Abstract
Remembering emotional autobiographical memories (AMs) is important for emotional well-being, and investigation of the role of emotion regulation (ER) during AM recollection has relevance for understanding mental health issues. Although significant progress has been made in understanding the brain mechanisms underlying ER and AM, less is known about the role of ER during AM recollection. The present study investigated how focusing away (or ‘distracting’) from the emotional content during AM recollection influences the subjective re-experiencing of emotions and the associated neural correlates, by manipulating the retrieval focus of participants who remembered emotional AMs while fMRI data were recorded. First, focusing away from emotion led to decreased self-reported emotional responses, along with increased engagement of ER-related regions (ventro-medial prefrontal cortex, vmPFC), and reduced activity in emotion-related regions (amygdala, AMY). Second, increased vmPFC activity was linked to reduced emotional ratings, during the non-emotional focus. Third, mediation analysis identified vmPFC as a functional hub integrating affective signals from AMY and mediating their impact on the subjective re-experiencing of emotion, according to the current retrieval focus. Collectively, these findings shed light on the neural mechanisms underlying the ability to effectively switch attentional focus away from emotions during AM recollections and have direct relevance for understanding, preventing and treating affective disorders, characterised by reduced ability to regulate emotions.
Keywords: personal memories, emotion control, ventro-medial PFC, emotional disorders
INTRODUCTION
Emotional autobiographical memories (AMs), such as the birth of a child, winning an award or failing an exam, play an important role in the construction of personal identity, in future planning and in decision-making. Hence, they are key factors in the personal emotional well-being. In some circumstances, excessive focus on the emotional aspects of negative personal experiences can have debilitating consequences and lead to psychiatric disorders. To avoid such consequences, it is important to be able to control our emotional responses by switching our attentional focus away from the emotional aspects of our memories, and maintain healthy cognitive and affective functioning. Although there has been recent progress in understanding the brain mechanisms underlying emotional control and emotional AM recollection, much less is known about how focusing on or away (‘distracting’) from the emotional content during the recollection of memories for life experiences might influence the subjective re-experience of emotion and the associated neural correlates. The present study addressed this issue by using functional magnetic resonance imaging (fMRI) in conjunction with an AM task that involved manipulation of the attentional focus during retrieval of emotional personal experiences and recording of participants’ subjective ratings of their emotional responses.
Understanding how people deal with emotional AMs has relevance for understanding both normal healthy functioning and the dysfunction and negativity bias observed in patients with affective disorders. Indeed, excessive focus on emotional aspects of unpleasant memories has been associated with increased susceptibility to affective disorders, such as depression and post-traumatic stress disorder (PTSD) (Brewin et al., 1999; Rubin et al., 2008b, 2011), which are characterised by impaired emotion regulation (ER) (Mayberg, 1997; Gotlib and Joormann, 2010). Recently the topic of ER has gained considerable interest, as the ability to cope adaptively with emotionally challenging situations is vital for physical and mental health, and understanding its mechanisms has important implications for understanding and treating affective disorders (Ochsner and Gross, 2005; Gross, 2008).
In general terms, attentional deployment involves shifts in attention to or away from the emotional aspects of emotion eliciting stimuli or events, which can be achieved by focusing either outwards (e.g. by engaging in a competing task) or inwards (e.g. by changing the focus of memories or thoughts), in order to alter the emotional responses (Gross, 2008). The effectiveness of ER strategies involving attentional deployment, such as distraction, has been confirmed by a recent meta-analysis (Webb et al., 2012). Regarding the neural correlates, available evidence points to down-regulation of emotion-sensitive brain regions (amygdala, AMY) by the engagement of cognitive (prefrontal cortex, PFC) and attentional (parietal cortex, PC) control regions during attentional deployment, similar to other ER strategies, such as reappraisal (Ochsner et al., 2004; Wager et al., 2008; McRae et al., 2010; Kanske et al., 2011).
Moreover, studies directly comparing neural correlates underlying cognitive distraction and reappraisal reported greater decreases in AMY activation and greater increases in activation in PFC and PC regions during distraction relative to reappraisal (McRae et al., 2010; Kanske et al., 2011). Interestingly, AMY down-regulation was impaired when using reappraisal but not when using distraction in remitted depressed participants (Kanske et al., 2012), thus suggesting that cognitive distraction, as a type of attentional deployment, could be particularly useful to immediately deal with emotional situations in depression. It should be noted, however, that in some cases if clear instructions are given, reappraisal could be relatively effective in unmedicated patients with depression (Dillon and Pizzagalli, 2013) and remitted bipolar disorder (Gruber et al., 2013).
The available brain imaging research tended to investigate ER strategies mainly during viewing emotional images, rather than during retrieval of real-life emotional memories, which are at the basis of maintaining depression and PTSD (Brewin et al., 1999; Rubin et al., 2008a, 2008b). Thus, the neural correlates of focusing away or ‘distracting’ from emotion during autobiographical recollection are not clear. The very few studies investigating ER in the context of emotional AM retrieval focused particularly on reappraisal (Kross et al., 2009; Fabiansson et al., 2012; Holland and Kensinger, 2012) and reported that reappraisal can lead to decrease in self-reported emotion. However, the accompanying changes in the neural response are not conclusive, particularly in emotion-related brain regions. For instance, Holland and Kensinger (2012) reported increased activity in cognitive control regions in the lateral and medial PFC when participants down-regulated their emotional reactions using reappraisal during the (re)construction of AMs, but this was also associated with increased, rather than decreased activity in emotion-related regions. The other two studies did not report specific activations related to reappraisal, which could be due to the use of a few memories repeatedly presented across different conditions and/or due to delayed instructions to regulate (i.e. after the engagement of memory retrieval) (Kross et al., 2009; Fabiansson et al., 2012).
These findings suggest that in the case of AM retrieval, down-regulation instructions should be given early, before the engagement in the retrieval process, especially because AM research revealed early involvement of emotion processing regions during recollection of personal events (Daselaar et al., 2008). However, to our knowledge, the use of other ER strategies than reappraisal during retrieval of emotional AMs has not been explored. Relevant for the applicability to clinical conditions, one might expect that using reappraisal to regulate emotional personal memories in affective disorders might be a costly strategy and more difficult to apply, due to overall decreased cognitive/executive abilities in these patient populations (Kanske et al., 2012). Therefore, it would be important to explore whether manipulation of attentional deployment, which has been shown to have immediate beneficial effects in reducing the emotional impact of viewing emotional images and to have clinical utility (Kanske et al., 2012), is also effective in down-regulating the subjective emotional re-experiencing of AMs and the associated activity in emotion-related regions.
The main goal of the present study was to investigate the neural correlates of focusing away from emotion during autobiographical recollection, by manipulating the attentional focus during AM retrieval. Manipulation of the attentional focus was performed by cueing participants, before engaging in the retrieval process, to focus either on emotional (Emotion condition) or on non-emotional contextual (Context condition) aspects of their memories, with the latter expected to decrease emotional responses during AM recollection (Philippot et al., 2003; Neumann et al., 2007). fMRI data were recorded while participants performed this AM task manipulating the retrieval focus. Based on previous evidence from ER studies, we made the following three predictions. First, we expected that diverting attention from emotion during AM recollection would be associated with reduced experiencing of emotional content of personal memories, reflected in a reduction of self-reported emotional responses. At the neural level we expected that this behavioural effect would be accompanied by increased involvement of ER-related brain regions (PFC) and reduced activity in emotion-related brain regions (AMY). Second, we expected a link between activity in ER-related brain regions, during the Context focus condition, and changes in self-reported emotional responses of the associated AM recollections. Third, we also expected that these effects would involve interactions between the PFC and AMY.
MATERIALS AND METHODS
Participants
Eighteen right-handed young healthy adults participated in this study (six men; age range 18–46 years, mean = 26 years, s.d. = 7.02). One subject dropped out the study after the first run of the fMRI session, hence data from 17 subjects (six men, mean age = 26.06 years; s.d. = 7.20) were analysed. All participants had English as first language and had no history of neurological, psychological or psychiatric illness. The experimental protocol was approved by the institutional Research Ethics Board, and all participants provided written informed consent and received payment for their participation.
Collection and selection of emotional AMs
Personal memories were elicited from each participant during an interview performed about 5 weeks prior to the fMRI session, similar to the procedure employed by other neuroimaging studies of AM (Markowitsch et al., 2000; Addis et al., 2004). This procedure allows increased control over the properties of the memories to be used in different trial types, compared with studies involving AM retrieval directly in the scanning session (Cabeza and St Jacques, 2007; St Jacques, 2012). It should also be noted that the disadvantage of reactivation is attenuated by interposing sufficient time between the pre-scan interview and the subsequent scanning session (Maguire and Mummery, 1999). We used an autobiographical memory questionnaire (AMQ) specifically constructed to target the assessment of emotional personal episodes and their recollective qualities (Denkova et al., 2012), which comprised a list of 115 written cues for distinct life events (e.g. ‘the birth of a family member’ and ‘being hospitalised’), resulting from a combination and extension of lists employed by other authors (Levine et al., 2002; Sharot et al., 2007). For each cue, participants were asked to remember a unique episode from their life, that occurred in a specific place and time (e.g. one instance when s/he played in a specific basketball game), rather than remembering general or repeated events (e.g. playing high school basketball). Upon recollection, participants were asked to provide a brief description of the memory, which was then used as a personalised memory cue during fMRI scanning; notably, at the time of collecting the AMs, participants were naïve to the specific purpose of the pre-scanning interview. To assess phenomenological characteristics of each event, participants dated their memories and rated them on several Likert scales, similar to other AM studies (Addis et al., 2004; Greenberg et al., 2005), as follows: emotional valence (on a 7-point scale: −3 = very negative, 0 = neutral and +3 = very positive), emotional intensity, personal significance, the amount of contextual details, vividness (i.e. the amount of visuo-perceptual details) and frequency of retrieval (all of latter used a 7-point scale: 1 = not at all, 7 = extremely).
Then, for each participant, the 40 most emotional memories (20 positive and 20 negative) were selected, based on the ratings provided in the AMQ (i.e. rated 2 or 3 and −2 or −3, respectively). Half of the memories were assigned to the Emotion AM condition and the other half of the memories were assigned to the Context AM condition. The memories in the two conditions were matched as closely as possible in order to avoid differences in terms of age and phenomenological properties, and to ensure that any differences between the two retrieval foci during the fMRI session would not be due to initial differences in the properties of the memories assigned to the two conditions (Table 1).
Table 1.
Average characteristics of the memories assigned to Context and Emotion conditions
| Context | Emotion | P-values | |
|---|---|---|---|
| Age (months) | 74.28 (37.88) | 70.76 (36.07) | 0.18 |
| Emotional intensity | 5.50 (0.61) | 5.47 (0.59) | 0.22 |
| Contextual details | 5.11 (1.13) | 5.16 (1.06) | 0.44 |
| Vividness | 5.32 (0.65) | 5.37 (0.65) | 0.29 |
| Personal significance | 4.53 (0.93) | 4.56 (0.93) | 0.54 |
| Frequency of rehearsal | 3.43 (0.86) | 3.49 (0.90) | 0.24 |
Standard deviations are given in parentheses; P-values are from paired t-tests.
fMRI tasks
The fMRI session comprised two AM tasks, differing according to the focus of retrieval (Context and Emotion), and a semantic memory (SM) control task. Immediately before performing the fMRI tasks, participants were given detailed instructions and performed practice trials in order to familiarise themselves with the tasks and ensure that they understood the instructions.
The AM task: Context vs Emotion focus
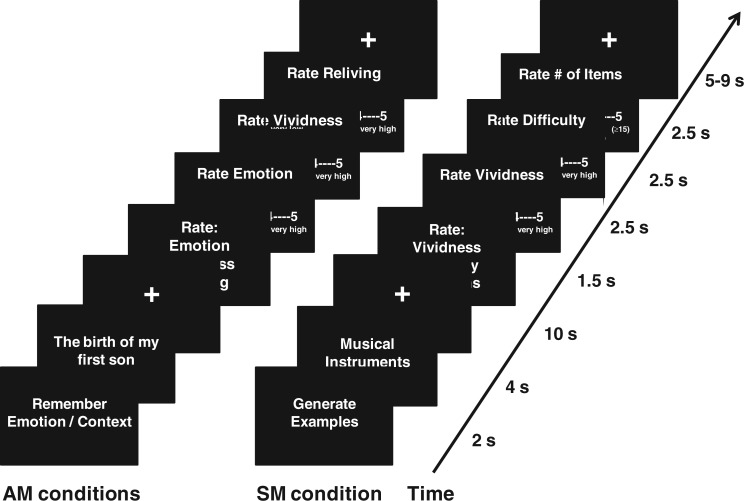
Participants were asked to retrieve the memories associated with each personalised memory cue by either focusing on non-emotional contextual (Context condition) or on emotional (Emotion condition) aspects of their memories (Figure 1). For the Context condition, participants were instructed to focus on the contextual aspects of their memories, by retrieving as many details as possible (e.g. about where and when the event occurred and who/what was present in the event). For the Emotion condition, participants were instructed to focus on the emotional aspects of their memories, including sensations and feelings that they may have triggered (e.g. butterflies in the stomach). Each memory cue was preceded by an instruction cue (‘Remember Context’ or ‘Remember Emotion’, for the Context and Emotion conditions, respectively). After each memory cue appeared on the screen, participants had to indicate by a button press once they recognised the cue as belonging to them, and then continued remembering details of the event until cued again to rate the recollected memory. Each recollection was rated on three 5-point Likert scales including emotional intensity, vividness and reliving (1 = very low and 5 = very high). Participants were instructed to make quick and accurate responses and to use the whole scale.
Fig. 1.
Diagram of the scanned tasks. During the AM conditions, the participants remembered highly emotional personal memories, by focusing either on emotional (Emotion focus) or on non-emotional contextual (Context focus) details of their recollections. Then, participants rated each recollected memory for emotional intensity, vividness and reliving, on 5-point Likert scales (1 = very low, 5 = very high). During the SM control condition, participants generated as many exemplars from a given semantic category as possible, and then rated each of them for vividness, difficulty and number of items on 5-point Likert scales.
Noteworthy, given that focusing away from or on emotional aspects of AMs was the main goal of ER manipulation in the present study, collecting ratings of emotional intensity was essential for assessing the effectiveness of this manipulation during AM retrieval on the subjective re-experiencing of the associated emotions. In addition, ratings of vividness and reliving were also collected, similar to other studies (Addis et al., 2004; Rubin and Siegler, 2004; Talarico et al., 2004; Daselaar et al., 2008).
The SM control task
In line with other AM functional neuroimaging studies (Greenberg et al., 2005; Young et al., 2013), we used a control condition involving SM retrieval, which involved generation of exemplars from 20 different semantic categories (e.g. musical instruments and sports) (Battig and Montague, 1969); also, similar to the AM retrieval task, the SM task involves searches in memory and extended retrieval time. The participants were presented with a semantic category name cue (e.g. fruits and vegetables) and instructed to recall as many exemplars as possible for each category. Each semantic category cue was preceded by an instruction cue (‘Generate Examples’). Once the category cue appeared on the screen, participants had to indicate by a button press that they started recalling exemplars from the category, and then they continued recalling until cued again for memory ratings. To be consistent with the AM conditions, each exemplar generation was rated on three 5-points Likert scales appropriate for SM generation, as follows: vividness, difficulty of the task (1 = very low; 5 = very high) and approximate number of recalled items (1 = 1–3 items; 2 = 4-7 items; 3 = 8-10 items; 4 = 11-14 items and 5 = 15 or more items).
fMRI design and procedure
The two AM conditions (Context and Emotion) and the SM control condition had the same general structure (Figure 1). Each trial began with an instruction screen for 2 s, immediately followed by a memory cue for 4 s. After the cue offset, a fixation screen was presented for 10 s, during which participants elaborated their personal memories or generated exemplars. The end of the retrieval period was marked by the presentation of an instruction screen for upcoming ratings, for 1.5 s. Then, each of the three ratings was presented for 2.5 s in a counterbalanced order across trials. The ratings were followed by an inter-trial interval of variable duration (2–9 s, average = 6 s), before the beginning of the next trial.
The scanning session was divided into two blocks of four runs. Each run started with 6 s of a fixation to allow stabilisation of the fMRI signal and comprised five trials from each condition (Emotion, Context and SM). To avoid induction of longer-lasting effects, the trials within each run were pseudo-randomised, so that no more than two consecutive trials of the same type were presented. To prevent possible biases from using the same run order, participants were assigned different run orders. Similar to other AM neuroimaging studies (Greenberg et al., 2005), in order to increase statistical power, the four runs from the first block were immediately repeated in the second block of the scanning session, and the order of runs was counterbalanced across participants. Stimuli were projected on a screen directly behind the subjects’ head within the scanner, which subjects viewed through a mirror.
All stimuli appeared in white letters against a black background created in Adobe Photoshop, and the CIGAL software (http://www.nitrc.org/projects/cigal/) was used for stimulus presentation and collection of behavioural responses during the fMRI session. All responses were made on a four-button MRI-compatible response box placed under the subject’s right hand; the fifth rating was indicated by the participants with a double click on button #1.
MRI data collection
MRI data were recorded using a 1.5-T Siemens Sonata scanner. The anatomical images were 3-D MP-RAGE anatomical series (repetition time [TR] = 1600 ms, echo time [TE] = 3.82 ms, field of view = 256 × 256 mm, number of slices = 112, voxel size = 1 × 1 × 1 mm). The functional images consisted of a series of images acquired axially using an echoplanar sequence (TR = 2000 ms, TE = 40 ms, field of view = 256 × 256 mm, number of slices = 28, voxel size = 4 × 4 × 4 mm), thus allowing for full-brain coverage.
Behavioural and fMRI data analyses
To investigate the effect of the retrieval focus on the qualities of the remembered memories, differences in ratings for emotional intensity, vividness and reliving between Emotion and Context conditions were assessed using repeated measures ANOVA and planned t-tests. All neuroimaging data were pre-processed and analyses were performed with SPM2 (Statistical Parametric Mapping). Standard pre-processing steps included quality assurance, TR alignment, motion correction, coregistration, normalisation and smoothing (8 mm full-width half maximum isotropic Kernel). At the individual level, each event was modelled by the canonical haemodynamic response function (hrf) and its temporal derivative. Movement parameters calculated during the realignment were included as parameters of no interest to control for movement artefacts. In both AM and SM conditions, the hrf was time-locked to the closest TR linked to the subjects’ responses signalling the recognition of the memory cues (for AMs) and the semantic categories (for SMs), and the beginning of elaborating AMs/generating SM exemplars. Specifically, the hrf was time-locked to 2 s (1TR) following the onset of the memory cues, in the Emotion and Context AM conditions, and 1 s (0.5TR) after the onset of the category cue, in the SM condition. This procedure was guided by the present RT data, which showed that the beginning of AM elaboration following the cues occurred at an average RT of 1.67 s (±0.44), and the beginning of SM exemplar generation at an average RT of 1.03 s (±0.40). Hence, this procedure allows comparisons of the fMRI signal associated with the AM and SM retrieval, by accounting for differences in the timing of memory identification and reducing the impact of initial processing of the cues per se. This is consistent with procedures used in previous neuroimaging AM studies, which typically do not include in the analyses the time spent on viewing/reading the memory cue, and hence account for differences in the temporal profiles of AM and SM responses (Addis et al., 2007; Spreng and Grady, 2009; Ford et al., 2011).
Individual analyses produced whole-brain activation maps for the contrasts of interest, identifying main effects of each AM condition relative to the SM control condition (i.e. Context vs SM and Emotion vs SM). These individual contrasts were then entered into group-level random-effects t-tests analyses, which allowed investigation of the common and dissociating effects for the Context and Emotion retrieval foci. The common effects were investigated through conjunction analyses (i.e. [Context vs SM] ∩ [Emotion vs SM]), in which the statistical activations maps of the two contributing contrasts (Context vs SM) and (Emotion vs SM) were inclusively masked with each other. This procedure allowed identification of the brain regions that showed increased activation in both conditions. The dissociating effects of the retrieval focus were investigated through interaction analyses using paired t-tests (i.e. [Context vs SM] > [Emotion vs SM] and [Emotion vs SM] > [Context vs SM]), whose outputs were also inclusively masked with the corresponding main effects (i.e. Context vs SM and Emotion vs SM, respectively). This procedure ensured that the interaction differences are due to existing differences in the contrasts of interest compared with the SM baseline (e.g. Context vs SM), and not because of differences going in opposite direction in the contrast being compared with (i.e. Emotion vs SM). For the medial temporal lobe (MTL) regions targeted as a priori regions of interest (amygdala-AMY and hippocampus-HC), we used anatomical ROI masks derived from Wake Forest University Pick Atlas toolbox. Overall, unless otherwise noted, for the analyses directly comparing each AM condition with the SM control condition (main effects), an intensity threshold of P < 0.001 uncorrected was used in each of the contributing maps to the conjunction, and for the interaction analyses an intensity threshold of P < 0.05 was used. The latter were further inclusively masked with the activation map of the corresponding main contrasts, set up at P < 0.001.
To further explore the link between brain activity and self-reported re-experience of emotion in each AM condition, linear regression analyses were performed between activity in the Context vs SM contrast and emotional ratings of the context-focused AMs and between activity in the Emotion vs SM contrast and the emotional ratings of the emotion-focused AMs. Again, to make sure that these correlations with ratings occurred in brain areas showing significant effects in the contrasts of interest, the resulting co-variation maps were further inclusively masked with the corresponding activation maps (Context vs SM and Emotion vs SM, respectively). The results of those analyses provided the basis for additional data-driven correlation and mediation analyses (see the ‘Results’ section for more details). A threshold of P < 0.05, uncorrected, was used for all correlations, and a threshold of P < 0.001 uncorrected was used for the masks (except for the AMY, targeted as a priori region of interest, for which a threshold of P < 0.05 was used). An extent threshold of 10 contiguous voxels was used in all analyses, except for the MTL regions, where due to their a priori selection as regions of interest, an extent threshold of five contiguous voxels was used.
Finally, the SPM analyses were complemented by analyses performed with in-house MATLAB tools (Dolcos and McCarthy, 2006), which allowed extraction of the fMRI signal and examination of the time course of activity related to different conditions, across the whole length of the trials.
RESULTS
Behavioural results
Decreased subjective re-experiencing of emotion when focusing away from emotional aspects of recollected AMs
As expected, the ANOVA examining the effect of the two retrieval foci (Emotion vs Context) on the three ratings (emotional intensity, vividness and reliving) identified a significant focus × ratings interaction (F(1,16) = 4.14, P = 0.025). Post hoc paired t-tests revealed a decrease in ratings for emotional intensity in the Context condition compared with the Emotion condition (P < 0.001), while there were no significant differences in the ratings for vividness (P > 0.25) and reliving (P > 0.20) between the two conditions (Figure 2). Thus, the ratings assessed immediately after recollection of AMs in the scanning sessions showed that our manipulation of the retrieval focus worked to reduce the subjective emotional experience, when the focus was on non-emotional aspects of the retrieved AMs, without affecting other ratings.
Fig. 2.
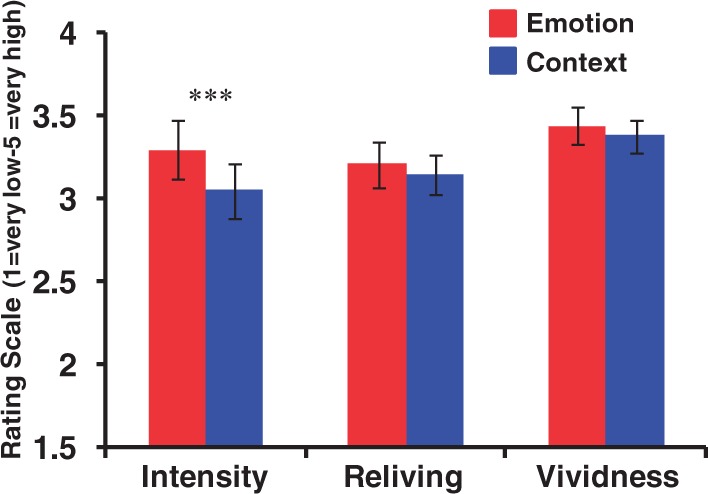
Decreased subjective re-experiencing of emotion during the Context focus. Self-reported ratings for emotional intensity were lower in the Context than in the Emotion AM condition (***P < 0.001), while the ratings for reliving and vividness were similar in the two conditions (P > 0.20 and P > 0.25, respectively).
fMRI results
Dissociable effects in ventro-medial PFC and AMY when focusing away from emotional content of AMs
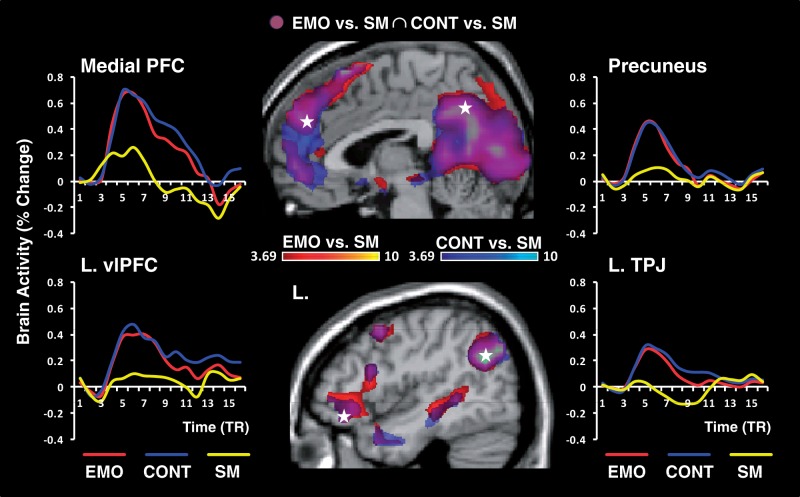
In the context of the overall common pattern of neural correlates corresponding to the typical AM retrieval network (Table 2 and Figure 3) (Svoboda et al., 2006), subtle manipulation of the retrieval focus also yielded differential involvement of emotion control (ventro-medial PFC, vmPFC) and basic emotion processing (AMY) brain regions, linked to whether participants were instructed to focus away from or on emotional aspects of their personal memories.
Table 2.
Brain regions common to Context and Emotion conditions
| Brain regions | Side | BA | Talairach coordinates |
T-values |
Cluster size | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Emotion vs SM | Context vs SM | AM vs SM | ||||||||
| x | y | z | (P < 0.001) | (P < 0.001) | (P < 0.05 FWE) | |||||
| Lateral frontal cortex | ||||||||||
| Middle frontal gyrus | L | 6 | −44 | 14 | 47 | 7.20 | 7.29 | 8.17 | 38 | |
| Inferior frontal gyrus | R | 45 | 55 | 27 | 6 | 8.53 | 4.91 | 7.53 | 23 | |
| Fronto-temporal junction | ||||||||||
| Inferior frontal gyrus | L | 47 | −48 | 34 | −15 | 6.64 | 6.32 | 7.32 | 158 | |
| Superior temporal gyrus | L | 38 | −44 | 18 | −21 | 5.42 | 4.04 | 5.22 | ||
| Middle frontal gyrus | R | 11 | 44 | 30 | −15 | 6.59 | 5.72 | 6.88 | 62 | |
| Inferior frontal gyrus | R | 47 | 40 | 27 | −11 | 5.50 | 4.14 | 7.10 | ||
| Superior temporal gyrus | R | 38 | 36 | 10 | −24 | 5.22 | 4.77 | 5.82 | ||
| Medial frontal cortex | ||||||||||
| Superior frontal gyrus | L | 6 | −8 | 19 | 62 | 8.60 | 6.74 | 8.59 | 197 | |
| Superior frontal gyrus | L | 9 | −8 | 56 | 30 | 7.07 | 5.63 | 6.91 | ||
| Medial frontal gyrus | L | 10 | 0 | 54 | −3 | 5.80 | 9.99 | 7.86 | ||
| Superior frontal gyrus | R | 8 | 4 | 37 | 46 | 6.84 | 4.04 | 6.44 | ||
| Lateral temporal cortex | ||||||||||
| Middle temporal gyrus | L | 22 | −59 | −35 | 2 | 7.72 | 6.03 | 7.45 | 334 | |
| Inferior temporal gyrus | L | 20 | −55 | −9 | −23 | 7.30 | 5.84 | 6.99 | ||
| Middle temporal gyrus | L | 21 | −51 | −1 | −23 | 6.72 | 6.97 | 7.22 | ||
| Middle temporal gyrus | R | 21 | 59 | −5 | −20 | 5.12 | 6.30 | 6.12 | 12 | |
| Medial temporal lobe | ||||||||||
| Parahippocampal Gyrus | R | 35 | 20 | −39 | −5 | 4.53 | 4.03 | 4.48 | 7 | |
| Hippocampus** | L | −20 | −27 | −5 | 4.38 | 3.58 | 4.29 | 20 | ||
| Hippocampus** | R | 20 | −24 | −9 | 3.68 | 3.98 | 4.08 | 9 | ||
| Amygdala* | L | −28 | 7 | −24 | 3.58 | 2.78 | 4.06 | 8 | ||
| Temporo-parietal junction | ||||||||||
| Angular gyrus | L | 39 | −48 | −57 | 32 | 7.78 | 9.55 | 9.13 | 185 | |
| Angular gyrus | R | 39 | 40 | −57 | 32 | 7.17 | 5.82 | 7.58 | 60 | |
| Posterior/occipito-parietal cortices | ||||||||||
| Cuneus | R | 18 | 8 | −81 | 15 | 9.39 | 9.14 | 9.59 | 1494 | |
| Lingual gyrus | R | 18 | 8 | −70 | 7 | 6.75 | 5.47 | 7.26 | ||
| Precuneus | R | 31 | 8 | −45 | 35 | 6.36 | 7.66 | 7.55 | ||
| Cuneus | L | 17 | −8 | −77 | 11 | 8.37 | 8.01 | 8.06 | ||
| Precuneus | L | 7 | −8 | −53 | 36 | 6.69 | 7.34 | 7.35 | ||
| Posterior cingulate | L | 29 | 0 | −42 | 9 | 6.48 | 6.14 | 7.06 | ||
The regions common to Emotion and Context focus are identified by conjunction analysis ([Emotion vs SM] ∩ [Context vs SM]). For this, the Emotion vs SM map was inclusively masked with the Context vs SM map. The intensity threshold was set up at P < 0.001 (t ≥ 3.69) in each of the contributing maps. The areas identified by the conjunction analysis are part of the autobiographical memory (AM) retrieval network, and most of them also survived a FWE-corrected threshold of P < 0.05 (t ≥ 6.78) for the general AM vs SM contrast (as indicated by the text in bold font in the AM vs SM column); for this analysis, the two AM conditions were merged together and compared with the SM condition. Activations in the MTL regions were identified using anatomical ROI masks (significance noted with asterisks), and also survived structural ROI corrections for the AM vs SM contrast (except for the right hippocampus). *Significant at P < 0.01 in each of the contributing maps, **Significant at P < 0.005 in each of the contributing maps; BA, Brodmann’s area; R, right; L, left.
Fig. 3.
The AM retrieval network. Compared with SM, retrieval of AMs, with either Emotion (EMO) or Context (CONT) focus, yielded overlapping increased activity in typical regions of the AM retrieval network. As also illustrated in Table 2, this network included midline cortical structures (medial PFC and precuneus), lateral frontal, temporal and parietal areas, as well as MTL structures (amygdala and hippocampus, data not shown). The activations maps for Emotion vs SM and Context vs SM were set up at a threshold of P < 0.001/t ≥ 3.69 (k ≥ 10 voxels), and superimposed on high-resolution brain images displayed in sagittal views. The coloured horizontal bars show the gradient of the t-values. The line graphs show the time course of responses from typical AM retrieval areas, as extracted from peak voxels (indicated by the white stars) reaching a FWE-corrected threshold of P < 0.05 (t ≥ 6.78) for the AM vs SM contrast (see Table 2, for all peak locations). PFC, prefrontal cortex; vlPFC, ventro-lateral PFC; TPJ, temporo-parietal junction; L, left; TR, repetition time (1 TR = 2 s).
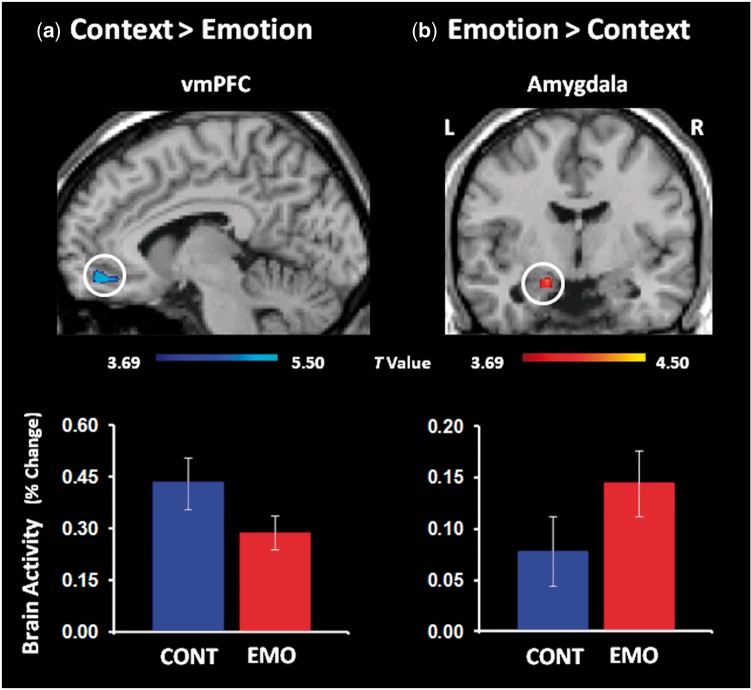
Specifically, focusing away (Context condition) compared with focusing on emotional details (Emotion condition) led to increased activity in ventral areas of the medial PFC (BA10/11) and decreased activity in the left AMY (Figure 4). Other brain regions showing either increased ([Context vs SM] > [Emotion vs SM]) or decreased ([Emotion vs SM] > [Context vs SM]) activity for the Context focus are reported in Table 3.
Fig. 4.
Dissociable patterns of brain activity in emotion control vs basic emotion processing regions during the Context focus. Focusing away from emotion during AM recollection was associated with increased engagement of the ventro-medial PFC (vmPFC) (a) and decreased engagement of the left amygdala (AMY) (b). To ensure that the interaction differences are due to existing differences in the contrasts of interest compared with the SM baseline (e.g. Context vs SM), the interaction maps were inclusively masked with the activation map for Context vs SM and Emotion vs SM, respectively; the resulting maps are superimposed on high-resolution brain images displayed in sagittal (vmPFC) and coronal (AMY) views. The interaction maps were set up at P < 0.05, uncorrected, and were also inclusively masked with the activation map of the corresponding main contrasts, set up at P < 0.001. The gradient colour bars start at P < 0.001 (t = 3.69). The bar graphs represent the percent signal changes extracted from the displayed regions. The error bars correspond to the standard errors of the means. CONT, Context; EMO, Emotion; L, Left; R, Right.
Table 3.
Brain regions dissociating Context and Emotion conditions
| Brain regions | Side | BA | Talairach coordinates |
T-values |
Cluster size | |||
|---|---|---|---|---|---|---|---|---|
| X | y | z | Interaction | Mask | ||||
| (A) [Context vs SM] > [Emotion vs SM] inclusively masked with [Context vs SM] | ||||||||
| Ventro-medial frontal cortex | ||||||||
| Medial frontal gyrus | R | 10/11 | 8 | 46 | −16 | 2.83 | 6.58 | 12 |
| Parietal cortex | ||||||||
| Inferior parietal lobule | R | 39 | 48 | −68 | 40 | 3.14 | 3.86 | 14 |
| Medial temporal cortex | ||||||||
| Parahippocampal Gyrus* | L | 37 | −32 | −39 | −5 | 2.17 | 2.88 | 5 |
| (B) [Emotion vs SM] > [Context vs SM] inclusively masked with [Emotion vs SM] | ||||||||
| Lateral frontal cortex | ||||||||
| Middle frontal gyrus | L | 6 | −48 | 6 | 48 | 2.79 | 3.81 | 15 |
| Fronto-temporal junction | ||||||||
| Inferior frontal gyrus | L | 47 | −32 | 19 | −8 | 4.24 | 4.61 | 130 |
| Inferior frontal gyrus | R | 47 | 40 | 31 | −5 | 4.09 | 4.30 | 101 |
| Dorso-medial frontal cortex | ||||||||
| Superior frontal gyrus | L | 8 | 0 | 30 | 50 | 3.97 | 5.48 | 173 |
| Superior frontal gyrus | R | 6 | 12 | 30 | 50 | 3.61 | 3.92 | |
| Superior/medial frontal gyrus | L | 10 | −16 | 59 | 8 | 3.58 | 5.20 | 21 |
| Lateral temporal cortex | ||||||||
| Superior temporal gyrus | L | 22 | −51 | −27 | 1 | 3.88 | 4.58 | 67 |
| Superior temporal gyrus | L | 41 | −40 | −39 | 6 | 3.17 | 4.23 | |
| Middle temporal gyrus | R | 21, 22 | 55 | −39 | 2 | 2.88 | 4.08 | 10 |
| Medial temporal lobe | ||||||||
| Parahippocampal gyrus | L | 28 | −20 | −20 | −6 | 4.17 | 4.14 | 11 |
| Amygdala | L | −20 | −5 | −13 | 5.11 | 4.50 | 5 | |
| Posterior/parieto-occipital cortices | ||||||||
| Precuneus | L | 7 | 0 | −63 | 58 | 4.19 | 4.78 | 37 |
| Lingual gyrus | L | 17 | −4 | −89 | −2 | 4.10 | 4.65 | 642 |
| Middle occipital gyrus | L | 18 | −20 | −81 | 19 | 3.04 | 4.51 | |
| Posterior cingulate | R | 31 | 24 | −65 | 18 | 3.61 | 5.59 | |
| Midbrain | ||||||||
| Hypothalamus | L | −4 | 0 | −7 | 3.86 | 4.51 | 17 | |
| Cerebellum | ||||||||
| Culmen | R | 16 | −47 | −4 | 2.87 | 4.17 | 13 | |
The regions dissociating Context and Emotion focus are identified by interaction analyses ([Context vs SM] > [Emotion vs SM] and [Emotion vs SM] > [Context vs SM]). The resulting interaction maps set up at P < 0.05 were inclusively masking with the corresponding main contrasts of interest (Context vs SM and Emotion vs SM, respectively) set up at P < 0.001; exceptions in the MTL are noted with asterisks. *Significant at P < 0.05 in the interaction and P < 0.01 in the mask; BA, Brodmann’s area; R, right; L, left.
Increased vmPFC activity linked to reduced self-reported emotional ratings
Investigation of brain–behaviour relations using correlation analyses between brain regions sensitive to Context (Context vs SM) or Emotion (Emotion vs SM) focus and the ratings of emotional intensity during recollection in Context and Emotion AM conditions, respectively, revealed a negative correlation (R = −0.66, P = 0.004) in the vmPFC (BA 10) for the Context condition only (Figure 5). Specifically, participants who had greater activity in this region also had lower emotional ratings while focusing on non-emotional aspects (Context) of recollected AMs, and this effect was not significant for the Emotion condition (R = −0.23, P = 0.37). The difference between the two R coefficients was also tested using the R to Z Fisher’s transformation, which is considered a very strict method. This comparison revealed a marginally significant difference between the two correlation coefficients (z = 1.48, P = 0.069). Interestingly, this medial PFC area showing the negative correlation is in close proximity to the vmPFC region showing specific increased engagement in the ([Context vs SM] > [Emotion vs SM]) contrast illustrated in Figure 4. Other brain regions associated with Context and Emotion conditions that showed significant correlations with the corresponding self-reported emotion ratings are reported in Table 4.
Fig. 5.
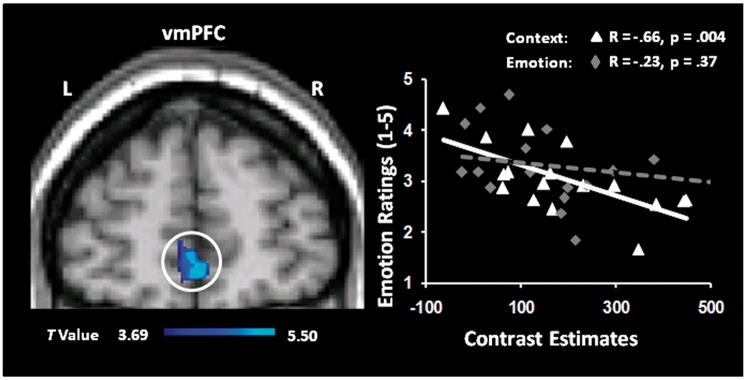
Activity in the vmPFC was linked to reduced emotional ratings during the Context focus. Increased activity in the vmPFC for Context vs SM correlated negatively with emotional ratings for the AMs retrieved with a Context focus. To ensure that these correlations with ratings occurred in brain areas showing significant effects in the contrasts of interest, the resulting co-variation maps were inclusively masked with the activation map for Context vs SM contrast; the resulting map is superimposed on a high-resolution brain image displayed in a coronal view. The scatterplots are based on the contrast estimates extracted from the peak voxel of the area showing the overlap between the co-variation and activation maps. To illustrate the specificity of this correlation to the Context focus condition, the correlation for the Emotion focus condition is also plotted. L, left; R, right.
Table 4.
Brain–behavioural co-variations for Context and Emotion conditions
| Brain regions | Side | BA | Talairach coordinates |
R-Values (P) |
T-Values |
Cluster size | |||
|---|---|---|---|---|---|---|---|---|---|
| x | Y | z | Correlation | Mask | |||||
| (A) Context | |||||||||
| Negative correlations | |||||||||
| Medial frontal gyrus | L | 10 | −4 | 54 | −9 | −0.66 (0.004) | 3.43 | 5.21 | 18 |
| Positive correlations | |||||||||
| Medial frontal gyrus | R | 9 | 8 | 44 | 20 | 0.70 (0.002) | 3.75 | 4.08 | 22 |
| Inferior parietal lobule | L | 40 | −51 | −52 | 43 | 0.60 (0.01) | 2.90 | 4.21 | 12 |
| Lingual gyrus | L | 18 | −12 | −73 | 7 | 0.53 (0.02) | 2.41 | 5.72 | 12 |
| (B) Emotion | |||||||||
| Positive correlations | |||||||||
| Inferior frontal gyrus | L | 47 | −32 | 19 | −11 | 0.78 (0.0002) | 4.79 | 5.30 | 16 |
| Superior frontal gyrus | L | 6 | 0 | 15 | 62 | 0.65 (0.005) | 3.29 | 6.02 | 11 |
| Cuneus | L | 18 | −12 | −81 | 15 | 0.62 (0.008) | 3.04 | 6.06 | 60 |
| Middle occipital gyrus | R | 18 | 28 | −78 | 1 | 0.57 (0.02) | 2.72 | 3.94 | 23 |
| Precuneus | R | 7 | 4 | −61 | 29 | 0.52 (0.03) | 2.35 | 5.50 | 34 |
The covariation maps, set up at P < 0.05, were inclusively masked with the statistical maps of the corresponding main contrasts (Context vs SM and Emotion vs SM, respectively), set up at P < 0.001. No negative correlations were observed for the Emotion condition. BA, Brodmann’s area; R, right; L, left.
vmPFC mediates the link between AMY activity and emotional ratings
Given the evidence of bidirectional influences between medial PFC and AMY (Ongur et al., 2003; Price, 2007) and of co-variation between activity in these regions (Urry et al., 2006; Johnstone et al., 2007), we also sought for evidence supporting the idea of functional interactions between vmPFC and AMY, in the present study. Specifically, to further investigate whether activity in the vmPFC is linked to activity in the AMY, data-driven correlation and mediation analyses were performed to elucidate possible relations between these brain regions, linked to the subjective experience of emotion. Again, the resulting correlation maps were inclusively masked with the activation maps of the corresponding main effects (see also the ‘Methods’ section). Finally, to ensure the specificity of these co-variations to the Context or Emotion focus conditions, the correlation maps were exclusively and reciprocally masked with each other. That is, the map for the Context condition was exclusively masked with the correlation map of the Emotion condition and vice versa.
The focus was on the vmPFC area associated with reduced emotional ratings during Context and the AMY region showing common engagement during the Context and Emotion conditions (Table 2). Specifically, we extracted the fMRI signal from the peak voxels in the two regions for each condition and correlated these values with each other. These analyses revealed a significant positive correlation between vmPFC and AMY for the Context condition (x = −28, y = −1, z = −20; R = 0.64, P = 0.006) and a similar effect for the Emotion condition (x = −28, y = 7, z = −24; R = 0.71, P = 0.001). In other words, participants showing increased AMY activity also had increased vmPFC activity, and this effect was observed for both Context and Emotion conditions.
Consistent with previous evidence regarding AMY–PFC interactions in ER (e.g. Dolcos et al., 2006), these findings suggest that the AMY (as a basic emotion processing region) signals the vmPFC (an emotion integration and ER brain region) about the emotional charge of retrieved AMs. In turn, the vmPFC takes action by exerting control over the experienced emotions according to the focus of the task (away from or on the emotional aspects). To test this idea, we further explored the possibility that the relation between AMY activity and emotional ratings is mediated by changes in vmPFC activity. This mediation analysis (Preacher and Hayes, 2004) explicitly tested whether the relation between AMY activity (the predictor variable, denoted as X in Figure 6) and emotional ratings (the outcome variable, denoted as Y) can be explained by activations in the vmPFC (denoted as M, the mediator). Following standard conventions for mediation analysis, path a refers to the X to M relation (AMY to vmPFC), path b refers to the M to Y relation controlling for X (vmPFC to emotional ratings, controlling for AMY), and the interaction between path a and path b (a × b) reflects the mediation effect (indirect effect).
Fig. 6.
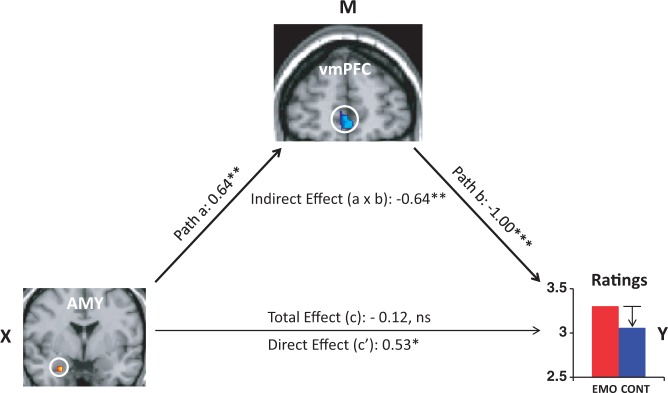
Ventro-medial PFC mediates the link between amygdala and emotional ratings, during the Context but not the Emotion focus. Path a refers to the X to M relation (i.e. Amygdala, AMY, to vmPFC), path b refers to the M to Y relation controlling for X (i.e. vmPFC to emotional ratings controlling for AMY), and the interaction between path a and path b (a × b) reflects the mediation effect (indirect effect). This analysis identified a significant (P = 0.009) negative mediation effect of vmPFC on the relation between the AMY and emotional ratings during context-focused retrieval, and a significant (P = 0.03) positive direct effect (path c′, X to Y controlling for M) between AMY and emotional ratings when controlling for vmPFC influence. The mediation effect was specific to the Context condition, as it was not significant for the Emotion condition (P = 0.22). Standardised coefficients and significance noted with asterisks are reported for each path. *P < 0.05; **P < 0.01; ***P < 0.001 (two-tailed); ns, not significant.
This analysis identified a significant (P = 0.009) negative mediation effect of vmPFC on the relation between the AMY and emotional ratings during context-focused retrieval, and a significant (P = 0.03) positive direct effect (path c′, X to Y controlling for M) between AMY and emotional ratings when controlling for vmPFC influence. The opposite signs in the indirect and direct effects (Wager et al., 2008; Kober et al., 2012) explain why the total effect of the AMY on emotional ratings (path c, X to Y, without considering M) is not significant (P = 0.66). Importantly, this mediation effect was specific to the Context condition, as it was not significant for the Emotion condition (P = 0.22).
DISCUSSION
The present study yielded three main novel findings regarding the neural correlates of ER during emotional autobiographical recollection. First, focusing away from emotion (Context focus) led to decreased self-reported emotional responses, along with increased engagement of ER brain regions (vmPFC) and reduced activity in basic emotion brain regions (AMY). Second, activity in the vmPFC was negatively correlated with subjective ratings of emotion during context-focused AM recollection. Third, mediation analysis identified a role of the vmPFC as a functional hub integrating affective signals from the AMY and mediating their impact on the subjective re-experiencing of emotion, according to the current retrieval focus. These findings will be discussed in turn below.
Dissociable effects in vmPFC and AMY when focusing away from emotional content of AMs
Decreased self-reported emotion and AMY activity in the Context condition extend previous ER studies (McRae et al., 2010; Kanske et al., 2011), by showing that focusing away from emotional aspects of memories is an efficient ER strategy during AM recollection. To our knowledge, the present study is the first to provide insights into the behavioural and neural effects of manipulation of attentional focus during the retrieval of emotional AMs (see also Denkova et al., 2013a, 2013b). The finding that simply focusing away from emotional aspects of AMs can reduce the subjective re-experiencing of emotion demonstrates that this is an efficient and easy to use ER strategy when remembering unwanted personal emotional memories.
Overall, the present finding has direct relevance for potential training avenues for affective disorders, which are characterised by excessive focus on emotional personal memories (Brewin et al., 1999; Rubin et al., 2008a, 2008b) and impaired use of ER strategies such as reappraisal (Aldao et al., 2010; Gotlib and Joormann, 2010; Joormann and Gotlib, 2010), along with a lack of flexibility in deploying attention to different aspects of representations active within working memory (Joormann and Gotlib, 2008; Gotlib and Joormann, 2010). Related to this idea, a recent fMRI study showed that ER strategies involving attentional deployment might, in fact, be more efficient in down-regulating emotion than reappraisal in remitted depressed patients (Kanske et al., 2012). It should be noted that focusing on non-emotional contextual details does not completely eliminate reactivation of emotional aspects, but rather it attenuates the effect of emotion as a result of subtly manipulating the attentional focus during retrieval away from the emotional aspects. This is reflected in the fact that overall both AM conditions produced greater AMY activity compared with the SM condition. Thus, the present difference between the two conditions is a matter of degree, rather than of ‘all or none responses’. This finding is also consistent with suggestions from the AM literature that effortful reconstruction of the contextual elements during AM retrieval might spontaneously attenuate the reliving of the associated emotion (Conway and Pleydell-Pearce, 2000; Philippot et al., 2003; Neumann et al., 2007) and may provide a possible explanation for inconsistent AMY activation reported across typical AM studies (Svoboda et al., 2006; Dolcos et al., 2012).
It is important to note that while in other ER studies manipulation of attentional deployment involves engagement in cognitively demanding tasks, to divert attention from the main emotional task and distract participants from the emotional aspects, the manipulation used here involves simply switching the retrieval focus while the main task remains the same (i.e. recollection of personal memories). This remarkably subtle manipulation has therefore the advantage of still changing the focus of AM recollections without distorting the actual memories. With this idea in mind, our findings could be linked to and extend recent preliminary studies showing that training to be more concrete and specific in recollecting memories might have beneficial effects in depression (Raes et al., 2009; Watkins et al., 2009). Therefore, our findings provide support for cognitive behavioural therapies involving ER training to ‘distract’ from emotional aspects of personal memories, by focusing on and elaborating non-emotional contextual aspects of retrieved AMs, which in turn leads to reduced self-experienced emotion.
Increased vmPFC activity linked to reduced self-reported emotional ratings
Regarding the neural correlates of the observed behavioural effect, the opposing patterns of response in vmPFC compared with that observed in the AMY suggest that changing the focus of recollections involves active engagement of ER-related brain regions, possibly reflecting the switch from emotional aspects of the memories to other contextual details (time, location, etc.). In the neuroimaging research, the medial PFC has been linked to a variety of functions (Amodio and Frith, 2006), including self-referential (Northoff et al., 2006; Wagner et al., 2012) and emotional (Kober et al., 2008) processing, mentalising (Frith and Frith, 2003; Denny et al., 2012) and felt rightness (Moscovitch and Winocur, 2002). Also relevant to the present study, medial PFC has been implicated in ER (Etkin et al., 2011; Ochsner et al., 2012) and particularly in distancing from emotions (Koenigsberg et al., 2010). In the present study, the activity in vmPFC was linked to switching between goal/context appropriate behaviours (focusing on emotional or non-emotional aspects of personal memories), which is in line with recent evidence highlighting its role as a functional hub in coordinating adaptive emotional behaviour (Roy et al., 2012).
Although the negative co-variation between vmPFC and emotional ratings in the Context condition is consistent with a role of this region in attenuating the emotional response when the focus is not on emotional aspects, the exact mechanisms are not clear. One possibility is that the vmPFC exerts top-down control on emotion generation regions, but it is also possible that the vmPFC itself is contributing to emotion generation. At any rate, the present results suggest a role of the vmPFC in switching the retrieval focus during AM recollection, which leads to reduced emotional ratings when the focus is on non-emotional aspects of the recollected memories. This idea is further supported by the results of the mediation analyses discussed below.
vmPFC mediates the link between AMY activity and emotional ratings
The present study also points towards a link between AMY response and emotional ratings, through a mediating effect of the vmPFC. Previous research also attributed a mediator role to the vmPFC, but linked to a top-down effect between this region and AMY (Urry et al., 2006; Johnstone et al., 2007). Namely, these studies showed that during ER, the vmPFC mediated the relationship between other PFC control regions and the AMY, such that activity in those PFC regions was found to be positively linked to vmPFC, which in turn was negatively linked to activity in the AMY. Here, we expand this finding by revealing a mediating role of the vmPFC, linked to a bottom-up effect from the AMY. Overall, the present data suggest that AMY is signalling the emotional relevance of the memories to the vmPFC, which is integrating and interpreting them according to the current goals, and as a result of switching the focus away from emotion in the Context condition it leads to decreased re-experienced emotions. The positive co-variation between the overall AMY activity (for both Context vs SM and Emotion vs SM) and the vmPFC activity is consistent with the idea that, given their emotional content, all AMs trigger AMY activity, which in turn signals vmPFC through bottom-up processing. This idea is supported by findings of positive co-variations between AMY and PFC (Dolcos et al., 2006) in AM (Greenberg et al., 2005) and ER (Wager et al., 2008) studies and could be related to the timing of presenting the instruction cues and the type of ER strategies (Wager et al., 2008).
The result of the mediation analysis is consistent with our overall interpretation of the present results, suggesting bottom-up effects from AMY to vmPFC, which in turn mediates the effect on emotional ratings in the Context focus. This is consistent with neuroimaging research suggesting a role of the vmPFC in integrating information from different systems, in assigning emotional significance and in coordinating adaptive behaviours (Dolan, 2007; Roy et al., 2012). Also, consistent with this idea, lesion research shows that vmPFC damage can lead to context-inappropriate emotional responses in both human and animals (Beer et al., 2003; Murray and Izquierdo, 2007). The fact that, when accounted for the vmPFC involvement in our mediation model, there is a significant positive relationship between the AMY and emotional ratings is consistent with the well-established role of this region in processing emotional arousal (Zald, 2003; McGaugh, 2005; Phelps, 2006). Finally, it is also possible that different AMY and vmPFC subregions are involved in slightly different aspects of processing engaged at different moments of the retrieval process. Although fMRI measures lack the most proper specificity in finely clarifying timing-related aspects, it is possible that the AMY–vmPFC relationship (path a) in our mediation model reflects earlier processing that informs the vmPFC about the emotional content of the AMs, whereas the vmPFC–Ratings relationship (path b) reflects later processing associated with the level of re-experienced emotion linked to the focus of AM recollection. Future studies targeting elucidation of sub-regional specificity in AMY and vmPFC, and investigating the timing of their interaction in the context of ER manipulation are needed to address this issue.
Caveats
One limitation of the present study is linked to the absence of objective measures to verify what exactly the subjects are doing, as it is often the case in ER and AM studies. Specifically, it is not clear whether participants are consistently diverting their attention to contextual details in the Context condition, which then resulted in reduced emotional ratings. Instead, it is possible that reduced emotional ratings in the Context compared with the Emotion condition were caused by potentially different task demands in the two conditions. However, this does not seem to be the case, given that no differences were observed between the Emotion and Context conditions in other ratings (vividness and reliving). Hence, it is more likely that the differences in emotional ratings were caused by differential experiencing of emotion when focusing on or away from the emotional content of the recollected AMs. Future investigations using additional psychophysiological recordings (e.g. skin conductance) could help further clarify this issue.
CONCLUSIONS
In summary, the present study sheds light on the neural underpinnings of focused attention as an effective ER strategy during emotional autobiographical recollection. Three main findings emerged from the present investigation. First, focusing away from emotion led to decreased self-reported emotional responses, along with increased engagement of vmPFC and reduced AMY activity. Second, increased activity in the vmPFC was linked to reduced self-reported ratings of emotion during the context-focused AM retrieval. Third, mediation analyses identified a role of the vmPFC as a functional hub integrating affective signals from the AMY and mediating their impact on the subjective re-experiencing of emotion, according to the current retrieval focus. Overall, by demonstrating the usefulness of focusing attention on non-emotional aspects of recollected AMs, these findings have direct relevance for understanding, preventing and treating affective disorders, and provide fruitful avenues for application of this ER strategy in both healthy and clinical groups.
Conflict of Interest
None declared.
Acknowledgments
The authors wish to thank Trisha Chakrabarty and Kristina Suen for assistance with data collection and analysis. This research was supported by funds from NARSAD (currently, the Brain & Behavior Research Foundation), CPRF (currently, Healthy Minds Canada) and the University of Illinois (to F.D.). E.D. was supported by a Wyeth-CIHR Post-Doctoral Fellowship and S.D. was supported by Post-Doctoral Fellowships from University of Alberta and University of Illinois.
REFERENCES
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14(6):752–62. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–77. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clinical Psychology Review. 2010;30(2):217–37. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Battig WF, Montague WE. Category norms of verbal items in 56 categories. A replication and extension of the Connecticut category norms. Journal of Experimental Psychology. 1969;80:1–46. [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85(4):594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Reynolds M, Tata P. Autobiographical memory processes and the course of depression. Journal of Abnormal Psychology. 1999;108(3):511–7. doi: 10.1037//0021-843x.108.3.511. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11(5):219–27. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107(2):261–88. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cerebral Cortex. 2008;18(1):217–29. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Denkova E, Dolcos S, Dolcos F. Reliving emotional personal memories: affective biases linked to personality and sex-related differences. Emotion. 2012;12(3):515–28. doi: 10.1037/a0026809. [DOI] [PubMed] [Google Scholar]
- Denkova E, Dolcos S, Dolcos F. The effect of the retrieval focus and valence on the medial temporal lobe activity during autobiographical recollections. Frontiers in Behavioural Neuroscience. 2013a;7:109. doi: 10.3389/fnbeh.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkova E, Dolcos S, Dolcos F. The effect of retrieval focus and emotional valence on the inferior frontal cortex activity during autobiographical recollection. Frontiers in Behavioral Neuroscience. 2013b;7:192. doi: 10.3389/fnbeh.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Pizzagalli DA. Evidence of successful modulation of brain activation and subjective experience during reappraisal of negative emotion in unmedicated depression. Psychiatry Research: Neuroimaging. 2013;212:99–107. doi: 10.1016/j.pscychresns.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1481):787–99. doi: 10.1098/rstb.2007.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience. 2006;26(7):2072–9. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Kragel P, Wang L, McCarthy G. Role of the inferior frontal cortex in coping with distracting emotions. Neuroreport. 2006;17(15):1591–4. doi: 10.1097/01.wnr.0000236860.24081.be. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Denkova E, Dolcos S. Neural correlates of emotional memories: a review of evidence from brain imaging studies. Special Issue on “Recent Advances of Functional Neuroimaging Studies on Episodic Memories”. Psychologia. 2012;55:80–111. [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiansson EC, Denson TF, Moulds ML, Grisham JR, Schira MM. Don’t look back in anger: neural correlates of reappraisal, analytical rumination, and angry rumination during recall of an anger-inducing autobiographical memory. Neuroimage. 2012;59(3):2974–81. doi: 10.1016/j.neuroimage.2011.09.078. [DOI] [PubMed] [Google Scholar]
- Ford JH, Addis DR, Giovanello KS. Differential neural activity during search of specific and general autobiographical memories elicited by musical cues. Neuropsychologia. 2011;49(9):2514–26. doi: 10.1016/j.neuropsychologia.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358(1431):459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, Labar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43(5):659–74. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotions. New York, NY: Guilford; 2008. pp. 497–512. [Google Scholar]
- Gruber J, Hay AC, Gross JJ. Rethinking emotion: cognitive reappraisal is an effective positive and negative emotion regulation strategy in bipolar disorder. Emotion. 2013;14(2):388–96. doi: 10.1037/a0035249. [DOI] [PubMed] [Google Scholar]
- Holland AC, Kensinger EA. The neural correlates of cognitive reappraisal during emotional autobiographical memory recall. Journal of Cognitive Neuroscience. 2012;25:87–108. doi: 10.1162/jocn_a_00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;15:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117:206–13. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cognition & Emotion. 2010;24(2):281–98. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex. 2011;21:1379–88. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: the influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage. 2012;61(3):686–93. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America. 2012;107(33):14811–6. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, et al. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48(6):1813–22. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biological Psychiatry. 2009;65(5):361–6. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychology of Aging. 2002;17(4):677–89. [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9(1):54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Thiel A, Reinkemeier M, Kessler J, Koyuncu A, Heiss WD. Right amygdalar and temporofrontal activation during autobiographic, but not during fictitious memory retrieval. Behavioral Neurology. 2000;12(4):181–90. doi: 10.1155/2000/303651. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry & Clinical Neurosciences. 1997;9(3):471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Emotional arousal and enhanced amygdala activity: new evidence for the old perseveration-consolidation hypothesis. Learning & Memory. 2005;12(2):77–9. doi: 10.1101/lm.93405. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22(2):248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The frontal cortex and working with memory. In: Stuss DT, Knight RT, editors. The Frontal Lobes. Oxford: Oxford University Press; 2002. pp. 188–209. [Google Scholar]
- Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Annals of the New York Academy of Sciences. 2007;1121:273–96. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- Neumann A, Blairy S, Lecompte D, Philippot P. Specificity deficit in the recollection of emotional memories in schizophrenia. Consciousness and Cognition. 2007;16(2):469–84. doi: 10.1016/j.concog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460(3):425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Philippot P, Schaefer A, Herbette G. Consequences of specific processing of emotional information: impact of general versus specific autobiographical memory priming on emotion elicitation. Emotion. 2003;3(3):270–83. doi: 10.1037/1528-3542.3.3.270. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavioral Research Methods, Instruments & Computers. 2004;36(4):717–31. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- Raes F, Williams JM, Hermans D. Reducing cognitive vulnerability to depression: a preliminary investigation of MEmory Specificity Training (MEST) in inpatients with depressive symptomatology. Journal of Behavior Therapy and Experimental Psychiatry. 2009;40(1):24–38. doi: 10.1016/j.jbtep.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16(3):147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC, Siegler IC. Facets of personality and the phenomenology of autobiographical memory. Applied Cognitive Psychology. 2004;18:913–30. [Google Scholar]
- Rubin DC, Berntsen D, Bohni MK. A memory-based model of posttraumatic stress disorder: evaluating basic assumptions underlying the PTSD diagnosis. Psychological Review. 2008a;115(4):985–1011. doi: 10.1037/a0013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC, Boals A, Berntsen D. Memory in posttraumatic stress disorder: properties of voluntary and involuntary, traumatic and nontraumatic autobiographical memories in people with and without posttraumatic stress disorder symptoms. Journal of Experimental Psychology: General. 2008b;137(4):591–614. doi: 10.1037/a0013165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC, Dennis MF, Beckham JC. Autobiographical memory for stressful events: the role of autobiographical memory in posttraumatic stress disorder. Consciousness and Cognition. 2011;20(3):840–56. doi: 10.1016/j.concog.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450(7166):102–5. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience. 2009;22(6):1112–23. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- St Jacques PL. Functional neuroimaging of autobiographical memory. In: Bernsten D, Rubin DC, editors. Understanding Autobiographical Memory: Theories and Approach. Cambridge, UK: Cambridge University Press; 2012. pp. 114–38. [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico JM, LaBar KS, Rubin DC. Emotional intensity predicts autobiographical memory experience. Memory and Cognition. 2004;32(7):1118–32. doi: 10.3758/bf03196886. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Haxby JV, Heatherton TF. The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdisciplinary Review: Cognitive Science. 2012;3:451–70. doi: 10.1002/wcs.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins ER, Baeyens CB, Read R. Concreteness training reduces dysphoria: proof-of-principle for repeated cognitive bias modification in depression. Journal of Abnormal Psychology. 2009;118(1):55–64. doi: 10.1037/a0013642. [DOI] [PubMed] [Google Scholar]
- Webb TL, Miles E, Sheeran P. Dealing with feeling: a meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin. 2012;138(4):775–808. doi: 10.1037/a0027600. [DOI] [PubMed] [Google Scholar]
- Young KD, Bellgowan PS, Bodurka J, Drevets WC. Functional neuroimaging of sex differences in autobiographical memory recall. Human Brain Mapping. 2013;34(12):3320–34. doi: 10.1002/hbm.22144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research: Brain Research Review. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]