Abstract
Recent research has shown that experiencing events that represent a significant threat to social bonds activates a network of brain areas associated with the sensory-discriminative aspects of pain. In the present study, we investigated whether the same brain areas are involved when witnessing social exclusion threats experienced by others. Using a within-subject design, we show that an ecologically valid experience of social exclusion recruits areas coding the somatosensory components of physical pain (posterior insular cortex and secondary somatosensory cortex). Furthermore, we show that this pattern of activation not only holds for directly experienced social pain, but also during empathy for social pain. Finally, we report that subgenual cingulate cortex is the only brain area conjointly active during empathy for physical and social pain. This supports recent theories that affective processing and homeostatic regulation are at the core of empathic responses.
Keywords: social exclusion, physical pain, empathy, fMRI, somatosensory cortex
INTRODUCTION
Two dimensions of pain
Pain is a fundamental sensory and affective state that informs us about the relevance of incoming external/internal signals and guides our behavior toward the maintenance of our own welfare and survival (Perl, 2007). Evolutionarily speaking, an efficient detection system of this state (for self and others) has developed in order to prioritize escape, recovery and healing (Williams, 2002). It is well known that a nociceptive stimulus applied to the body activates a broad network of brain areas usually referred to as the ‘pain matrix’ (Iannetti and Mouraux, 2010), which consists of two distinct yet interacting parts: one coding for the sensory-discriminate features of the stimulus (location, intensity and duration) and the other coding for the affective-motivational component of the painful experience (unpleasantness, negative affect; Davis, 2000; Peyron et al., 2000). While the former involves mainly the primary and secondary somatosensory cortex (SI, SII), and the posterior insula (pINS), the latter is mainly represented in the anterior insula (aINS) and the anterior-mid part of the cingulate cortex (aMCC/pACC nomenclature according to Vogt, 2005).
Far from having only a ‘physical’ dimension, pain is also an experience that can occur without direct somatic stimulation. Probably we all are familiar with unpleasant situations after which we feel ‘hurt’ or ‘in pain’ even if we were not physically harmed. This kind of pain, which in the field of social psychology has been referred to as ‘social pain’, is instantiated by events that represent a threat to social relationships (e.g. bereavement, relationship break-up, and exclusion from social activities) and to the attachment system in general (Bowlby, 1999). The use of ‘physical’ terms in everyday language to describe the feelings related to painful experiences provides a clue of the strong similarities between physical and social pain (see Eisenberger (2012) for a review).
In the case of social pain, which has mostly been studied by eliciting feelings of exclusion during interactive games (Williams et al., 2000), cerebral activations have been predominantly found in the affective part of the pain matrix (aINS, aMCC, pACC, extending to the more ventral section of the cingulate cortex; Eisenberger et al., 2003; Dewall et al., 2010; Bolling et al., 2011). This suggests that the negative emotional state induced by pain of a social nature does not necessarily involve the activation of the sensory-discriminative part; therefore, excluding one of the hallmarks of the neural response to physically induced pain.
Common substrates for physical and social pain
However, the comparison of neural activations triggered by these two types of pain has so far mainly been based on independent investigations, which either assessed physical or social pain. Therefore, it remains an open question what neural mechanisms they share. One way to overcome this limitation is to measure neuronal and behavioral responses in the same individuals when undergoing the two types of pain.
To date, only one study has addressed this issue by using a within-subjects design. Kross et al. (2011) observed neural responses in participants undergoing both physical painful stimulation and social threat. In the social pain task, they were exposed to photos of ex-partners with whom participants had recently experienced an unwanted breakup. Results showed that the neural activity related to the two tasks overlapped not only in the part of the pain network coding for the affective-motivational component of pain (i.e. aMCC and aINS), but also in the dorsal part of the posterior insula (dpINS) and in the parietal operculum (SII), which are areas associated with the sensory-discriminative component of pain. The authors concluded that when social pain is powerfully elicited, it is capable of activating areas that so far were linked only to painful physical experiences.
However, the experience of an unwanted break-up is a rather singular and complex event carrying a multitude of emotional and cognitive consequences. It thus remains to be shown whether everyday experiences of social exclusion activate areas associated with the somatosensory component of physical pain as well. Notably, previous fMRI studies on social exclusion have relied on the Cyberball task (Williams et al., 2000), in which participants supposedly interact with other players in a virtual ball tossing game, indicated on screen by schematic depictions of these players. It might be argued that this setup is not naturalistic enough to induce strong and ecologically valid feelings of exclusion, due to its computer-game-like appearance. Indeed, previous studies have shown that distinct neural substrates are recruited for perception and representation of real and virtual agents (e.g. cartoons), with the former more capable of allowing mental inferences about others’ states and intentions (Han et al., 2005; Mar et al., 2007). These and other findings have recently called on researchers to shift to more ecological paradigms to better approximate real-life social interactions (Kingstone et al., 2008; Risko et al., 2012). In the present study, we therefore developed a version of the Cyberball game by displaying videos of real players tossing the ball to participants, or deliberately excluding them.
Empathy for physical pain and empathy for social pain
The experience of pain has a fundamental role not only for the protection and the survival of the organism, but also for the social relationship among human beings. In fact, part of the nervous system has evolved to detect pain in other individuals, recognize their emotional state and produce behavioral responses appropriate for the social context (Decety, 2011). Given its relevance, in the past few years, functional neuroimaging studies have been mainly focusing on the observation of physical pain inflicted on others in order to provide insights into the mechanisms by which empathy is implemented in the nervous system (de Vignemont and Singer, 2006; Decety and Lamm, 2006; Bastiaansen et al., 2009; Singer and Lamm, 2009; Zaki and Ochsner, 2012).
While the neural underpinnings of empathy for physical and social pain have been extensively explored separately (Singer et al., 2004; Jackson et al., 2005; Lamm et al., 2011, for physical pain, Beeney et al., 2011; Masten et al., 2011b; Meyer et al., 2012, for social pain), it remains unclear to which extent the two experiences share common neural substrates. The most consistent finding of these studies is that empathy for physical pain recruits a core network consisting of aINS and aMCC (Lamm et al. (2011) for a recent meta-analysis). These brain structures jointly seem to be engaged in the representation of emotional states, and in the behavioral and autonomic nervous system regulation required by these states. Hence, it has been suggested that some sort of ‘embodied simulation’ lies at the root of empathizing with the painful experiences of others, that mainly entail the reactivation of the emotional aspects related to the painful experience (Singer and Lamm, 2009), but under some specific circumstances also the sensorial component (Avenanti et al., 2005; Hein and Singer, 2008; Keysers et al., 2010).
Conversely, witnessing another person suffering from pain of a social nature results in the activation of what has been referred to as the ‘mentalizing network’ (Mitchell et al., 2005; Amodio and Frith, 2006; Frith and Frith, 2006), but not of the pain network—unless the target of the social exclusion is a person affectively close to the observer, which has been shown to activate the affective-motivational component of the pain network (i.e. MCC and mid-INS; Masten et al., 2011b; Meyer et al., 2012).
One possible interpretation of this distinction between empathy for physical vs social pain is that while the vicarious experience of physical pain relies on low-level, automatic processes that are easily and automatically activated by means of bottom-up processes such as perception-for-action coupling mechanisms (Preston and de Waal, 2002; Decety and Lamm, 2006), witnessing another person suffering from social pain may require more abstract types of reasoning due to the less aversive and less directly perceivable nature of the social stimulus itself. This will more likely require a deliberate effort of understanding the mental state of the other person rather than triggering a direct affective resonance with her (Eisenberger, 2012).
It is however also possible that the experimental paradigms that have been used so far were not particularly effective in inducing sufficiently strong empathic responses for social pain, and that the observed differences between the vicarious experiences of physical and social pain are due to differences in the intensity and ecological validity of empathic experiences. In order to avoid this shortcoming, we developed a more realistic and ecologically valid version of the classical social pain paradigm (Cyberball), to address two main questions.
Aims of the study
First, in light of the results obtained by Kross and colleagues, we aimed at exploring to what extent first person experiences of physical and social pain overlap. Secondly, in addition to what has been reported by Kross and colleagues, we explored commonalities and differences related to the vicarious experience of physical and social pain.
To achieve these aims, we used a within-subjects design in which brain and behavioral responses of female participants were observed during a physical pain task and a social pain task, both including a condition in which the participant was the target of the painful experience (hitherto ‘self’) and a condition in which she was witnessing another person being in pain (hitherto ‘other’). We hypothesized that the vicarious and first-hand experiences of social exclusion share hemodynamic activity in regions of the brain devoted to the processing of the affective-motivational aspects of pain and that it could extend to the activation of somatosensory areas, usually associated with processing of pain of physical nature, regardless the target of the social exclusion.
METHODS
Subjects
A total of 23 female participants took part in the fMRI experiment. Female participants of the same age range were recruited to act as confederates in the experiment. Confederates were previously informed about the study and instructed to act as real participants, outside the scanner room. The mean age of the participants was 22.4 years (s.d. = 2.0, range = 20–28). All participants gave informed consent and the study was approved by the Ethics Committee of ‘Santa Maria della Misericordia’, Udine, Italy. Instructions about the experiment were provided to the participant and the confederate simultaneously to ensure that the participant believed that the confederate would also partake in the experiment. General empathic traits and alexithymic traits were measured with self-report questionnaires (the Interpersonal Reactivity Index; Davis (1980) and the Bermond-Vorst Alexithymia Questionnaire; Vorst and Bermond (2001)).
fMRI design
The study consisted of two sessions entailing two runs each, performed on the same day. In one session, participants performed the physical pain task and in the other session, the social pain task. Both sessions included a ‘self’ and ‘other’ condition. The order of the two sessions was counterbalanced across participants. Therefore, the tasks were organized in a 2 × 2 × 2 within-subjects factorial design, with the factors TARGET (self and other), TYPE of pain (physical and social) and INTENSITY of pain (pain and no-pain). In order to increase the ecological validity of the empathy sessions, participants were paired with a real person (confederate) as the target of the ‘other’ condition (see Singer et al., 2004).
Physical pain task
Stimulus set and apparatus
Electrical pain stimuli were delivered by a bipolar concentric surface electrode (stimulation area: 20 mm2), which depolarizes predominantly Aδ-fibers, applied on the back of the participants’ left hand. We delivered a 100-Hz train of electrical pulses of 2 ms pulse duration (square pulse waveform) for 1 s via a direct current stimulator (Digitimer Electronics, model DS7, Hertfordshire, UK). Current amplitude was delivered in a range from 0.1 to 2.0 mA, with steps of 0.1 mA.
Experimental paradigm
The experimental paradigm (based on Singer et al., 2004) consisted of two parts: in the first, participant’s and confederate’s pain thresholds were determined and in the second, the participant entered the scanner and the actual experiment took place. During the pain thresholds determination, participant and confederate had to judge the painfulness of each received stimulus, using a 10-point intensity ratings scale (0 = ‘don’t feel anything’, 1 = ‘can feel something but not painful’, 2 = ‘mildly painful’, 8 = ‘maximum tolerable pain’, 10 = ‘worst imaginable pain’). The intensities of the stimulations that the participant and confederate rated as 1 and 8 were noted and then used as stimuli for the ‘no-pain’ and ‘pain’ conditions, respectively.
During the fMRI experiment, visual stimuli were presented via goggles connected to the workstation in the MRI console room. Visual stimuli consisted of colored arrows pointing either to participant’s hand or away from it. The color of the arrow was an indicator of the target and intensity of the stimulation: dark blue and light blue for, respectively, painful stimulation (self pain) and non-painful stimulation (self no-pain), delivered to the participant in the scanner, while dark pink and light pink for, respectively, painful stimulation (other pain) and non-painful stimulation (other no-pain), delivered to the confederate in the MRI console room. In reality, the confederate did not receive any stimulation.
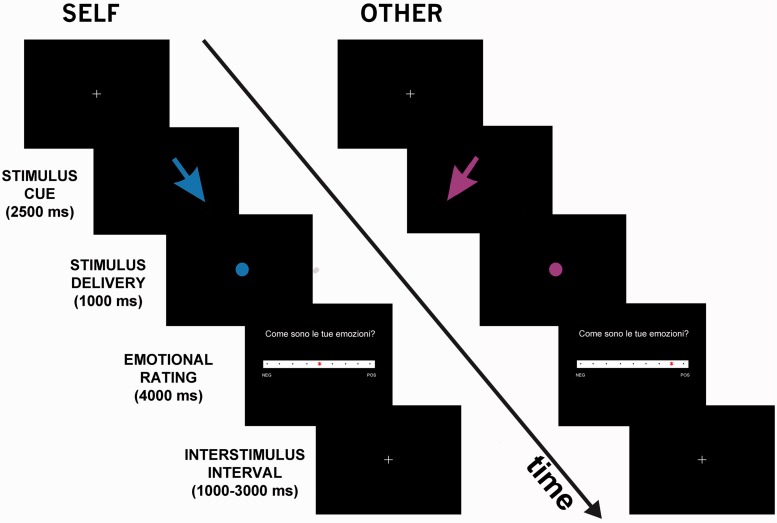
Each stimulation trial started with a fixation cross in the middle of the screen. Then the arrow appeared and stayed on the screen for 2500 ms, before a circle of the same color appeared (1000 ms), representing the actual delivery of the stimulus. At the end of each stimulus, the participant was asked to rate the valence of emotions felt on a Likert-type rating scale with nine discrete values, from −4 = ‘very negative’ over 0 to +4 = ‘very positive’ (4000 ms). The response was given by moving an asterisk from a random initial position toward the chosen position using the left and right keys on a response pad that the participant held in her right hand (Figure 1).
Fig. 1.
fMRI design for the physical pain task. In each trial, participants were first presented with colored arrows as cues indicating the target, either the participant (self) or the confederate (other) and the intensity (painful or non-painful) of the incoming stimulation. Specifically, dark colors indicated a painful stimulus, whereas light colors were paired with non-painful stimuli (in the figure only dark-colored cues are shown). The actual delivery of the stimulus was signaled by a dot of the same color of the arrow, appearing after 2500 ms. Participants judged their own emotion on a 9-points Likert scale, displayed for 4000 ms, immediately after the stimulation period (1000 ms). Interstimulus interval was randomly jittered (1000–3000 ms).
The session was divided in two separate runs of 40 randomized stimulations each (10 self pain, 10 self no-pain, 10 other pain and 10 other no-pain).
Social pain task
The social pain task was designed on the basis of the well-known Cyberball task (Williams et al., 2000), but using records of real people playing the game instead of animated cartoons and adopting the same manipulation of Singer et al. (2004) for the empathy condition. In particular, by replacing cartoons with real people and using a real confederate for the empathy part, we aimed to make the task more ecological and realistic. Videos were recorded using a Digital Video Camcorder (Canon Legria FS406, Tokyo, Japan) and then edited with Final Cut X software (Apple, Cupertino, CA, USA) in order to create black and white silhouettes (see Supplementary Video).
Participants were told that they and the confederate, with whom they were paired, would have been alternatively connected via computer network to other participants controlling the decisions of the other two players visible in the videos, located in adjacent rooms of the building. Therefore, neither the participants nor the confederate met the other players.
During the game, the participant was given the opportunity to decide to whom to throw the ball every time she was in possession of it by pressing either the left or the right keys on the pad that she held in her right hand.
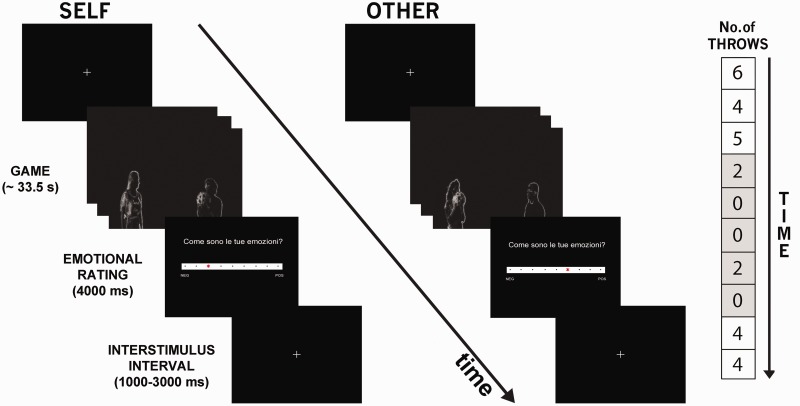
The session consisted of two runs: in the first one, the participant herself was engaged in the game; in the second one, she watched the game played by the confederate seated in the MRI console room (while in reality the decisions of the confederate were computer controlled). In both runs, 10 blocks with 12 passes each were performed. The blocks were equally assigned to two conditions: ‘social inclusion’ and ‘social exclusion’. The five blocks that we regarded as ‘social inclusion’ were the blocks in which the player, either the participant or the confederate, received at least one-third of the total passes (four passes); the remaining five, regarded as ‘social exclusion’, were the blocks in which the player received less than one-third of the total passes (Figure 2). The order of the blocks was fixed, with the first three and the last two blocks belonging to the inclusion condition. The decision to add inclusion blocks at the end of the session (differently from previous studies) was to minimize temporal order effects. Each block lasted an average duration of 33.5 s (range 30–40 s). At the end of each block, the participant was asked to rate the valence of the emotion felt during the game on a Likert-type rating scale with nine discrete values, from −4 = ‘very negative’ over 0 to +4 = ‘very positive’ (4000 ms). The response was given using the same keys used for throwing the ball.
Fig. 2.
fMRI design for the social pain task. During each trial, participants could receive (or observe receiving for the ‘other’ condition) the ball from the other two players and decide to whom to throw the ball by pressing the left or the right key on the pad. Each round ended after 12 throws of the ball. Immediately after, they were asked to judge their own emotion on a 9-points Likert scale, displayed for 4000 ms. Interstimulus interval was randomly jittered (1000–3000 ms). On the right, the number of passes received by the player (either the participant or the confederate) in each of the 10 rounds is indicated. Inclusion rounds are depicted in white, exclusion rounds in gray.
At the end of the scanning session, participants were informally asked about the credibility of the entire experiment and debriefed about the deception involved in the Cyberball game. None of them reported to have been suspicious about the setup of the experiment. We acknowledge though that the use of an ad hoc questionnaire or a structured funnel debriefing would have been a more suitable probe to quantify their level of suspiciousness.
fMRI acquisition and pre-processing
A 3 Tesla Philips Achieva whole-body MR Scanner at the Hospital ‘Santa Maria della Misericordia’ (Udine, Italy), equipped with an 8-channel head coil, was used for MRI scanning. Structural images were acquired as 180 T1-weighted transverse images (0.75 mm slice thickness). Functional images were acquired using a T2*-weighted echo-planar imaging (EPI) sequence with 33 transverse slices covering the whole brain (slice thickness 3.2 mm; interslice gap 0.3 mm; TR/TE = 2000/35 ms; flip angle = 90°, field of view = 230 × 230 mm2; matrix size = 128 × 128, SENSE factor 2).
Data were analyzed with SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). All functional volumes were realigned to the first volume, segmented in gray matter, white matter and cerebrospinal fluid tissues, spatially normalized to the standard EPI template, and smoothed using a Gaussian kernel with full width at half maximum (FWHM) of 10 mm3 (6 mm smoothing at first, 8 mm at second level). Following pre-processing, statistical analysis was carried out using a general linear model approach. High-pass temporal filtering with a cut-off of 128 s was used to remove low-frequency drifts. Regressors of interest were convolved with the canonical hemodynamic response function. The Anatomy Toolbox version 1.6 (Eickhoff et al., 2005) was used for anatomical and cytoarchitectonic interpretation. Whole-brain analyses were thresholded at P < 0.05, FWE corrected at the cluster level.
fMRI analysis
Physical pain
In the first-level analysis data were analyzed, separately for each subject. Two separate regressors (stimulation period and rating) were defined for each condition (‘self pain’, ‘self no-pain’, ‘other pain’ and ‘other no-pain’) for a total of eight regressors for each run. Residual effects of head motion were corrected by including the six estimated motion parameters of each participant as regressors of no interest in the design matrix.
Neural activation related to conditions of interest was determined by entering the parameter estimates for the stimulation period regressors into a flexible factorial design ANOVA model (as implemented in SPM8), for random effect inference at the group level (Penny and Holmes, 2004). Linear contrasts of the repeated measure ANOVA with two within-subjects factors: TARGET (self and other) and INTENSITY (pain and no-pain) were used to assess main effects and interactions. Conjunction analyses (Nichols et al., 2005) of the contrasts high vs low pain for the ‘self’ and ‘other’-related conditions were used in order to identify brain regions commonly activated during the direct and the vicarious experience of physical pain.
Social pain
In the first-level analysis, data were analyzed separately for each subject. Two separate first-level regressors (interaction period and rating) were defined for each condition (‘inclusion’ and ‘exclusion’) for a total of four regressors for each of the two runs (‘self’ and ‘other’). Residual effects of head motion were corrected by including the six estimated motion parameters of each participant as regressors of no interest in the design matrix for each of the two runs (‘self’ and ‘other’).
Neural activation related to conditions of interest (split up by intensity and target) was determined by entering the parameter estimates for the stimulation period regressors into a flexible factorial design, for random effect inference at the group level (Penny and Holmes, 2004). Linear contrasts of the repeated measure ANOVA with two within-subjects factors: TARGET (self and other) and INTENSITY (exclusion and inclusion) were used to assess main effects and interactions. Conjunction analyses (Nichols et al., 2005) of the contrasts exclusion vs inclusion for the ‘self’ and ‘other’-related conditions were used in order to identify brain regions commonly activated during the direct and the vicarious experience of social pain.
Physical and social pain
Finally, in order to investigate neural responses shared by the two kinds of pain, the overall contrast images resulting from the first-level analyses of the two task were entered in a new flexible factorial design ANOVA with the factors: TARGET (self and other), INTENSITY (pain and no-pain) and TASK (physical and social). Conjunction analyses (Nichols et al., 2005) of the contrasts exclusion vs inclusion and pain vs no-pain for the ‘self’ and ‘other’-related conditions were used in order to identify brain regions commonly representing the direct and the vicarious experience of both types of pain.
RESULTS
Behavioral results
Physical pain task
Participants were stimulated with current intensities ranging from 0.1 to 2.0 mA (overall mean of non-painful stimulations: 0.3 (s.d. = 0.2); overall mean of painful stimulations: 0.9 (s.d. = 0.6)).
Emotional ratings given by the participants during the physical pain task were analyzed through a repeated measure ANOVA with two within-subjects factors: TARGET (self and other) and INTENSITY (pain and no-pain) using SPSS 20 (IBM software).
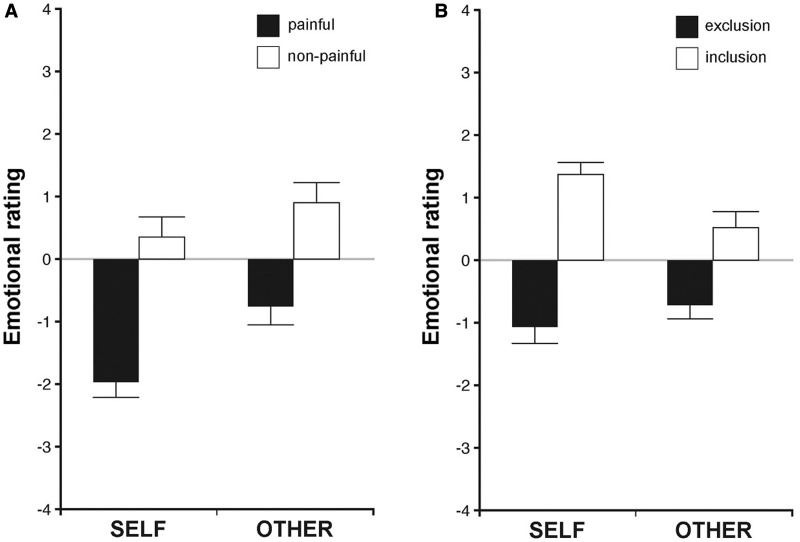
The analysis showed that the task was able to induce clearly distinct emotions according to the different conditions (Figure 3A). In particular, participants judged the stimuli applied to their own hands as more unpleasant than the stimuli applied to the confederate (main effect of TARGET, F(1,22) = 9.806, P = 0.005); furthermore, they rated the painful stimulations compared with the non-painful ones as more unpleasant (main effect of INTENSITY, F(1,22) = 36.661, P < 0.001). A trend toward significance was observed for the interaction between TARGET and INTENSITY (F(1,22) = 4.027, P = 0.057), indicating that painful trials generated more negative judgments in the ‘self’ condition compared with the other condition (paired-samples t-tests, t = −3.255, df = 22, P = 0.004), while ratings in the non-painful trials only showed a trend toward significance (t = −2.013, df = 22, P = 0.057) with the ‘other’ condition being judged as more positive.
Fig. 3.
Emotional ratings for the physical pain (A) and social pain (B) tasks. Graphs represent means and standard errors.
Notably, a correlational analysis showed that the difference between non-painful and painful stimulation ratings for the ‘self’ condition correlated with the same difference calculated for the ‘other’ condition (r21 = 0.594, P = 0.003, see Supplementary Figure S1), suggesting that participants judged the direct experience of painful stimulations (compared with non-painful stimulations) similarly to the experience of witnessing the suffering of another person.
Social pain task
Emotional ratings given by the participants during the social pain task were analyzed through a repeated measure ANOVA with two within-subjects factors: TARGET (self and other) and INTENSITY (exclusion and inclusion) (Figure 3B). The analysis showed that the task was effective in eliciting negative affect following the exclusion from the game. In particular, participants rated more negatively the exclusion (painful) blocks compared with the inclusion (non-painful) ones (main effect of INTENSITY, F(1,22) = 50.990, P < 0.001). Furthermore, an interaction between TARGET and INTENSITY was observed (F(1,22) = 18.353, P < 0.001), resulting from inclusion blocks generating more positive judgments in the ‘self’ condition compared with the other condition (paired-samples t-tests, t = −1.318, df = 22, P = 0.007). No difference was found between ratings in the exclusion conditions (t = 2.950, df = 22, P = 0.201). Finally, no significant main effect of TARGET was observed (F(1,22) = 1.037, P = 0.320).
An additional correlation was performed in order to investigate the relationship of the two variables: number of received passes and emotional ratings. The results show that the two variables are significantly correlated in both the ‘self’ condition (r = 0.941, P < 0.001) and the ‘other’ condition (r = 0.959, P < 0.001) (see Supplementary Figure S2), confirming the association between exclusion from the game and negative affect for both first person and vicarious experience of social pain.
Notably, similarly to the physical pain task, participants judged the experience of being excluded (compared with being fairly treated in the game) and the experience of witnessing another person being excluded in a similar fashion (significant correlation between the difference between inclusion and exclusion ratings in the ‘self’ and in the ‘other’ condition, r = 0.533, P = 0.009, see Supplementary Figure S3).
Physical and social pain tasks
Emotional ratings given by the participants during the two pain tasks were analyzed through a repeated measure ANOVA with three within-subjects factors: TARGET (self and other), INTENSITY (pain and no-pain) and TASK (physical and social).
On top of the main effects already reported in the previous sections, the analysis showed that the two tasks were comparable in eliciting negative affect, as indicated by the non-significant two-way interaction INTENSITY × TASK (F(1,22) = 0.267, P = 0.610) and non-significant three-way interaction TARGET × INTENSITY × TASK (F(1,22) = 1.438, P = 0.243), suggesting that the difference between painful and not painful trials and between exclusion and inclusion blocks was similar for both ‘self’ and the ‘other’ condition. Furthermore, correlational analysis between ratings given during the physical and social pain tasks for ‘self’ and ‘other’ conditions showed a significant correlation between empathy for physical and social pain (r = 0.571, P = 0.004, see Supplementary Figure S4). No significant correlation between the two types of pain for the self (r = 0.107, P = 0.623) was observed.
fMRI results
Physical pain task
Main effect of pain: self (pain > no-pain)
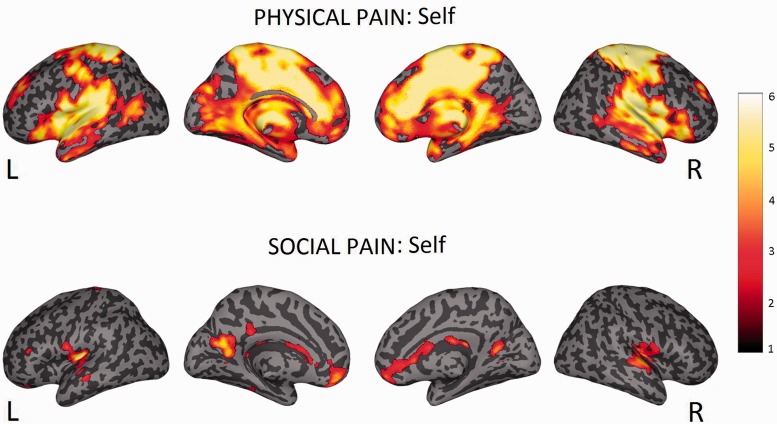
Comparison of hemodynamic responses associated with painful vs non-painful trials in the ‘self’ condition revealed increased activity in the regions classically associated with pain: anterior mid cingulate cortex (aMCC), posterior mid cingulate cortex (pMCC), bilateral anterior, mid and posterior insula (a, m, p -INS), bilateral postcentral gyrus (SI), thalamus and cerebellum. Other brain areas activated were: left mid frontal gyrus, right precentral gyrus, bilateral superior temporal gyrus, right superior temporal pole, left cuneus (P < 0.05, cluster-level corrected, see Supplementary Table S1 and Figure 4).
Fig. 4.
Top part: neural activations for the first person experience of physical pain (contrast: self (pain > no-pain)). Bottom part: neural activations for the first person experience of social exclusion (contrast: self (exclusion > inclusion)). Statistical maps are superimposed on a standard inflated surface (medial and lateral views are showed for each hemisphere). Maps are thresholded at P < 0.005 uncorrected, for illustrative purposes.
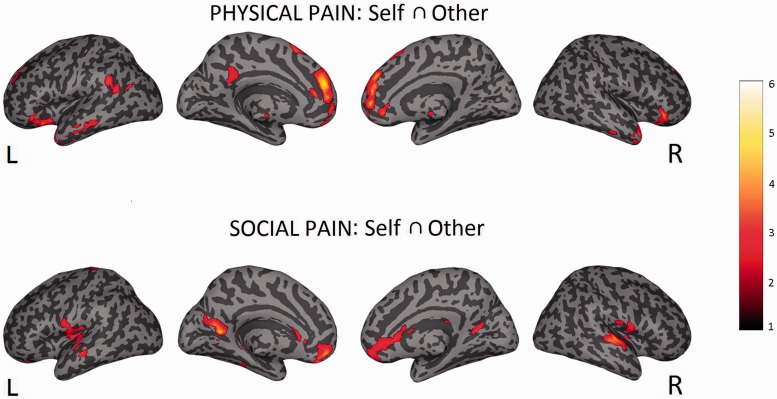
Conjunction: self ⋂ other (pain > no-pain)
In order to test shared activations between ‘self’ and ‘other’ for painful vs non-painful trials, a conjunction analysis was performed. In line with previous findings, perigenual anterior cingulate cortex (pACC) and bilateral aINS were revealed, which are two key areas associated with pain shared between self and other (e.g. Lamm et al., 2011). In addition to these areas of the pain network, we observed significant clusters in right mid superior frontal gyrus, left superior frontal gyrus, left gyrus rectus, right inferior orbitofrontal gyrus, right mid temporal gyrus, right superior temporal pole and right mid temporal pole (P < 0.05, cluster-level corrected, see Supplementary Table S3 and Figure 5). Note that the main effect of pain: other (pain > no-pain) is available in the supplementary materials (Supplementary Table S2 and Supplementary Figure S5).
Fig. 5.
Top part: neural activations for empathy for physical pain (contrast: self ⋂ other (pain > no-pain)). Bottom part: neural activations for empathy for social exclusion (contrast: Self ⋂ Other (exclusion > inclusion)). Statistical maps are superimposed on a standard inflated surface (medial and lateral views are showed for each hemisphere). Maps are thresholded at P < 0.005 uncorrected, for illustrative purposes.
Social pain task
Main effect of pain: self (exclusion > inclusion)
Comparison of hemodynamic responses between exclusion vs inclusion trials in the ‘self’ condition revealed enhanced activity in the following regions: left pINS extending to Rolandic Operculum (SII), right pINS, right subgenual anterior cingulate cortex (sACC), left mid orbitofrontal gyrus, right superior temporal gyrus, left mid temporal gyrus, left calcarine gyrus, caudate bilaterally (P < 0.05, cluster-level corrected, see Supplementary Table S4 and Figure 4).
Conjunction: self ⋂ other (exclusion > inclusion)
To test for shared brain networks between the direct and vicarious experience of social exclusion, a conjunction analysis was performed. Commonly activated areas belonging to the pain network were: right sACC, bilateral pINS and left Rolandic Operculum (SII). In addition, we observed left mid superior frontal gyrus, right medial orbitofrontal gyrus, bilateral gyrus rectus, bilateral superior temporal gyrus and left mid temporal gyrus (P < 0.05, cluster-level corrected, see Supplementary Table S6 and Figure S5). Note that the main effect of pain: other (exclusion > inclusion) is available in the supplementary materials (Supplementary Table S4 and Supplementary Figure S5).
Shared networks for physical and social pain
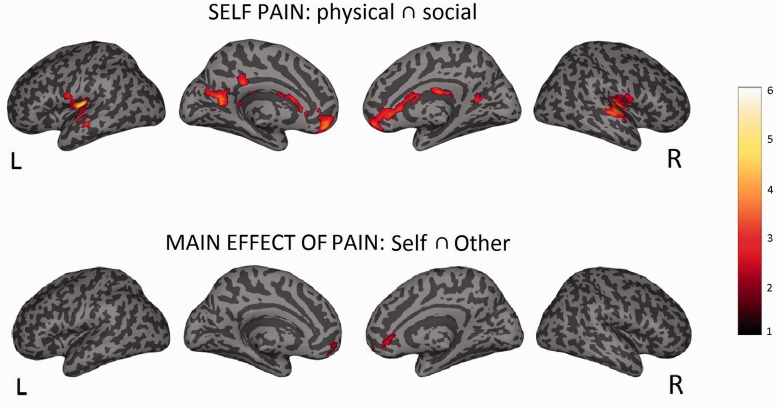
Conjunction: self (pain > no-pain) ⋂ self (exclusion > inclusion)
In order to test to which extent brain activity associated with physical and social pain is shared, a conjunction analysis was performed between areas recruited during the physical pain and the social exclusion task. Commonly activated areas of the pain network were right sACC, bilateral pINS and left Rolandic Operculum (SII). In addition, we observed left mid orbitofrontal gyrus, right superior temporal gyrus, left mid temporal gyrus, bilateral caudate (P < 0.05, cluster-level corrected, see Supplementary Table S7 and Figure 6).
Fig. 6.
Top part: common neural activations for physical and social pain (contrast: self (pain > no-pain) ⋂ self (exclusion > inclusion)). Bottom part: common neural activations for empathy for physical and social pain (contrast: self (main effect pain > no-pain and exclusion > inclusion) ⋂ other (main effect pain > no-pain and exclusion > inclusion)). Statistical maps are superimposed on a standard inflated surface (medial and lateral views are showed for each hemisphere). Maps are thresholded at P < 0.005 uncorrected, for illustrative purposes.
Conjunction: self (pain > no-pain) ⋂ self (exclusion > inclusion) ⋂ other (pain > no-pain) ⋂ other (exclusion > inclusion)
The question about which brain areas commonly represent empathy for social and physical pain was assessed by an overall conjunction analysis. This revealed activation in right sACC and left mid orbitofrontal gyrus (P < 0.001, uncorrected, see Supplementary Table S8 and Figure 6).
Difference between empathy for physical and social pain
In order to test which brain areas were selectively engaged in empathy for physical and social pain, respectively, we formally compared the two conditions.
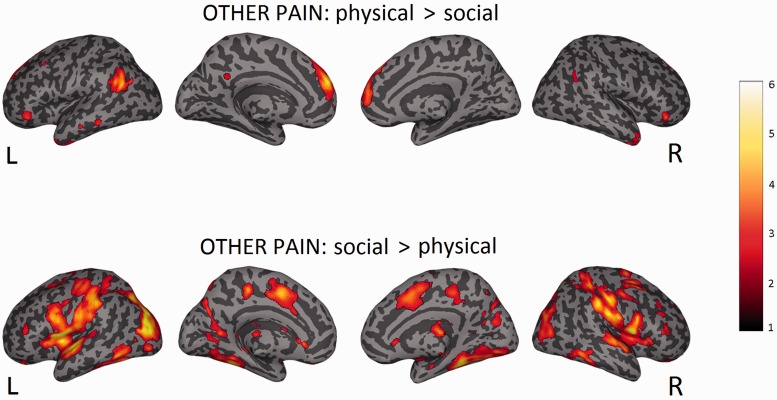
Other (pain > no-pain) > other (exclusion > inclusion)
Higher activity in empathy for physical compared with social pain was observed in left mid superior frontal gyrus, right superior frontal gyrus, left inferior temporal gyrus, left angular gyrus and left temporo-parietal junction (P < 0.05, cluster-level corrected, see Supplementary Table S9 and Figure 7).
Fig. 7.
Difference in neural activation between physical and social pain for the empathy condition. Top part: brain areas more active during the witnessing of the other person suffering from physical pain than from social pain (contrast: other (pain > no-pain) > other (exclusion > inclusion)). Bottom part: brain areas more active during the witnessing of the other person suffering from social pain than from physical pain (contrast: other (exclusion > inclusion) > other (pain > no-pain)). Statistical maps are superimposed on a standard inflated surface (medial and lateral views are showed for each hemisphere). Maps are thresholded at P < 0.005 uncorrected, for illustrative purposes.
Other (exclusion > inclusion) > other (pain > no-pain)
Higher activity during empathy for social compared with physical pain was observed in several regions, among them: left pMCC, left mINS, bilateral Rolandic Operculum, right supramarginal gyrus, bilateral postcentral gyrus, right superior temporal gyrus, left inferior parietal gyrus, left precuneus, bilateral fusiform gyrus, left mid occipital gyrus, right lingual gyrus, left calcarine gyrus and cerebellum (P < 0.05, cluster-level corrected, see Supplementary Table S10 and Figure 7).
DISCUSSION
The question to which extent physical and social pain rely on similar neural mechanisms is of growing interest in social neuroscience. In order to address the common and distinct neural substrates of social and physical pain, it needs to be considered whether the subjective experiences of physical and social pain are comparable. Previous studies investigating the neural correlates of first-person experiences of social pain have either used paradigms such as the exclusion from a virtual ball-tossing game (Eisenberger et al., 2003; Masten et al., 2012), or strong experiences of social loss like bereavement and romantic rejection (Kersting et al., 2009; Fisher et al., 2010). While the former studies revealed activation in the affective-motivational component of the pain network (aMCC, pACC and aINS), the latter also observed the involvement of somatosensory areas (pINS, PAG and thalamus, see Eisenberger (2012) for a review). These inconsistencies might stem from a different degree of emotional involvement and unpleasantness triggered by the different scenarios. Hence, it might be that only bereavement and romantic rejection are powerful enough to elicit feelings of distress that can activate areas related to painful physical experiences.
Apart from differences in emotion involvement, a further complication when trying to identify the shared neural substrates of physical and social pain stems from the fact that these two types of pain have so far mainly been investigated in independent samples. However, evidence that social pain shares activation with the sensory-discriminative part of physical pain has recently been strengthened by Kross et al. (2011). Using a within-subject design, these authors observed that the neural activity related to two tasks involving different types of pain (physical and social) overlapped not only in the part of the pain network coding for the affective-motivational component (i.e. aMCC and aINS), but also in areas associated with the sensory-discriminative one (dpINS and SII). The authors concluded that when social pain is powerfully elicited, in this case by romantic rejection, it is capable of activating areas that so far were linked only to painful physical experiences.
However, as these findings differ from what has been reported in the social rejection literature so far (Eisenberger et al., 2003; Krill and Platek, 2009; Dewall et al., 2010; Masten et al., 2012), the involvement of the somatosensory cortex during social rejection by Kross et al. might relate to the intensity of the social pain experience (and not only to the fact that their within-subject design might have been more sensitive). Recalling the experience of being subjected to the rejection of the partner is a very particular event and certainly more powerful than being excluded from a virtual game. The question whether everyday experiences of social exclusion activate areas associated with the somatosensory component of physical pain as well therefore remained unclear, so far.
Our study, however, using a within-subjects design as well, shows that a modified version of the Cyberball social exclusion game reveals similar findings as during romantic rejection in the involvement of the somatosensory component of the experience. Cyberball is a successfully used approximation of real-life experiences of social exclusion and causes negative affect, as shown by behavioral findings and the consistent recruitment of affective areas such as aMCC, p- and s- ACC and aINS in previous research (Eisenberger et al., 2003; Krill and Platek, 2009; Dewall et al., 2010; Masten et al., 2012). Nevertheless, the strength of the unpleasant experience might be dampened by its computer-like appearance. The first aim of the present study was therefore to test a new and more ecological paradigm for investigating social pain, in order to elicit an aversive emotional response comparable to the one elicited by a physical threat.
The paradigm used video clips of people rather than cartoon manikins, as in Cyberball. It was indeed able to induce aversive feelings during exclusion trials of comparable size to the unpleasantness induced by painful physical stimulation, as indicated by the similar difference between high and low painful stimulation ratings for both types of pain. At the neural level, the first-person experience of social exclusion resulted in increased activity in the sACC, a region that has been found in other Cyberball studies (Masten et al., 2009, 2011; Bolling et al., 2011, 2012; Moor et al., 2012) as part of a pool of areas (aMCC and pACC) involved in experiencing rejection (Eisenberger, 2012; Premkumar, 2012) and that has been associated to self-reported distress in response to social exclusion (Masten et al., 2009; Onoda et al., 2009), although this correlation was not observed in the present study.
sACC has been generally implicated in the processing of sadness (Mayberg et al., 1999; Phan et al., 2002) and negative affect (Drevets et al., 2008; Shackman et al., 2011).
Interestingly, in the specific case of the Cyberball task, sACC has been mainly observed in studies targeting adolescents (Masten et al., 2009; Masten et al., 2011a; Moor et al., 2012) or in paradigms where the excluding players on the screen were represented with photos of real people (Bolling et al., 2011, 2012), leading to the question of the specific role of this structure in the processing of pain of a social nature.
Besides sACC, the first-hand experience of social exclusion resulted in increased activity also in regions coding for its somatosensory representation, such as pINS and SII. It is crucial to note the use of a within subject design allowed us to assess whether the overlap between the first-hand experience of physical and social pain reflects the recruitment of similar neural processes. This was the case, as shown by the conjunction analysis, which revealed that largely overlapping areas in the somatosensory areas were activated by the two types of pain.
Recent studies addressing the functional organization of the insular cortex have shown that this region can be divided in two or three subdivisions (anterior and mid-posterior, or anterior, mid and posterior, respectively), each associated with different functions (Mutschler et al., 2009; Kurth et al., 2010; Kelly et al., 2012). Specifically, the anterior insula has been mainly linked to emotional-cognitive processes, and the mid-posterior insula to sensorimotor processes, involving the coding of the intensity and the localization of pain, as well as primary interoceptive bodily representation (Craig, 2009).
Therefore, one possible interpretation of this pattern of results is that the increased ecological validity of the present version of the Cyberball task is associated to a more intense experience of social exclusion. The negative emotional experience of being excluded by participants represented on the screen as real people, with human motions and gestures, might have exacerbated the painful consequences of the social exclusion beyond the affective domain to the extent of being perceived as physically painful. However, a rigorous comparison between different versions of the Cyberball task is still lacking. Further studies are needed to clarify the impact of the presentation’s modality on perceived negative affect and intensity of the emotion felt.
It is interesting to note, though, that our paradigm did not show the classical affective regions observed in most of the social exclusion studies, such as aINS and pACC/aMCC (Eisenberger, 2012). These regions have been associated not only with painful or aversive events, but in general with the processing of emotional stimuli and cognitive control (Kelly et al., 2012; Shackmann et al., 2011). One possible explanation could therefore be that similar activations during inclusion and exclusion trials alike prevented us from observing the classical affective network when formally comparing them. Indeed, that interpretation was confirmed by our data: in the ‘self’ condition, inclusion trials showed similar activation strength as exclusion trials in both aINS and aMCC (see Supplementary Figure S6). It is also possible that the order of the exclusion and inclusion blocks adopted in the present study could have played a role. Differently from the majority of previously published studies using the Cyberball paradigm, we decided to minimize temporal order effects by splitting the inclusion blocks in two parts, before and after the exclusion blocks, thus avoiding exclusion blocks being always at the end. Indeed, a repeated measure ANOVA on the emotional ratings of the inclusions trials, with the within factors: TIME (pre-exclusion and post-exclusion) and TARGET (self and other) show that ratings became less positive during post-exclusion trials (main effect of TIME: F(1,22) = 8.587, P = 0.008) for both ‘self’ and ‘other’ conditions (TARGET × TIME: F(1,22) = 0.725, P = 0.404). The result suggests that exclusion trials or habituation/fatigue could have dampened positive feelings associated with the re-inclusion in the game. Interestingly, neurophysiological data speak for the second hypothesis. In particular, if the last two blocks are perceived more negatively because of the preceding exclusion, we expect to observe increased activation in areas coding for negative affect (such as aMCC and aINS) in the contrast post-exclusion vs pre-exclusion. This in turn would explain why we failed to observe these areas when contrasting exclusion vs inclusion. A post hoc analysis indeed revealed that by comparing the last two blocks with the first three blocks of inclusion, no significant increased activation was observed in any of the pain-network regions during the post-exclusion trials, both for ‘self’ and ‘other’ conditions. On the contrary, during pre-exclusion trials, increased activation was found in the right pINS (44 − 14 2) during the ‘self’ condition and in the aMCC (−6 14 28) and in the aINS (34 26 14) (P < 0.05, cluster-level corrected) for the ‘other’ condition (see Supplementary Figure S7). The data therefore suggest that sequence order cannot explain why we did not observe the affective regions classically found in most of the social exclusion studies. Conversely, a possible explanation of this pattern of results is that inclusion shows a general decrease of activations with time, with general arousal effects mainly at the beginning. This interpretation would be in line with the proposed hypothesis of similar activation of the affective network for inclusion and exclusion blocks. However, given the low number of available trials, further clarification about the effect of temporal presentation of stimuli on perceived social exclusion is needed.
The second goal of our study was to address whether the vicarious experience of social pain ‘equally hurts’. This was achieved by comparing neural and behavioral responses when being socially excluded oneself, and when witnessing the exclusion of another person. Our results show that empathy for another person undergoing social discrimination elicits an aversive response that is subserved by the same somatosensory areas that are also involved in the first-hand experience of social exclusion.
According to the few previous neuroscientific studies on empathy for social exclusion, witnessing another person suffering from pain of a social nature generally results in the activation of what has been referred to as the ‘mentalizing network’ (Mitchell et al., 2005; Amodio and Frith, 2006; Frith and Frith, 2006). In addition, the affective-motivational component associated with pain (i.e. aMCC, pACC and aINS) is activated only if the target of the social exclusion is a person affectively close to the observer ( Meyer et al., 2012). Here, we were able to show that the first-person and vicarious experience of social exclusion not only overlaps in areas belonging to the ‘mentalizing’ network (like the vmPFC), but also in areas processing negative affect (sACC) as well as, more interestingly, the sensori-discriminative component of the painful experience, such as SII and pINS. These findings suggest that some experiences of social exclusion can trigger the same neural reaction for both self- and other-related experiences. This extends models of empathy proposing that this social skill relies on a partial sharing of the affective experiences of others, based on one’s own emotional representations in similar experiences (Singer et al., 2004; Bastiaansen et al., 2009). We believe that along with the increased ecological value of our version of Cyberball, the presence of a real confederate as excluded player might have played a role in the emotional resonance process. A final intriguing question addressed in the present work relates to the relationship between empathy for physical and social pain. The conjunction analysis revealed common activation only in one region: the sACC. This area has not been classically associated with empathy for physical or social pain, but mainly with the processing of sadness (Mayberg et al., 1999; Phan et al., 2002) and negative emotions (Drevets et al., 2008; Shackman et al., 2011). Nevertheless, the finding reinforces previous evidence suggesting that the cingulate cortex, including its more rostral portions, plays a pivotal role in the processing of vicarious negative affect. For instance, while recent meta-analyses of empathy mainly stressed the role of medial cingulate cortex, they also indicate engagement of more rostral and subgenual cingulate areas in specific contrasts requiring cognitive skills such as overt evaluation of other emotions (Fan et al., 2011; Lamm et al., 2011; Shackman et al., 2011; Torta and Cauda, 2011). The idea of a common underlying mechanism for empathic responses to any type of pain receives additional supported by our finding of a significant correlation between emotional ratings given by participants for vicarious experiences of both types of pain.
In line with previous neuroscientific findings (Singer et al., 2004; Jackson et al., 2005; Lamm et al., 2011; Fan et al., 2011), our study also showed that witnessing another person suffering from physical pain reactivates areas restricted to the affective part of the pain network (aINS and pACC, in a portion slightly more anterior than the one classically observed though), while the sensorimotor component is not engaged. Conversely, empathy for pain of a social nature activated a more posterior portion of insular cortex and SII. This difference could be related to the different type of paradigm used to induce empathic responses. In particular, while an abstract cue-based paradigm (adapted from Singer et al., 2004) was used to indicate the painfulness and the target of stimulation, during the physical pain task, the social pain task involved the direct witnessing of the other’s exclusion. It has recently been argued that cue-based paradigms engage top-down processes for the representation and coding of other’s pain, rather than bottom-up sensory-based processes engaged by explicit depictions of painful situations and stimulations (picture-based), or their ensuing bodily expressions (Keysers et al., 2010; Lamm et al., 2011). In fact, when the somatic cause of the pain of the target is attended by the observer (for instance, seeing others’ hands painfully stimulated), regions of neural overlap between this experience and the first-person experience are found also in the somatosensory cortices (see Keysers et al. (2010) for a review). The difference between empathy for physical and social pain with respect to somatosensory sharing could therefore be explained with the different way of triggering the empathic responses in the two tasks we used. While in the former, empathy is instantiated by semantic representations and abstract reasoning (top-down processes, mapped to TPJ and dMPFC), the latter used direct observations of the unpleasant event (bottom-up processes, mapped in primary visual and sensorimotor cortex and mid-posterior INS). Consequently, the more picture-based nature of the social pain task could have disclosed the somatosensory resonance with the target, in addition to the affective one. Further studies using comparable paradigms for investigating empathy for painful events are needed to clarify the actual differences between the different types of pain.
LIMITATIONS
The present study addresses important questions related to the neural substrates of physical and social pain and of the empathic responses for both the experiences. The within-subjects design was chosen in order to see the extent of neural overlap between all the conditions, and eventually it proved to convey interesting results.
On the other hand, the paradigm we use leads itself to the problem of spurious generalizations. In fact, it is possible that responses to the different types of pain are enhanced in a situation in which a combination of physical and social negative stimuli is delivered so closely in time.1
Similarly, empathic responses, especially in the social pain task could have been possibly increased by people facing that same situation first, since in the present study participants always witnessed the other participant being excluded after experiencing exclusion at first hand. Further studies should address these problems, investigating the extent of vicarious responses without previous exposure to the same type of experience and separating in time the different types of pain.
Another limitation of the current study lies in the generalization of the results to the whole population. In fact, in order to increase statistical homogeneity, the present study investigated only female participants. Further research is needed to extend the validity of results to the male population.
CONCLUSIONS
In summary, our study provides evidence that experiences of social rejection can activate regions of the brain so far observed during experiences of physical pain and possibly responsible for coding the intensity of the threatening event. Furthermore, for the first time, we showed that this pattern of brain activation extends to the witnessing of the same type of social pain in others. Our findings provide fresh support to models of empathy proposing a partial sharing of the affective experiences of others based on one’s own emotional representations in similar experiences. Finally, the version of the Cyberball task developed in the present study represents a more ecological tool for the investigation of social pain that could be used in settings and populations (e.g. in autism and childhood) where other ways of powerful social exclusion such as romantic rejection or bereavement could not be used.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank Migena Haskocelaj for help in running the experiment and Claus Lamm for useful comments and discussions on an earlier version of the manuscript. We finally thank the two anonymous reviewers for their invaluable comments and suggestions. This research was partially funded by the Viennese Science and Technology Fund (WWTF, CS11-016).
Footnotes
1 Interestingly that was the case: by comparing participants (labeled PS henceforth, N = 14) that underwent the physical pain task first, and participants (labeled SP henceforth, N = 9) that performed the social pain task first, we observed order effects. Specifically, we found higher activation for the social pain task in the PS group, in the sACC [4 12 −6], caudate [14 20 −6], right medial orbitofrontal gyrus [6 46 −10], right superior orbitofrontal gyrus [12 66 −16], right inferior orbitofrontal gyrus [28 34 −16], right insula [40 24 −8] (P < 0.05, cluster-level corrected). Interestingly, aMCC [16 24 28] was also found activated at threshold of P < 0.001, uncorrected. No evidence for activation differences was observed when comparing the physical pain task. These findings could be interpreted as a possible spillover effect of the unpleasant experience of physical pain to the unpleasantness of social exclusion. It is also possible that the observed difference between the two groups in the social pain task is not related to the nature of the preceding task (physical pain) but rather to the order of presentation of the task itself. Given the small sample size and the impossibility to disentangle these two hypotheses, further experiments targeting these issues are needed.
REFERENCES
- Amodio D, Frith C. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nature Neuroscience. 2005;8(7):955–60. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Keysers C. Evidence for mirror systems in emotions. Philosophical Transactions of the Royal Society B: Biological Science. 2009;364(1528):2391–404. doi: 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeney JE, Franklin RG, Jr, Levy KN, Adams RB., Jr I feel your pain: emotional closeness modulates neural responses to empathically experienced rejection. Social Neuroscience. 2011;6(4):369–76. doi: 10.1080/17470919.2011.557245. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, et al. Dissociable brain mechanisms for processing social exclusion and rule violation. NeuroImage. 2011;54(3):2462–71. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pelphrey KA, Vander Wyk BC. Differential brain responses to social exclusion by one’s own versus opposite-gender peers. Social Neuroscience. 2012;7(4):331–46. doi: 10.1080/17470919.2011.623181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. New York: Basic Books; 1999. Attachment and loss. Attachment (vol.1) (2nd ed.). . [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Davis KD. The neural circuitry of pain as explored with functional MRI. Neurological Research. 2000;22(3):313–7. doi: 10.1080/01616412.2000.11740676. [DOI] [PubMed] [Google Scholar]
- Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends in Cognitive Sciences. 2006;10(10):435–41. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Decety J. The neuroevolution of empathy. Annals of the New York Academy of Sciences. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. Human empathy through the lens of social neuroscience. ScientificWorldJournal. 2006;6:1146–63. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewall CN, Macdonald G, Webster GD, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychological Science. 2010;21(7):931–7. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13(8):663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nature Reviews. Neuroscience. 2012;13(6):421–34. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35(3):903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Fisher HE, Brown LL, Aron A, Strong G, Mashek D. Reward, addiction, and emotion regulation systems associated with rejection in love. Journal of Neurophysiology. 2010;104(1):51–60. doi: 10.1152/jn.00784.2009. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50(4):531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Han S, Jiang Y, Humphreys GW, Zhou T, Cai P. Distinct neural substrates for the perception of real and virtual visual worlds. NeuroImage. 2005;24(3):928–35. doi: 10.1016/j.neuroimage.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Current Opinion in Neurobiology. 2008;18(2):153–8. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back) Experimental Brain Research. 2010;205(1):1–12. doi: 10.1007/s00221-010-2340-1. [DOI] [PubMed] [Google Scholar]
- Jackson P, Meltzoff A, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage. 2005;24(3):771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kelly C, Toro R, Di Martino A, et al. A convergent functional architecture of the insula emerges across imaging modalities. NeuroImage. 2012;61(4):1129–42. doi: 10.1016/j.neuroimage.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersting A, Ohrmann P, Pedersen A, et al. Neural activation underlying acute grief in women after the loss of an unborn child. The American Journal of Psychiatry. 2009;166(12):1402–10. doi: 10.1176/appi.ajp.2009.08121875. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Reviews Neuroscience. 2010;11(6):417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Smilek D, Eastwood JD. Cognitive Ethology: a new approach for studying human cognition. British Journal of Psychology. 2008;99(Pt 3):317–40. doi: 10.1348/000712607X251243. [DOI] [PubMed] [Google Scholar]
- Krill A, Platek SM. In-group and out-group membership mediates anterior cingulate activation to social exclusion. Frontiers in Evolutionary Neuroscience. 2009;1:1. doi: 10.3389/neuro.18.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(15):6270–5. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010;214(5–6):519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54(3):2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Mar RA, Kelley WM, Heatherton TF, Macrae CN. Detecting agency from the biological motion of veridical vs animated agents. Social Cognitive and Affective Neuroscience. 2007;2(3):199–205. doi: 10.1093/scan/nsm011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Colich NL, Rudie JD, Bookheimer SY, Eisenberger NI, Dapretto M. An fMRI investigation of responses to peer rejection in adolescents with autism spectrum disorders. Developmental Cognitive Neuroscience. 2011a;1(3):260–70. doi: 10.1016/j.dcn.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, Eisenberger NI. An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. NeuroImage. 2011b;55(1):381–8. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Social Cognitive and Affective Neuroscience. 2012;7(1):106–14. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. The American Journal of Psychiatry. 1999;156(5):675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Meyer ML, Masten CL, Ma Y, et al. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Social Cognitive and Affective Neuroscience. 2012;8(4):446–454. doi: 10.1093/scan/nss019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. NeuroImage. 2005;28(4):757–62. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Moor BG, Guroglu B, Op de Macks ZA, Rombouts SA, Van der Molen MW, Crone EA. Social exclusion and punishment of excluders: neural correlates and developmental trajectories. NeuroImage. 2012;59(1):708–17. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, et al. Functional organization of the human anterior insular cortex. Neuroscience Letters. 2009;457(2):66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25(3):653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Society for Neuroscience. 2009;4(5):443–54. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes A. Random-effects analysis. In: Penny W, Holmes A, Friston KJ, editors. Human Brain Function. San Diego, CA: Elsevier; 2004. pp. 843–50. [Google Scholar]
- Perl ER. Ideas about pain, a historical view. Nature Reviews Neuroscience. 2007;8(1):71–80. doi: 10.1038/nrn2042. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiologie Clinique. 2000;30:263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Premkumar P. Are you being rejected or excluded? Insights from neuroimaging studies using different rejection paradigms. Clinical Psychopharmacology and Neuroscience. 2012;10(3):144–54. doi: 10.9758/cpn.2012.10.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FBM. Empathy: its ultimate and proximate bases. Journal of Behavioral and Brain Science. 2002;25:1–72. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Risko EF, Laidlaw K, Freeth M, Foulsham T, Kingstone A. Social attention with real versus reel stimuli: toward an empirical approach to concerns about ecological validity. Frontiers in Human Neuroscience. 2012;6:143. doi: 10.3389/fnhum.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Annals of the New York Academy of Sciences. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not the sensory components of pain. Science. 2004;303:1157–61. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. NeuroImage. 2011;56(4):2157–72. doi: 10.1016/j.neuroimage.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorst H, Bermond B. Validity and reliability of the Bermond-Vorst alexithymia questionnaire. Personality and Individual Differences. 2001;30(3):413–34. [Google Scholar]
- Williams AC. Facial expression of pain: an evolutionary account. Behavioral and Brain Sciences. 2002;25(4):439–55. doi: 10.1017/s0140525x02000080. discussion 455–88. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. Cyberostracism: effects of being ignored over the Internet. Journal of Personality and Social Psychology. 2000;79(5):748–62. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience. 2012;15(5):675–80. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.