
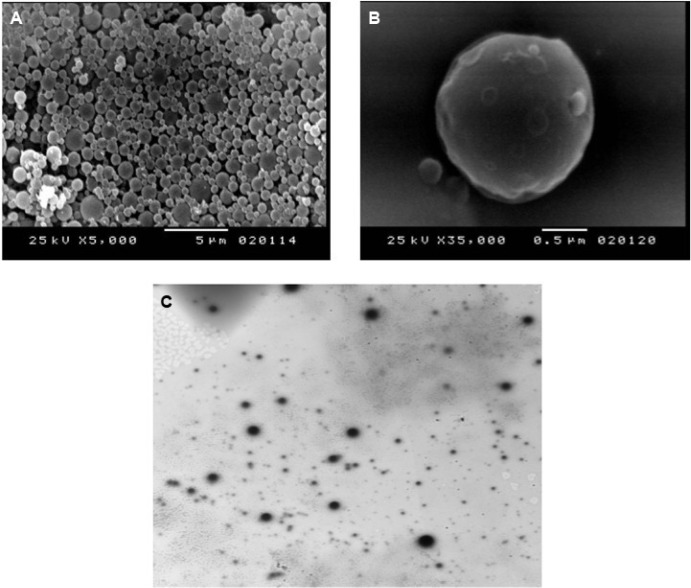
The surface topography of the nanosponges was studied by scanning electron microscopy. In addition, the morphology of the selected nanosponge formulation integrated in the carbopol 940 hydrogel, F9, was studied using transmission electron microscopy. Representative scanning electron microscopy and transmission electron microscopy photographs are shown in Figure 2. It is evident that the prepared nanosponges possess a spherical uniform shape with a spongy structure. The presence of the fine orifices on the surface could be caused by the diffusion of the dichloromethane from the surface of the formed nanoparticles during preparation.38 Transmission electron microscopy confirms that the integration of the nanosponges into the hydrogel did not affect the integrity of the formed nanosponges.
Figure 2.
Morphological studies of the selected formulation F9.
Notes: (A) Scanning electron photograph of dried nanosponges (×5,000). (B) Scanning electron photograph of dried nanosponges (×35,000). (C) Transmission electron photograph of hydrogel integrating lemongrass oil-loaded nanosponges (×2,500).
Abbreviation: f, formulation.
Citral content
The HPLC method, used for determination of citral content, was validated. A good linear relationship was observed between LGO concentrations and peak areas in the range of 0.002–0.016 μL/mL, with a correlation coefficient (r) of 0.998. The lower limit of detection and lower limit of quantification were found to be 0.0004 and 0.001 μL/mL, respectively. The method was evaluated for specificity, where none of the excipients showed interference at the detection wavelength. The method showed acceptable precision, with a relative standard deviation for the intraday and interday assay of less than 2%. In addition, the mean percentage recovery was found to be 97.81%±0.86%, indicating accuracy of the method. All the prepared formulations showed satisfactory total citral content (neral and geranial isomers), ranging from 94.14%±3.22% to 98.40%±3.00%.
In vitro release
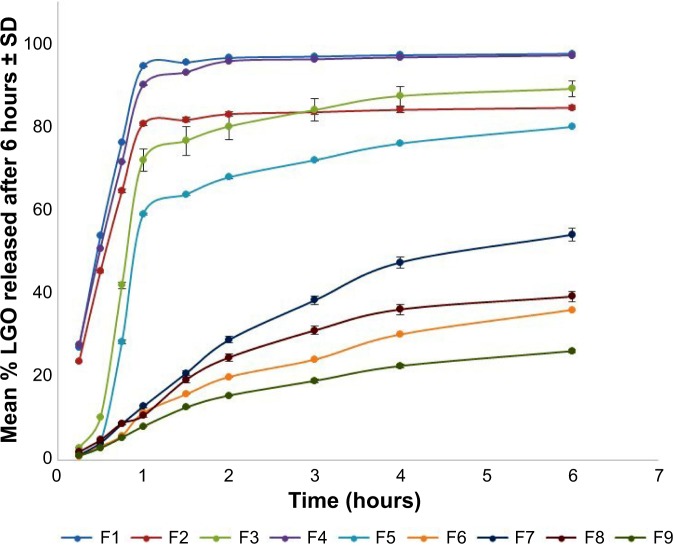
The in vitro release profiles of LGO from carbopol hydrogels integrating nanosponges showed marked variations among different formulations, as illustrated in Figure 3. The percentage of drug released after 6 hours ranged from 26.25%±0.39% (F9) to 97.51%±0.06% (F1). Statistical analysis was performed using analysis of variance to compare LGO release from different formulations and to assess the significance of varying EC:PVA ratio and/or the stirring rate on the percentage LGO released after 6 hours. The response surface plot of the effect of EC:PVA ratio (X1) and the stirring rate (X2) on the percentage LGO released after 6 hours is presented in Figure 4. The suggested sequential model was linear, rather than two-factor interaction or polynomial, indicating the absence of interaction between the two factors. The linear equation relating the percentage LGO released after 6 hours (Y2) to the investigated independent variables in terms of coded values is presented by:
| (2) |
Figure 3.
In vitro release profiles of lemongrass oil (LGO) from topical hydrogels integrating LGO-loaded nanosponges in phosphate buffer (pH 6.8) at 37°C±0.5°C.
Note: Results are presented as mean ± SD (n=3).
Abbreviations: f, formulation; SD, standard deviation.
Figure 4.
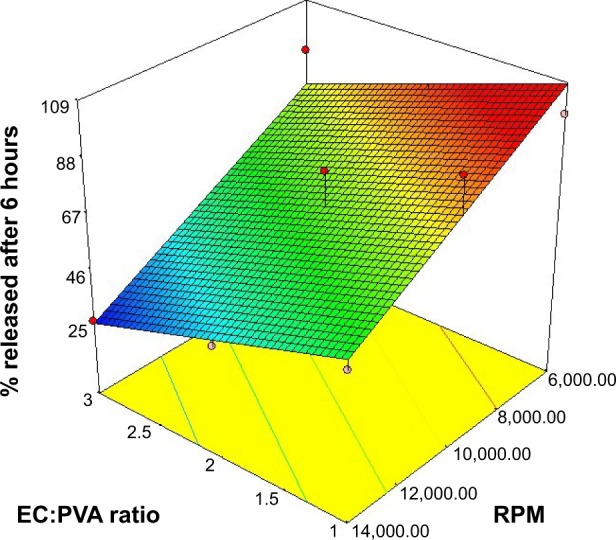
Response surface plot of the effect of ethyl cellulose (EC):polyvinyl alcohol ratio (PVA), (X1) and stirring rate (X2) on the mean percentage of lemongrass oil released after 6 hours (Y2).
The negative coefficient of both the terms X1 and X2 indicates that increasing both the EC:PVA ratio and the stirring rate had a negative effect on the percentage LGO released after 6 hours (Y2). The observed effects were found to be significant at P≤0.05. The significant decrease in the percentage LGO released after 6 hours with the increase of the EC:PVA ratio could be due to the increase in the thickness of the nanosponges’ matrix wall. This could lead to a longer diffusional path, and consequently reduced LGO release rates.38,39 Moreover, the decrease in the percentage LGO released after 6 hours with increasing the stirring rate might be explained on the basis of decreased porosity of the nanosponges formed at higher stirring rates, as evidenced by the scanning electron microscopy photographs (representative samples shown in Figure 2). According to Mateovic et al40 larger microspheres exhibit higher porosity and higher release rates. In our work, the increase in the stirring rate is accompanied by a reduction in the nanosponges’ size. Thus, this increase might be accompanied by decreased porosity and increased entrapment of oil within the hydrophilic matrix, with the possible consequence of decreased release rates. Moreover, the higher porosity of the larger particles would allow for the leakage of the volatile oil to the hydrogel during preparation, resulting in faster release rates. It is worthy to note that because of the plastic and hydrophobic nature of EC, drug particles near the surface of the matrix could be initially released into the surrounding medium, resulting in the increase in the pores and thus facilitating further drug release and the initial burst effect.41
Skin irritation
The scores for erythema were recorded for all rats at 24, 48, and 72 hours. The mean erythemal score was found to be 0.00, indicating that the developed formulation F9 showed no erythema or edema on the shaved rats’ skin.
In vivo antifungal activity
The antifungal effect of the selected formulation, F9, was determined using C. albicans (Figure 5). All the rats in the study showed well-defined infection in the range of 3.48–3.56 log10 CFU/mL before the application of the formulation (day 0). Both groups of animals showed an insignificant decrease in the CFU/mL values after 2 days of treatment compared with in day 0. However, after 3 consecutive days of treatment, the treated group showed a marked significant decrease in C. albicans count compared with the control group killed on the same day (P<0.05). The observed ability of the selected formulation to reduce the C. albicans burden in the skin could be considered as evidence for its antifungal effectiveness in the oil concentration used in the hydrogel. This is probably because of the possible penetration and accumulation of the nanoparticles loaded with the oil in the skin layers. As a consequence, this could result in prolonged and enhanced antifungal action of the oil.34
Figure 5.
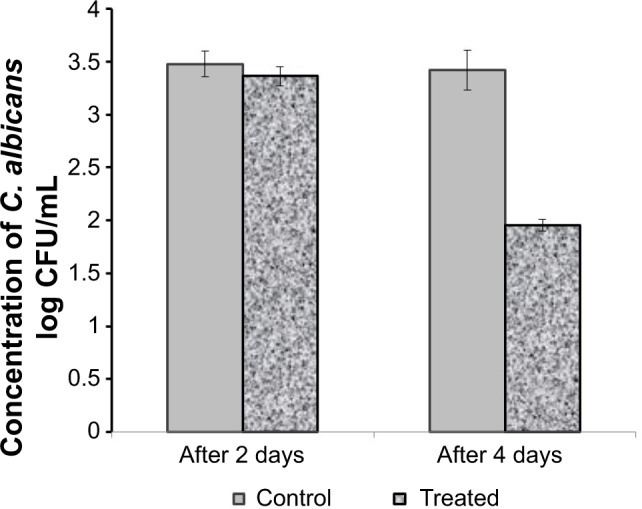
Antifungal activity of the selected hydrogel formulation integrating lemongrass oil-loaded nanosponges; F9 after 2 and 4 days of treatment.
Note: Values are expressed as mean ± standard deviation (n=6).
Abbreviation: f, formulation.
Conclusion
LGO was successfully incorporated in an EC nanosponge, using the emulsion solvent evaporation method, followed by their integration into carbopol hydrogel. Among the nine prepared hydrogels integrating lemongrass-loaded nanosponges, The F9 formulation was chosen for further study on the basis of its particle size and controlled release profile. F9 was subjected to surface investigation, using scanning and transmission electron microscopy, which revealed the spongy structure with minute pores and the sustained integrity of the nanosponge structure when incorporated in the hydrogel. The selected formula showed no skin irritation and in vivo antifungal activity in male albino rats. These results could be promising with respect to the practical application of the incorporation of LGO in pharmaceutical formulations with the benefit of decreasing the hazards of its use in folk medicine in the crude form.
Acknowledgments
This Project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under the grant no. (1433/249/61). The authors, therefore, acknowledge with thanks DSR technical and financial support.
The authors also express their gratitude to Nayera Moneib, Professor of Microbiology, and Abeer Hanafy, Associate Professor of Pharmacology for their sincere help.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cheel J, Theoduloz C, Rodríguez J, Schmeda-Hirschmann G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf.) J Agric Food Chem. 2005;53(7):2511–2517. doi: 10.1021/jf0479766. [DOI] [PubMed] [Google Scholar]
- 2.Campos J, Schmeda-Hirschmann G, Leiva E, et al. Lemon grass (Cymbopogon citratus (DC) Stapf) polyphenols protect human umbilical vein endothelial cell (HUVECs) from oxidative damage induced by high glucose, hydrogen peroxide and oxidised low-density lipoprotein. Food Chem. 2014;151:175–181. doi: 10.1016/j.foodchem.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Khan MS, Ahmad I. In vitro antifungal, anti-elastase and anti-keratinase activity of essential oils of Cinnamomum-, Syzygium- and Cymbopogon-species against Aspergillus fumigatus and Trichophyton rubrum. Phytomedicine. 2011;19(1):48–55. doi: 10.1016/j.phymed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Carmo ES, Pereira FO, Cavalcante NM, Gayoso CW, Lima EO. Treatment of pityriasis versicolor with topical application of essential oil of Cymbopogon citratus (DC) Stapf – therapeutic pilot study. An Bras Dermatol. 2013;88(3):381–385. doi: 10.1590/abd1806-4841.20131800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar P, Mishra S, Malik A, Satya S. Housefly (Musca domestica L.) control potential of Cymbopogon citratus Stapf. (Poales: Poaceae) essential oil and monoterpenes (citral and 1,8-cineole) Parasitol Res. 2013;112(1):69–76. doi: 10.1007/s00436-012-3105-5. [DOI] [PubMed] [Google Scholar]
- 6.Tadtong S, Watthanachaiyingcharoen R, Kamkaen N. Antimicrobial constituents and synergism effect of the essential oils from Cymbopogon citratus and Alpinia galanga. Nat Prod Commun. 2014;9(2):277–280. [PubMed] [Google Scholar]
- 7.Blanco MM, Costa CA, Freire AO, Santos JG, Jr, Costa M. Neurobehavioral effect of essential oil of Cymbopogon citratus in mice. Phytomedicine. 2009;16(2–3):265–270. doi: 10.1016/j.phymed.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Francisco V, Costa G, Figueirinha A, et al. Anti-inflammatory activity of Cymbopogon citratus leaves infusion via proteasome and nuclear factor-κB pathway inhibition: contribution of chlorogenic acid. J Ethnopharmacol. 2013;148(1):126–134. doi: 10.1016/j.jep.2013.03.077. [DOI] [PubMed] [Google Scholar]
- 9.Gbenou JD, Ahounou JF, Akakpo HB, et al. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Mol Biol Rep. 2013;40(2):1127–1134. doi: 10.1007/s11033-012-2155-1. [DOI] [PubMed] [Google Scholar]
- 10.Thangam R, Suresh V, Kannan S. Optimized extraction of polysaccharides from Cymbopogon citratus and its biological activities. Int J Biol Macromol. 2014;65:415–423. doi: 10.1016/j.ijbiomac.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Rauber CS, Guterres SS, Schapoval EE. LC determination of citral in Cymbopogon citratus volatile oil. J Pharm Biomed Anal. 2005;37(3):597–601. doi: 10.1016/j.jpba.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Weisheimer V, Miron D, Silva CB, Guterres SS, Schapoval EE. Microparticles containing lemongrass volatile oil: preparation, characterization and thermal stability. Pharmazie. 2010;65(12):885–890. [PubMed] [Google Scholar]
- 13.Nur Ain, AH Farah, Diyana MH, Zaibunnisa AH. Encapsulation of lemongrass (cymbopogon citratus) oleoresin with β-cyclodextrin: phase solubility study and its characterization; Proceedings of the 2nd International Conference on Biotechnology and Food Science; 2011 Apr 1–3; Bali Island, Indonesia. Singapore: LACSIT Press; 2011. [Google Scholar]
- 14.Rungsardthong Ruktanonchai U, Srinuanchai W, Saesoo S, Sramala I, Puttipipatkhachorn S, Soottitantawat A. Encapsulation of citral isomers in extracted lemongrass oil with cyclodextrins: molecular modeling and physicochemical characterizations. Biosci Biotechnol Biochem. 2011;75(12):2340–2345. doi: 10.1271/bbb.110523. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian S, Singireddy A, Krishnamoorthy K, Rajappan M. Nanosponges: a novel class of drug delivery system – review. J Pharm Pharmaceut Sci. 2012;15(1):103–111. doi: 10.18433/j3k308. [DOI] [PubMed] [Google Scholar]
- 16.Cavalli R, Trotta F, Tumiatti W. Cyclodextrin-based Nanosponges for Drug Delivery. J Incl Phenom Macrocycl Chem. 2006;56(1–2):209–213. [Google Scholar]
- 17.Sharma R, Pathak K. Polymeric nanosponges as an alternative carrier for improved retention of econazole nitrate onto the skin through topical hydrogel formulation. Pharm Dev Technol. 2011;16(4):367–376. doi: 10.3109/10837451003739289. [DOI] [PubMed] [Google Scholar]
- 18.Aldawsari H, Badr-Eldin SM. Microsponges as promising vehicle for drug delivery and targeting: preparation, characterization and applications. African J Pharm Pharmacol. 2013;7(17):873–881. [Google Scholar]
- 19.Ahmed RZ, Patil G, Zaheer Z. Nanosponges – a completely new nano-horizon: pharmaceutical applications and recent advances. Drug Dev Ind Pharm. 2013;39(9):1263–1272. doi: 10.3109/03639045.2012.694610. [DOI] [PubMed] [Google Scholar]
- 20.Gangadharappa HV, Gupta NV, Prasad MSC, Shivakumar HG. Current trends in microsponge drug delivery system. Curr Drug Deliv. 2013;10(4):453–465. doi: 10.2174/1567201811310040010. [DOI] [PubMed] [Google Scholar]
- 21.Minelli R, Cavalli R, Ellis L, et al. Nanosponge-encapsulated camptothecin exerts anti-tumor activity in human prostate cancer cells. Eur J Pharm Sci. 2012;47(4):686–694. doi: 10.1016/j.ejps.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Murtaza G. Ethylcellulose microparticles: a review. Acta Pol Pharm. 2012;69(1):11–22. [PubMed] [Google Scholar]
- 23.Jelvehgari M, Siahi-Shadbad MR, Azarmi S, Martin GP, Nokhodchi A. The microsponge delivery system of benzoyl peroxide: preparation, characterization and release studies. Int J Pharm. 2006;308(1–2):124–132. doi: 10.1016/j.ijpharm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Amrutiya N, Bajaj A, Madan M. Development of microsponges for topical delivery of mupirocin. AAPS Pharm Sci Tech. 2009;10(2):402–409. doi: 10.1208/s12249-009-9220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiti S, Kaity S, Ray S, Sa B. Development and evaluation of xanthan gum-facilitated ethyl cellulose microsponges for controlled percutaneous delivery of diclofenac sodium. Acta Pharm. 2011;61(3):257–270. doi: 10.2478/v10007-011-0022-6. [DOI] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute . Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. (CLSI document M27-A3). [Google Scholar]
- 27.Pinto E, Pina-Vaz C, Salgueiro L, et al. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J Med Microbiol. 2006;55(Pt 10):1367–1373. doi: 10.1099/jmm.0.46443-0. [DOI] [PubMed] [Google Scholar]
- 28.Pinto E, Hrimpeng K, Lopes G, et al. Antifungal activity of Ferulago capillaris essential oil against Candida, Cryptococcus, Aspergillus and dermatophyte species. Eur J Clin Microbiol Infect Dis. 2013;32(10):1311–1320. doi: 10.1007/s10096-013-1881-1. [DOI] [PubMed] [Google Scholar]
- 29.Bouzabata A, Bazzali O, Cabral C, et al. New compounds, chemical composition, antifungal activity and cytotoxicity of the essential oil from Myrtus nivellei Batt. and Trab., an endemic species of Central Sahara. J Ethnopharmacol. 2013;149(3):613–620. doi: 10.1016/j.jep.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 30.Weisheimer V, Miron D, Silva CB, Guterres SS, Schapoval EE. Microparticles containing lemongrass volatile oil: preparation, characterization and thermal stability. Pharmazie. 2010;65(12):885–890. [PubMed] [Google Scholar]
- 31.Hoffmeister CR, Durli TL, Schaffazick SR, et al. Hydrogels containing redispersible spray-dried melatonin-loaded nanocapsules: a formulation for transdermal-controlled delivery. Nanoscale Res Lett. 2012;7(1):251. doi: 10.1186/1556-276X-7-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fahmy RH, Badr-Eldin SM. Novel delivery approach for ketotifen fumarate: dissofilms formulation using 32 experimental design: in vitro/in vivo evaluation. Pharm Dev Technol. 2014;19(5):521–530. doi: 10.3109/10837450.2013.800108. [DOI] [PubMed] [Google Scholar]
- 33.Shah KA, Date AA, Joshi MD, Patravale VB. Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. Int J Pharm. 2007;345(1–2):163–171. doi: 10.1016/j.ijpharm.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 34.Gupta M, Vyas SP. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem Phys Lipids. 2012;165(4):454–461. doi: 10.1016/j.chemphyslip.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Leite MCA, Bezerra APB, de Sousa JP, Guerra FQS, Lima EO. Evaluation of Antifungal Activity and Mechanism of Action of Citral against Candida albicans. Evidence-Based Complement Altern Med. 2014;2014:378280. doi: 10.1155/2014/378280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivas P, Sreeja K. Formulation and Evaluation of Voriconazole Loaded Nanosponges for Oral and Topical Delivery. Int J Drug Dev Res. 2013;5(1):55–69. [Google Scholar]
- 37.Nokhodchi A, Jelvehgari M, Siahi MR, Mozafari MR. Factors affecting the morphology of benzoyl peroxide microsponges. Micron. 2007;38(8):834–840. doi: 10.1016/j.micron.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Nokhodchi A, Jelvehgari M, Siahi MR, Dastmalchi S. The effect of formulation type on the release of benzoyl peroxide from microsponges. Iranian J Pharm Sci. 2005;1(3):131–142. [Google Scholar]
- 39.Comoğlu T, Gönül N, Baykara T. Preparation and in vitro evaluation of modified release ketoprofen microsponges. Farmaco. 2003;58(2):101–106. doi: 10.1016/s0014-827x(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 40.Mateovic T, Kriznar B, Bogataj M, Mrhar A. The influence of stirring rate on biopharmaceutical properties of Eudragit RS microspheres. J Microencapsul. 2002;19(1):29–36. doi: 10.1080/02652040010055289. [DOI] [PubMed] [Google Scholar]
- 41.Lekshmi UMD, Poovi G, Reddy PN. In vitro observation of repaglinide engineered polymeric nanoparticles. Digest J Nanomat Biostructures. 2012;7(1):1–18. [Google Scholar]