Abstract
Creativity is crucial to the progression of human civilization and has led to important scientific discoveries. Especially, individuals are more likely to have scientific discoveries if they possess certain personality traits of creativity (trait creativity), including imagination, curiosity, challenge and risk-taking. This study used voxel-based morphometry to identify the brain regions underlying individual differences in trait creativity, as measured by the Williams creativity aptitude test, in a large sample (n = 246). We found that creative individuals had higher gray matter volume in the right posterior middle temporal gyrus (pMTG), which might be related to semantic processing during novelty seeking (e.g. novel association, conceptual integration and metaphor understanding). More importantly, although basic personality factors such as openness to experience, extroversion, conscientiousness and agreeableness (as measured by the NEO Personality Inventory) all contributed to trait creativity, only openness to experience mediated the association between the right pMTG volume and trait creativity. Taken together, our results suggest that the basic personality trait of openness might play an important role in shaping an individual’s trait creativity.
Keywords: openness to experience, trait creativity, creativity assessment packet, voxel-based morphometry
INTRODUCTION
Creativity is imperative to the progression of human civilization and is essential to cultural life. It is characterized by the formation of something that is both novel and useful (Sternberg and Lubart, 1993; Jung et al., 2013). Throughout the history of human civilization, there have been many creative individuals, such as Albert Einstein, Thomas Alva Edison, Sir Isaac Newton, or Charles Dickens, who made outstanding contributions to society. Eminent creators like these also share certain personality traits (e.g. curiosity and imagination) and creative cognition (e.g. divergent thinking) that make them more creative than individuals who lack of these characteristics (Oldham and Cummings, 1996; Feist, 1998; Piffer, 2012). In fact, creative potential can be separated into those aspects related to personality and those related to cognition (Rhodes, 1961; Gough, 1979; Amabile, 1996; Runco, 2007; Piffer, 2012). Thus, based on these findings, trait creativity might be a set of aptitude or personality variables that influence an individual’s creativity, whereas creative cognition refers to cognitive processes and metacognitive strategies during creative production, such as divergent thinking (Satzinger et al., 1999; Zeng et al., 2009). Here, we use structural magnetic resonance imaging (sMRI) to investigate the brain structures underlying individual differences in trait creativity.
As early as 1969, Williams suggested a cognitive-affective model of creativity and developed a corresponding creativity assessment packet (CAP, Williams, 1969, 1980). The CAP included a divergent thinking test [similar to the figural Torrance Tests of Creative Thinking (TTCT)] and a divergent feeling test (including four aptitude elements: imagination, risk-taking, curiosity and challenge) (Williams, 1993; Hwang et al., 2007; Liu et al., 2011). The divergent feeling test [also known as the Williams creativity aptitude test (WCAT); Lin and Wang (1994)] is a self-report creative personality assessment that is widely used in creativity studies (Fekken, 1985; Williams, 1993; Chan, 2005; Claxton et al., 2005; Maker and Schiever, 2005; Cai and Zhu, 2007; Miller, 2009). Sternberg (1999) also suggested that these aptitudes (trait creativity) can have an impact on creative problem solving ability. Studies of creative personality have indicated that trait creativity is associated with certain personality characteristics, such as norm doubting, ambition, impulsivity, willing to take risks, autonomy, imagination, hostility, curiosity and self-confidence (Davis, 1992; Eysenck, 1997; Feist, 1998; Cassandro and Simonton, 2010; Piffer, 2012). In addition, many studies have investigated the relationship between creativity [as measured by divergent thinking tests, creative achievement questionnaires (CAQs) or creative personality assessments] and the five basic factors of personality [as measured by the NEO Personality Inventory: openness to experience, conscientiousness, extraversion, agreeableness and neuroticism (Costa and McCrae, 1992)]. The central finding is that openness to experience and extraversion are positive predictors of creativity, whereas neuroticism is a negative predictor of creativity (McCrae, 1987; Aguilar-Alonso, 1996; King et al., 1996; Dollinger et al., 2004; Chamorro-Premuzic and Reichenbacher, 2008; Furnham et al., 2009; Hoseinifar et al., 2011). These findings indicate that creative potential might be positively related to some basic dimensions of personality (e.g. openness to experience and extraversion).
Recent investigations into creativity have utilized brain imaging techniques, such as functional MRI (fMRI) and sMRI to study the neural correlates of creative cognition (for reviews, see Arden et al., 2010; Dietrich and Kanso, 2010; Jung et al., 2013). To some extent, previous fMRI studies have indicated that the hippocampus, anterior cingulate cortex, rostrolateral prefrontal cortex (PFC), right anterior superior temporal gyrus, parieto-temporal brain regions, precuneus and inferior occipital gyrus are associated with creative thinking (Bowden et al., 2005; Fink et al., 2009; Ellamil et al., 2012). However, there is little overlap in patterns of brain activation reported by studies that employed different measures of creative cognition (Arden et al., 2010; Dietrich and Kanso, 2010; Piffer, 2012).
Further evidence comes from sMRI studies that have explored the relationships between brain anatomy and creative cognition. These studies measured brain structure using volume, concentration and thickness index. For example, Moore et al. (2009) found that the score of a visuo-spatial divergent thinking task, as measured by the TTCT, was correlated negatively with the size of the splenium region of the corpus callosum. In addition, Jung et al. (2010) linked the measure of divergent thinking [as scored by composite creativity index (CCI)] and CAQ to cortical thickness. Their results showed that the left lingual gyrus, right cuneus, right inferior parietal gyrus, right angular gyrus and fusiform gyrus were negatively correlated with CCI, whereas the right posterior cingulate was positively correlated with CCI. In addition, the right angular gyrus was associated positively with CAQ score whereas the left lateral orbitofrontal gyrus was associated negatively with the CAQ score. Takeuchi et al. (2010b) also found positive correlations between regional gray matter volume (rGMV) and individual creativity (as assessed by S-A creativity test) in the right dorsolateral PFC (DLPFC), bilateral precuneus, bilateral caudate and right midbrain regions using voxel-based morphometry (VBM). Gansler et al. (2011) subsequently found that creative performance on the figural TTCT to be positively related to the gray matter volume (GMV) of the right parietal lobe gray matter, which might be related to global attention and visuo-spatial processing. In sum, brain structures that have been positively correlated with creativity are the right DLPFC, right posterior cingulate, right parietal lobe, bilateral caudate and right midbrain regions (Moore et al., 2009; Jung et al., 2010; Takeuchi et al., 2010b), whereas brain structures that have been inversely correlated with creativity are the left lateral orbitofrontal gyrus, lingual gyrus, inferior parietal gyrus and fusiform gyrus (Jung et al., 2010; Gansler et al., 2011). Thus, the anatomical correlates of creativity is not ‘limited to one lobe of the brain, nor to one hemisphere, nor to the “more is better” notion’ (Jung et al., 2010). Although there have been inconsistent findings in these studies, both increased and decreased brain regions might be consistent with the high complexity of human creativity which requires diverse cognitive abilities (Dietrich, 2004; Dietrich and Kanso, 2010; Takeuchi et al., 2010b; Jung et al., 2013).
An increasing amount of research has focused on inter-individual differences in behavior (e.g. those related to stable personality characteristics) and associated variations in brain anatomy using non-invasive sMRI (Jung et al., 2010; Takeuchi et al., 2010a; Kanai and Rees, 2011). Trait creativity is likely to be relatively stable and reliable as opposed to creative thinking. The examination of anatomical features using structural imaging may be more efficacious for investigations of (stable) trait creativity than fMRI. Therefore, this study employs VBM to identify the anatomical correlates of individual trait creativity, as measured by the WCAT (Lin and Wang, 1994). In light of previous neuroscience findings on creativity (Moore et al., 2009; Jung et al., 2010, 2013; Takeuchi et al., 2010b; Gansler et al., 2011), we hypothesized that individual differences in trait creativity (higher trait creativity) would be associated with larger volume in the DLPFC a brain region that has been linked to the inhibition of control (challenge and risk-taking) (Takeuchi et al., 2010b) and the right posterior superior temporal sulcus, which has been implicated in novelty seeking (curiosity and imagination) (Knoch et al., 2006; Krain et al., 2006). In addition, higher trait creativity might be associated with decreased volume in the lateral orbitofrontal gyrus (novel goal-directed behavior; Rolls et al., 2005; Jung et al., 2010). We further examine which basic personality trait (e.g. openness to experience or extraversion) would be able to mediate the relationship between brain structure and trait creativity.
MATERIALS AND METHODS
Subjects
In this study, 252 healthy college students (114 men, mean age = 21.65 years) from Beijing Normal University (China) participated in this study. The experimental protocol was approved by the Institutional Review Board of Beijing Normal University. Written informed consent was obtained from all participants prior to the experiment. In addition, participants were paid for their participation.
Two participants were excluded because of incomplete or erroneous questionnaire data. Four participants were omitted from further analyses due to excessive artifacts or abnormal brain structure (e.g. unusually large ventricles). Therefore, 246 of the students were included in further analyses (112 men, aged 18–25 years, mean = 21.70 ± 1.02 years; 134 women, aged 19–24 years, mean = 21.63 ± 1.06 years).
Specifically, the majority of the remaining participants (n = 230) was right-handed based on their self-report on handedness and all reported no history of neurological deficits. Excluding non-right-handedness participants did not change the correlation between rGMV and SWCAT (for details, see the Results section). Thus, all the participants were included in this study.
Assessment of individual trait creativity
Trait creativity was assessed using the test of divergent feelings, which is part of the CAP developed by Williams (1980). Specifically, this study utilized the Chinese version of the scale developed by Lin and Wang (1994) known as the WCAT. This scale, which contains 50 items (e.g. an item of imagination such as: ‘If the final page of a storybook is missing, I will make up the story’s ending myself’), provides scores for imagination, curiosity, challenge and risk-taking. Subjects were asked to rate the extent to which they agree or disagree with each item on a six-point Likert scales ranging from strongly disagree to strongly agree. The total score of the WCAT (SWCAT) was calculated by adding the responses of the 50 items (the eight reverse item scores were subtracted from the seven prior in order to obtain the total score). The higher this score, the higher creative potential. The split half reliability of the WCAT is 0.41–0.92, the Cronbach alpha coefficient is 0.40–0.87, and the test–retest reliability is 0.49–0.81 (Hwang et al., 2007). The Cronbach alpha coefficient in this study was 0.88. In the GMV analysis, only the total scores were included. The scores for each individual factor were omitted from this analysis because of the high correlations among the total scores, the factor scores and inter-factor correlations.
Assessment of personality
The Revised NEO Personality Inventory (NEO-PI-R; Costa and McCrae, 1992) is a 120-item self-report questionnaire based on the five-factor model of personality (Digman, 1990; Costa and McCrae, 1995). Respondents are required to indicate (on a five-point Likert scale) the extent to which they agree or disagree with each statement (ranging from strongly disagree to strongly agree) (Ortet et al., 2012). This inventory provides summary scores for five different domains of personality: neuroticism, extraversion, openness to experience, agreeableness and conscientiousness.
Assessment of general intelligence
We used the Raven’s Advanced Progressive Matrix (RAPM; Raven, 1998) to measure participants’ general intelligence (Takeuchi et al., 2010a, 2011) in order to control for the influence of general intelligence on brain gray matter (Colom et al., 2006; Jung and Haier, 2007; Takeuchi et al., 2011). This scale contains 36 nonverbal items. For each item, the participant is required to select the missing piece of a 3 × 3 matrix from one of the eight alternatives (Takeuchi et al., 2010b). The score of this psychometric test, which is used as an index of individual intelligence, is equal to the number of correct answers given by participants within a 30 min period (Takeuchi et al., 2010a).
Data acquisition
Participants were scanned using a Siemens 3 T scanner (MAGENTOM Trio, a Tim System) with a 12-channel phased-array head coil located at the BNU Imaging Center for Brain Research, Beijing, China. sMRI images were acquired using a 3D magnetization-prepared rapid gradient-echo T1-weighted sequence (repetition time = 2530 ms; echo time = 3.39 ms; inversion time = 1100 ms; flip angle = 7°, field of view = 256 × 256 mm). In total, 128 contiguous sagittal slices were acquired with 1 × 1 mm in-plane resolution and 1.33 mm slab thickness for whole brain coverage.
Data preprocessing
VBM was employed to characterize the differences in GMV to determine the neuroanatomical correlates of behavioral performance across participants (Ashburner and Friston, 2000). VBM was performed using Statistical Parametric Mapping version 8 (Wellcome Department of Imaging Neuroscience, London, UK), with an optimized VBM protocol (Good et al., 2001) on T1-weighted structural images. First, image quality was assessed by visual inspection. A total of four participants, whose images had excessive scanner artifacts or showed gross anatomical abnormalities, were excluded. Second, the image was reoriented manually to the anterior commissure for each participant. Third, images were segmented into four distinct tissue classes, namely gray matter, white matter, cerebrospinal fluid and everything else (e.g. skull and scalp) using a unified segmentation approach (Ashburner and Friston, 2005). Fourth, gray matter images for each participant were normalized to a study-specific template in MNI152 space using the Diffeomorphic Anatomical Registration through Exponential Lie algebra (DARTEL) registration method (Ashburner, 2007). The DARTEL registration involves repetitively computing the study-specific template based on the average tissue probability maps from all participants followed by warping all participants’ tissue maps into the generated template to improve the alignment. Fifth, gray matter voxel values were modulated by multiplying the Jacobian determinants derived from the normalization procedure in order to preserve the volume of tissue in each structure after warping. The modulated gray matter images were then smoothed with an 8 mm full width at half maximum isotropic Gaussian kernel. Finally, to exclude noisy voxels, the modulated images were masked using an absolute masking with a threshold of 0.2. The masked modulated gray matter images were used for further statistical analyses.
Statistical analysis
Behavioral data analysis
All behavioral data were analyzed using the statistical software SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Pearson correlations were carried out to examine the relationships between the SWCAT (total score) and the WCAT subscale score, the SWCAT and the five NEO-PI-R subscale scores, the SWCAT and age, and the SWCAT and the RAPM score. The differences in the SWCAT, RAPM and subscales scores of NEO-PI-R between males and females were also computed.
MRI data analysis
Statistical analysis of the MRI data was conducted using a general linear model. To investigate the anatomical correlates of individual differences in creativity, the SWCAT was entered as the covariate of interest, whereas the effect of gender, age, the score of RAPM and the total GMV (tGMV) were treated as the covariates of no interest. To perform the multiple comparisons correction, the voxel-wise intensity threshold was set at P < 0.005 and a cluster-level threshold was calculated using the AlphaSim program in AFNI, with Monte Carlo simulation (Cox, 1996; Ward, 2000). AlphaSim program is one of the methods for multiple comparison correction combining voxel intensity and cluster extent. Effects were deemed to be significant when the volume of a cluster was greater than the minimum cluster size on whole brain GMV (determined using the Monte Carlo simulation; 129 voxels, 1032 mm3), in which case the probability of a type I error was <0.05. Finally, the results were displayed using FSLviewer (http://fsl.fmrib.ox.ac.uk/fsl/fslview/). Generally, AlphaSim is widely used in previous literatures about VBM data analysis (DeYoung et al., 2010; Schwartz et al., 2010; Ding et al., 2012; Zou et al., 2012; Farb et al., 2013; Kong et al., 2013; Yang et al., 2013). Although the results corrected by Monte Carlo simulation might have some limitations reported in the study of Silver et al. (2011), it can provide the appropriate cluster-level threshold to achieve the desired false-positive rate.
RESULTS
Behavioral results
The mean, standard deviation and data distribution range for age, the SWCAT, RAPM scores and NEO-PI-R scores are shown in Table 1.
Table 1.
Participant demographics (N = 246; men = 112, women = 134)
| Measure | Mean | s.d. | Range |
|---|---|---|---|
| Age | 21.66 | 1.04 | 18–25 |
| SWCAT | 201 | 20.67 | 129–276 |
| RAPM | 26 | 4.22 | 5–35 |
| NEO-PI-R | |||
| Agreeableness | 62 | 7.57 | 40–86 |
| Conscientiousness | 60 | 10.88 | 32–87 |
| Extraversion | 50 | 9.08 | 24–77 |
| Neuroticism | 46 | 11.71 | 13–75 |
| Openness to experience | 57 | 8.61 | 29–86 |
N, number; s.d., standard deviation.
As the total SWCAT was highly correlated with the WCAT subscale scores (curiosity: r = 0.88, P < 0.001; risk-taking: r = 0.81, P < 0.001; challenge: r = 0.87, P < 0.001; imagination: r = 0.76, P < 0.001), only the SWCAT was used as the measure of individual trait creativity in the VBM analysis.
There were no statistically significant differences between males and females (Ps > 0.1) in terms of the SWCAT (mean standard deviation for females was 199.55 ± 22.75, and for males was 203.21 ± 17.78, P = 0.159), RAPM scores (females 26.31 ± 3.60, males 25.41 ± 4.83, P = 0.106) or NEO-PI-R subscale scores (agreeableness: females 62.48 ± 7.52, males 61.10 ± 7.60, P = 0.156; conscientiousness: females 60.51 ± 11.20, males 59.37 ± 10.50, P = 0.411; extraversion: females 50.84 ± 9.27, males 49.74 ± 8.85, P = 0.342; neuroticism: females 46.22 ± 11.77, males 44.77 ± 11.65, P = 0.332; and openness: females 56.37 ± 9.28, males 57.34 ± 7.74, P = 0.370). Thus, differences in the distributions of gender, age, RAPM score and NEO-PI-R subscale scores did not contribute to the gray matter analysis findings.
The Pearson correlation coefficient was calculated, respectively, between intelligence, age and trait creativity as measured by WCAT. The results revealed no correlation between the SWCAT and age (r = −0.03, P = 0.644) as well as between the SWCAT and the RAPM score (r = 0.09, P = 0.179). The Pearson correlation coefficient between the SWCAT and subscale of the NEO-PI-R was also carried out. The results showed that the scores for the dimensions of conscientiousness (r = 0.18, P = 0.004) and extraversion (r = 0.31, P < 0.001) were weakly but significantly positively correlated with the SWCAT. However, there was a stronger positive correlation between openness to experience and the SWCAT (r = 0.53, P < 0.001), which suggests a closer relationship between openness to experience and trait creativity as opposed to conscientiousness and extraversion as measured by the WCAT.
Structural imaging results
Correlation between rGMV and the SWCAT
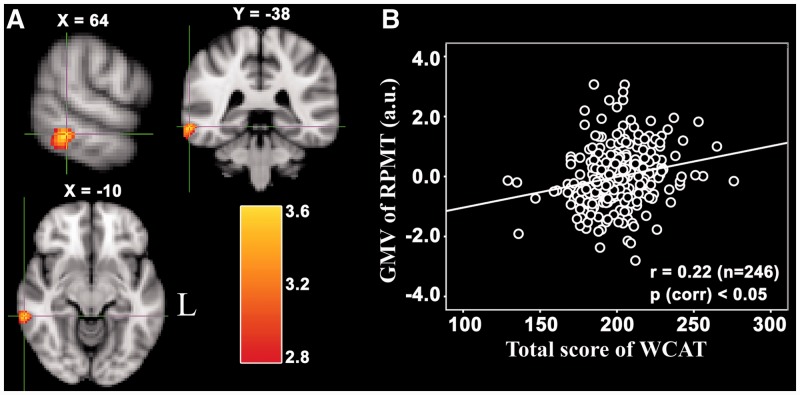
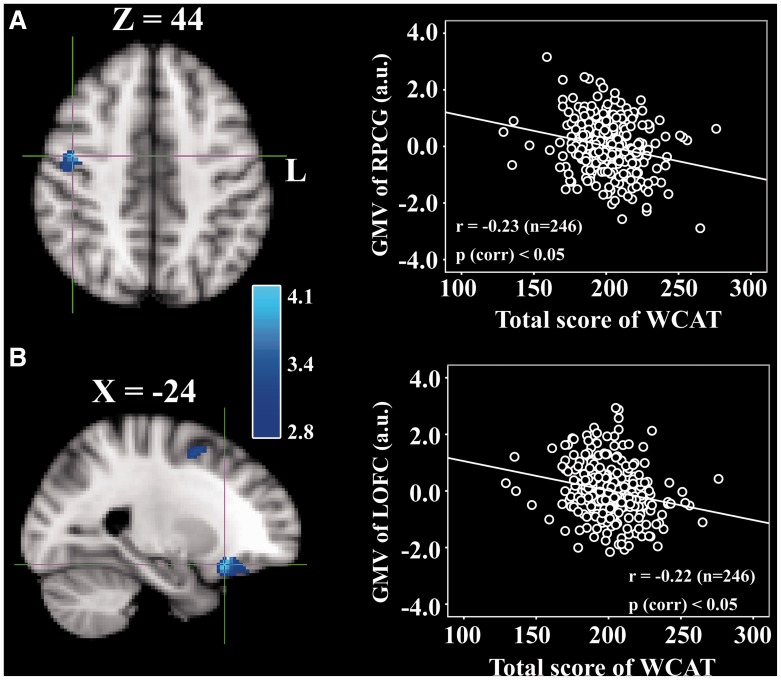
We investigated the association between brain structure, measured in terms of GMV, and creativity (the SWCAT). Gender, age, the score of RAPM and tGMV of individual brains were included as covariates of no interest and regressed out. The multiple regression analysis revealed that the SWCAT was significantly positively correlated with rGMV in a cluster that mainly included areas in the right posterior middle temporal gyrus (pMTG) and extended to the inferior temporal gyrus (ITG) (see Figure 1 for the peak coordination regions). In addition, significant negative correlations were found between the SWCAT and rGMV in (i) a cluster that mainly included areas in the right precentral gyrus (PCG) and extended to the posterior middle frontal gyrus (pMFG), and (ii) a cluster that mainly included regions in the left lateral orbitofrontal cortex (OFC) (Figure 2). There was no significant difference between these brain regions in terms of gender. Information regarding the regions that were (positively or negatively) correlated with the SWCAT is shown in Table 2.
Fig. 1.
Brain regions that positively correlated with the SWCAT. (A) The right pMTG in which rGMV was significantly positively correlated with the SWCAT after adjusting for gender and tGMV. The significant cluster is shown at P < 0.05 (corrected by the AlphaSim program in AFNI with a combined threshold of P < 0.005 for each voxel and a cluster size > 129 voxels). (B) The corresponding partial correlation scatterplot illustrates this relationship (for display purposes only). The x-axis of the scatterplot represents the total WCAT score and the y-axis represents standardized residuals of GMV. For standardized residual measures, gender and tGMV were regressed out. Statistical inference was based on the multiple comparison correction using the 3dClustSim (AFNI) program.
Fig. 2.
Brain regions that negatively correlated with the SWCAT. The PCG (A) and OFC (B), for which rGMV was negatively correlated with the SWCAT after adjusting for gender and tGMV. The significant cluster is shown at P < 0.05 (corrected by the AlphaSim program in AFNI with a combined threshold of P < 0.005 for each voxel and a cluster size > 129 voxels). The corresponding partial correlation scatterplot illustrates this relationship (for display purposes only). The x-axis of the scatterplot represents the total WCAT score and the y-axis represents standardized residuals of the GMV. For standardized residual measures, gender and tGMV were regressed out. Statistical inference was based on the multiple comparison correction using the 3dClustSim (AFNI) program.
Table 2.
Brain regions significantly correlated with the SWCAT
| Brain regions | H | BA | Peak coordination (MNI) |
Cluster volume (mm3) | Z | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Positive correlation | |||||||
| Middle temporal gyrus/ITG | R | 21 | 64 | −38 | −10 | 1176 | 3.59 |
| Negative correlation | |||||||
| PCG/middle frontal gyrus | R | 6 | 44 | −6 | 44 | 1720 | 4.00 |
| OFC/inferior frontal gyrus | L | 47 | −24 | 22 | −16 | 1064 | 4.03 |
H, hemisphere; L, left; R, right; BA, Brodmann area.
Moreover, we examined these areas showing an association between rGMV and trait creativity as measured by WCAT after excluding all participants who were not right-handed (n = 16). After controlling for age, sex and score of RAPM, the regression analysis revealed that the results using data from only right-handedness were similar to those using data from all participants. The statistical values and coordinates of the peak voxel in the right pMTG positively correlated with WCAT change as x, y, z = 64, −40, −10, z = 3.33. The statistical values and coordinates of the peak voxel in the PCG and the OFC that inversely correlated with WCAT were as follows: x, y, z = 44, –6, –44, z = 4.48; x, y, z = −24, 22, −16, z = 4.18. Although there were small variations in cluster size, significant regions were identical to those identified in the whole sample analyses (n = 246). These results might support the view that there was no direct relationship between right- or left-handedness and creativity (Shobe et al., 2009). In the future, the handedness-specific relationship between rGMV and creativity needs to be investigated further.
Openness to experience mediated the relationship between brain structure and the SWCAT
To test our hypothesis about the relationship between personality, trait creativity and brain structure, we first examined the association between personality factors (neuroticism, extraversion, openness, agreeableness and conscientiousness) and GMV in clusters that correlated trait creativity (pMTG, OFC and PCG), with gender, age, intelligence and tGMV as covariates. As expected, we found that openness was positively correlated with the right pMTG cluster (r = 0.22, P < 0.001) and negatively correlated with the OFC cluster (r = −0.13, P = 0.040). All other personality factors showed no significant correlation with these clusters. This indicated a closer association among openness, trait creativity and brain structure compared with other personality dimensions.
The results above indicated that openness, trait creativity and brain structure were linked closely to one another. But the role of openness in the relationship between the rGMV in the right pMTG or OFC and creativity remains unknown. So, as a second step, we examined the association between the GMV in the right pMTG or OFC and trait creativity while controlled openness personality factor. The results indicated that there was no significant correlation between the right pMTG and SWCAT (P = 0.067), whereas the association between the OFC and SWCAT did not change (P = 0.006). This indicated a partial role of openness between the rGMV in the right pMTG and trait creativity.
To establish whether in fact this was the case, a mediation analysis was then conducted using an INDIRECT macro implemented in SPSS (Preacher and Hayes, 2008) to examine whether openness mediated the relationship between brain structure and trait creativity. While adding gender, age, the score of RAPM and tGMV of individual brains as covariates in the model, the mediation analysis showed that there was no significant effect of the right pMTG GMV on trait creativity (β = 0.13, P = 0.067) after including openness as a mediator in the model. In contrast, the direct relationship was significant (β = 0.27, P < 0.001). Bootstrap simulation (n = 10 000) further confirmed that this reduction (i.e. the indirect effect through openness) was statistically significant (P < 0.05) with 95% confidence interval range from 0.07 to 0.24. That is, the mediation analysis confirmed our notion that the personality trait of openness partially mediated the correlation between the right pMTG volume and trait creativity (Figure 3).
Fig. 3.
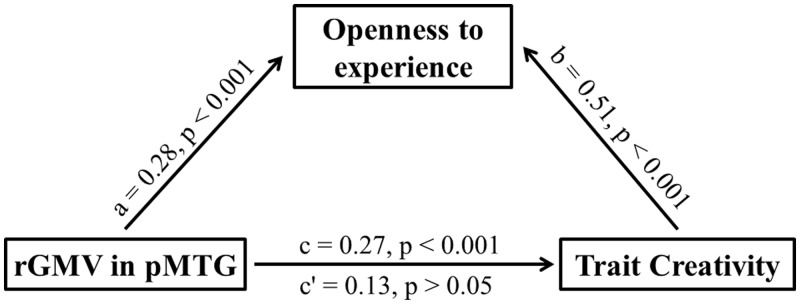
Mediation analysis: the personality trait of openness to experience partially mediated the correlation between the rGMV of right pMTG and trait creativity.
DISCUSSIONS
In this study, we investigated the associations between brain structure and individual trait creativity as measured by the WCAT. Behavioral results showed that the total score of WCAT was related to three domains of personality: openness to experience, conscientiousness and extraversion. VBM results showed that the rGMV of the right pMTG/ITG (BA21) was positively correlated with individual trait creativity, whereas the rGMV of the right PCG/pMFG (BA6) and the left lateral OFC (BA47) were inversely correlated with individual trait creativity. Interestingly, we found that openness to experience (as measured using the NEO-PI-R) partially mediated the relationship between the rGMV of right pMTG and trait creativity. These results indicate that the specific personality trait of openness to experience might play an important role in shaping an individual’s trait creativity.
The relationship between creativity and personality has received a great deal of attention for a long time (Woodman, 1981; Feist, 1998; Ma, 2009). Obviously, certain cognitive ability and personality characteristic would modulate individual differences in creativity (Gibson et al., 2009). Specifically, numerous studies consistently revealed that creativity had a positive association with openness to experience (McCrae, 1987; King et al., 1996; Dollinger et al., 2004; Carson et al., 2005; Miller and Tal, 2007). For example, Feist (1998) found that openness to experience was a distinguishing feature of creative people by contrasting scientists and nonscientists, more creative and less creative scientists, as well as artists and non-artists. In addition, conscientiousness had a positive relationship with scientific performance and a negative one with artistic performance (Feist, 1999). As early as 1987, McCrae (1987) investigated the associations between creativity as measured by Creative Personality Scale (CPS; Gough, 1979) and personality and found a stronger relation between CPS score and openness than between CPS score and extraversion/conscientiousness. Moreover, King et al. (1996) found individual creative ability as measured by TTCT verbal test also positively correlated with openness and extraversion. In this study, the strong correlation between SWCAT and openness (0.53) was also consistent with these previous findings (Feist, 1998), and indicated that conscientiousness and extraversion might indeed be important personality variables that influence an individual’s creativity.
It is suggested that increased rGMV in the right pMTG/ITG may contribute to higher trait creativity (e.g. curiosity and imagination) through ‘semantic processing’ functions that are associated with this region (Dronkers et al., 2004; Binder et al., 2009; Turken and Dronkers, 2011). That is, greater rGMV in the right pMTG in subjects with higher SWCAT might aid the generation of novel, unique connections between ideas. Many previous studies have indicated that the lateral temporal cortex (including the right pMTG) might play key roles in semantic processing such as metaphor understanding (the right posterior middle/superior temporal gyrus) (Goel and Dolan, 2001; Cardillo et al., 2012), semantic representation and control (Badre et al., 2005; Whitney et al., 2011), conceptual integration and comprehension (pMTG) (Hickok and Poeppel, 2004; Turken and Dronkers, 2011), and novel association (the right posterior superior temporal sulcus) (Jung-Beeman et al., 2004; Kröger et al., 2012). In addition, Abraham et al. (2012) have recently stated that divergent thinking could involve brain regions implicated in declarative memory and semantic cognition, such as the lateral inferior temporal cortex (BA 20/21), hippocampus and medial PFC (Cappa, 2008; Binder et al., 2009). Thus, our results indicate that individual differences in trait creativity (e.g. curiosity and imagination) might be linked with rGMV variations in the right pMTG.
In 2005, Kaasinen et al. (2005) showed a positive correlation between rGMV in the temporal, parietal and frontal cortices, and self-transcendence as measured using the Temperament and Character Inventory. High scores on self-transcendence are related to open, unusual and divergent thoughts, which might be similar to curiosity and imagination (novelty seeking), and their results suggested that high self-transcendence might be associated with relatively greater rGMV in the middle temporal region (Kaasinen et al., 2005). Chávez-Eakle et al. (2007) also found that subjects with high creative performance (originality, fluency and flexibility) showed greater cerebral blood flow in the ITG. In addition, investigations of word-generation found that the posterior temporal cortex might be related to object-concept representations and object category retrieval (novel ideas generation; Martin and Chao, 2001; Dobbins and Wagner, 2005). Moreover, Whitney et al. (2011) also indicated that the right posterior temporal gyrus might be related to semantic representation and control during ambiguous semantic associations. Based on these findings, and our own results, we suggest that higher trait creativity (e.g. curiosity and imagination) might be associated with enhanced semantic processing skills (e.g. novel association and conceptual expansion), which may be positively correlated with the rGMV in the pMTG.
Moreover, lower rGMV in the PCG/pMFG (BA 6) and OFC (BA 47) in subjects with higher trait creativity might be associated with reduced response inhibition and increased intuitive thinking, leading to challenge and risk-taking, which are two characteristics of trait creativity. The PCG/pMFG (sometimes named the ‘inhibitory motor area’) (Kreitzer and Malenka, 2008; Brass et al., 2009; Kozasa et al., 2012) has been implicated in tasks that require stimulus-response associations. Westerhausen et al. (2010) also provided evidence that the PCG/pMFG might be involved in the top-down control of attention processes (e.g. conflict-resolution, top-down and bottom-up processing bias integration) (Dwyer et al., 2009; Hill and Miller, 2010; Gautam et al., 2011). The MFG might also play a role in working memory during the manipulation of actively maintained information (Bunge and Zelazo, 2006; Woodward et al., 2006; DeYoung et al., 2010). These findings suggest that the reduced rGMV in the PCG/pMFG revealed in this study might be associated with reduced inhibition control, which may be associated with particular characteristics of higher trait creativity such as challenge and risk-taking. Additionally, a recent study found that the premotor region was a critical region for goal-directed actions (Gremel and Costa, 2013). Other fMRI studies also indicated that the premotor cortex is involved in diverse creative activities (Bengtsson et al., 2007; Chávez-Eakle et al., 2007; Aziz-Zadeh et al., 2012). In addition, Mostofsky et al. (2002) found that boys with attention-deficit/hyperactivity disorder (ADHD), which were known for being excessively impulsive, hyperactive and off-task (inattentive), had decreased volume in the premotor cortex. That is, decreased GMV in the PCG/pMFG might be associated with challenge and risk-taking actions during goal-directed process.
The OFC has been found to be related to the motivational control of goal-directed behavior (OFC activity signaled the expected rewards/punishments of a choice) (Decety et al., 2004; Schoenbaum et al., 2009; Plassmann et al., 2010), reversal learning (brain activations in the OFC reflected the disparity between the expected reward/punishment and the actual reward/punishment) (Schoenbaum and Roesch, 2005; Rudebeck and Murray, 2008; Tsuchida et al., 2010), and autonomic function (inhibitory and excitatory) regulation (Cavada and Schultz, 2000). For example, some disorders of executive functioning and impulse control, such as obsessive–compulsive disorder and trichotillomania, may be affected by dysregulation within the OFC (Tekin and Kircaali-Iftar, 2002; Menzies et al., 2008; Chamberlain et al., 2009). In addition, Jung et al. (2010) found that higher creative achievement (as measured using the CAQ) was associated with lower left lateral orbitofrontal volume, which might be related to novel goal-directed behavior (Rolls et al., 2005). Recently, Takeuchi et al. (2012) also found that an individual’s need for uniqueness (associated with the intrinsic satisfaction derived from the individual’s perception of themselves as different from the masses) was correlated with smaller rGMV in the right inferior frontal gyrus and the ventral part of the PCG, which might be related to reduced response inhibition function. In fact, the OFC projects to the inferior frontal gyrus (Area 47) and belongs to the ventro-lateral PFC (VLPFC). Many previous studies have observed that the VLPFC is critical for creativity (Goel and Vartanian, 2005; Qiu et al., 2010). Specifically, the VLPFC is a brain region extending from the anterior premotor cortex (BA 6) to the posterior frontal pole (BA 10), which had been implicated in cognitively controlled access to relevant information from semantic memory (Petrides and Pandya, 2002; Croxson et al., 2005). Together, our results and those of previous studies suggest that reduced rGMV in the PCG/pMFG and the OFC might be associated with increased intuitive thinking and risk-taking actions (reduced response inhibition and novel goal-directed behavior), and disrespect for social customs and rules.
Interestingly, this study found that openness to experience (a dimension of the NEO-PI-R) can partially mediate the relationship between the rGMV of the right pMTG and trait creativity (as measured using the WCAT). However, this mediation effect was not observed for the other factors of personality, such as extraversion and conscientiousness, which were also correlated with the SWCAT. Many previous studies have indicated that openness to experience is a stable personality factor and that is strongly related to creativity in the real world (Funder, 2001; Hu et al., 2011; Taki et al., 2013). For example, Soldz and Vaillant (1999) found that openness to experience scores were significantly correlated across 45 years in a sample of men. Our behavioral data also showed that the score for openness to experience had a strongly significant correlation with the SWCAT. Based on previous findings (Graziano and Ward, 1992; Fleeson, 2001; Lee et al., 2005), we suggest that openness to experience might induce some particular patterns of cognitive processing associated with intuition, imagination, curiosity and fantasy through ‘semantic processing’ functions related to the right pMTG (Dronkers et al., 2004; Binder et al., 2009; Turken and Dronkers, 2011). That is, the basic personality features related to the trait of openness to experience might play an important role in shaping an individual’s trait creativity. Although we cannot determine the direction of causation between openness to experience, trait creativity (based on the SWCAT) and the right pMTG volume, it might be hypothesized that the openness to experience capacity (which is stable and enduring in healthy adults) is associated with characteristics of trait creativity (e.g. curiosity, imagination and intuitive thinking), which enhances creative thinking and creative achievement. On the basis of this hypothesis, one could infer that the cultivation of the basic personality features of openness to experience in children and adolescents may increase an individual’s trait creativity and, thereby, facilitate divergent thinking and creative achievement.
CONCLUSION
This study used VBM to identify the GMV correlates of individual trait creativity as measured by the WCAT. We found a positive correlation between rGMV in the right pMTG and the SWCAT. In addition, rGMV in the PCG/pMFG and the OFC were negatively correlated with the SWCAT. These results indicate that higher trait creativity might be related to enhanced curiosity, imagination, challenge and risk-taking facilitated by semantic processing skills and reduced inhibition control. In addition, we found that openness to experience partially mediated the relationship between the right pMTG volume and trait creativity. This specific personality trait may therefore play an important role in shaping an individual’s trait creativity. However, because we used young healthy college students with a high-level of education and mainly included right-handedness, our interpretations may have some limitations. In addition, we cannot determine the direction of causation between openness to experience, trait creativity and the right pMTG volume. The implementation of longitudinal or intervention studies may help to elucidate the complex relationships between openness to experience, trait creativity and brain structure further.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31070900; 31271087), the Program for New Century Excellent Talents in University (2011) by the Ministry of Education, the Fundamental Research Funds for the Central Universities (SWU1209101) and the Key Discipline Fund of National 211 Project (TR201208-1). The authors declare no competing interests.
REFERENCES
- Abraham A, Pieritz K, Thybusch K, et al. Creativity and the brain: uncovering the neural signature of conceptual expansion. Neuropsychologia. 2012;50(8):1906–17. doi: 10.1016/j.neuropsychologia.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Aguilar-Alonso A. Personality and creativity. Personality and Individual Differences. 1996;21(6):959–69. [Google Scholar]
- Amabile TM. Creativity in Context. New York: Westview; 1996. [Google Scholar]
- Arden R, Chavez RS, Grazioplene R, Jung RE. Neuroimaging creativity: a psychometric view. Behavioural Brain Research. 2010;214(2):143–56. doi: 10.1016/j.bbr.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Liew SL, Dandekar F. Exploring the neural correlates of visual creativity. Social Cognitive and Affective Neuroscience. 2013;8(4):475–480. doi: 10.1093/scan/nss021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47(6):907–18. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Csíkszentmihályi M, Ullén F. Cortical regions involved in the generation of musical structures during improvisation in pianists. Journal of Cognitive Neuroscience. 2007;19(5):830–42. doi: 10.1162/jocn.2007.19.5.830. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden EM, Jung-Beeman M, Fleck J, Kounios J. New approaches to demystifying insight. Trends in Cognitive Sciences. 2005;9(7):322–8. doi: 10.1016/j.tics.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Brass M, Wenke D, Spengler S, Waszak F. Neural correlates of overcoming interference from instructed and implemented stimulus–response associations. The Journal of Neuroscience. 2009;29(6):1766–72. doi: 10.1523/JNEUROSCI.5259-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Zelazo PD. A brain-based account of the development of rule use in childhood. Current Directions in Psychological Science. 2006;15(3):118–21. [Google Scholar]
- Cai X, Zhu Y. The correlation of adolescent’s creative inclination, intelligence and academic achievement. Psychological Development and Education. 2007;23(2):36–41. [Google Scholar]
- Cappa SF. Imaging studies of semantic memory. Current Opinion in Neurology. 2008;21(6):669–75. doi: 10.1097/WCO.0b013e328316e6e0. [DOI] [PubMed] [Google Scholar]
- Cardillo ER, Watson CE, Schmidt GL, Kranjec A, Chatterjee A. From novel to familiar: tuning the brain for metaphors. Neuroimage. 2012;59(4):3212–21. doi: 10.1016/j.neuroimage.2011.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson SH, Peterson JB, Higgins DM. Reliability, validity, and factor structure of the creative achievement questionnaire. Creativity Research Journal. 2005;17(1):37–50. [Google Scholar]
- Cassandro VJ, Simonton DK. Versatility, openness to experience, and topical diversity in creative products: an exploratory historiometric analysis of scientists, philosophers, and writers. The Journal of Creative Behavior. 2010;44(1):9–26. [Google Scholar]
- Cavada C, Schultz W. The mysterious orbitofrontal cortex. Foreword. Cerebral Cortex. 2000;10(3):205–205. doi: 10.1093/cercor/10.3.205. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Müller U, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biological Psychiatry. 2009;65(7):550–5. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Chamorro-Premuzic T, Reichenbacher L. Effects of personality and threat of evaluation on divergent and convergent thinking. Journal of Research in Personality. 2008;42(4):1095–101. [Google Scholar]
- Chan KW. Creative Teaching and Learning. Hong Kong: Infolink Publishing Ltd; 2005. [Google Scholar]
- Chávez-Eakle RA, Graff-Guerrero A, García-Reyna J-C, Vaugier V, Cruz-Fuentes C. Cerebral blood flow associated with creative performance: a comparative study. NeuroImage. 2007;38(3):519–28. doi: 10.1016/j.neuroimage.2007.07.059. [DOI] [PubMed] [Google Scholar]
- Claxton AF, Pannells TC, Rhoads PA. Developmental trends in the creativity of school-age children. Creativity Research Journal. 2005;17(4):327–35. [Google Scholar]
- Colom R, Jung RE, Haier RJ. Distributed brain sites for the g-factor of intelligence. NeuroImage. 2006;31(3):1359–65. doi: 10.1016/j.neuroimage.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Professional Manual: Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Costa PT, McCrae RR. Domains and facets: hierarchical personality assessment using the Revised NEO Personality Inventory. Journal of Personality Assessment. 1995;64(1):21–50. doi: 10.1207/s15327752jpa6401_2. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, et al. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. Journal of Neuroscience. 2005;25(39):8854–66. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GA. Creativity Is Forever. 3rd edn. Dubuque, IA: Kendall/Hunt; 1992. [Google Scholar]
- Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: an fMRI investigation. Neuroimage. 2004;23(2):744–51. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing predictions from personality neuroscience: brain structure and the Big Five. Psychological Science. 2010;21(6):820–8. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A. The cognitive neuroscience of creativity. Psychonomic Bulletin & Review. 2004;11(6):1011–26. doi: 10.3758/bf03196731. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kanso R. A review of EEG, ERP, and neuroimaging studies of creativity and insight. Psychological Bulletin. 2010;136(5):822–48. doi: 10.1037/a0019749. [DOI] [PubMed] [Google Scholar]
- Digman JM. Personality structure: emergence of the five-factor model. Annual Review of Psychology. 1990;41(1):417–40. [Google Scholar]
- Ding H, Qin W, Jiang T, Zhang Y, Yu C. Volumetric variation in subregions of the cerebellum correlates with working memory performance. Neuroscience Letters. 2012;508(1):47–51. doi: 10.1016/j.neulet.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cerebral Cortex. 2005;15(11):1768–78. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Dollinger SJ, Urban KK, James TA. Creativity and openness: further validation of two creative product measures. Creativity Research Journal. 2004;16(1):35–47. [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–77. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Dwyer L, Edwards D, Mistilis N, Roman C, Scott N. Destination and enterprise management for a tourism future. Tourism Management. 2009;30(1):63–74. [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. NeuroImage. 2012;59(2):1783–94. doi: 10.1016/j.neuroimage.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. Creativity and personality. In: Runco M, editor. The Creativity Research Handbook. Cresskill, NJ: Hampton Press; 1997. pp. 41–6. [Google Scholar]
- Farb, Norman A. S., Grady, Cheryl L., Strother, Stephen, Tang-Wai, David F., Masellis, Mario, Black, Sandra, et al. (2013). Abnormal network connectivity in frontotemporal dementia: Evidence for prefrontal isolation. Cortex, 49(7), 1856–73. [DOI] [PubMed]
- Feist GJ. A meta-analysis of personality in scientific and artistic creativity. Personality and Social Psychology Review. 1998;2(4):290–309. doi: 10.1207/s15327957pspr0204_5. [DOI] [PubMed] [Google Scholar]
- Feist GJ. The influence of personality on artistic and scientific creativity. In: Sternberg RJ, editor. Handbook of Creativity. Cambridge, UK: Cambridge University Press; 1999. pp. 273–96. [Google Scholar]
- Fekken GC. Barron-Welsh Art Scale. In: Sweetland DJKRC, editor. Test Critiques. Vol. IV. Kansas City, MO: Test Corporation of America; 1985. pp. 58–67. [Google Scholar]
- Fink A, Graif B, Neubauer AC. Brain correlates underlying creative thinking: EEG alpha activity in professional vs. novice dancers. NeuroImage. 2009;46(3):854–62. doi: 10.1016/j.neuroimage.2009.02.036. [DOI] [PubMed] [Google Scholar]
- Fleeson W. Toward a structure- and process-integrated view of personality: traits as density distribution of states. Journal of Personality and Social Psychology. 2001;80(6):1011–27. [PubMed] [Google Scholar]
- Funder DC. Personality. Annual Review of Psychology. 2001;52:197–221. doi: 10.1146/annurev.psych.52.1.197. [DOI] [PubMed] [Google Scholar]
- Furnham A, Crump J, Batey M, Chamorro-Premuzic T. Personality and ability predictors of the “Consequences” Test of divergent thinking in a large non-student sample. Personality and Individual Differences. 2009;46(4):536–40. [Google Scholar]
- Gansler DA, Moore DW, Susmaras TM, Jerram MW, Sousa J, Heilman KM. Cortical morphology of visual creativity. Neuropsychologia. 2011;49(9):2527–32. doi: 10.1016/j.neuropsychologia.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Gautam S, Sood AD, Puri RK, Aichelin J. Sensitivity of the transverse flow to the symmetry energy. Physical Review C. 2011;83(3):034606. [Google Scholar]
- Gibson C, Folley BS, Park S. Enhanced divergent thinking and creativity in musicians: a behavioral and near-infrared spectroscopy study. Brain and Cognition. 2009;69(1):162–9. doi: 10.1016/j.bandc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. The functional anatomy of humor: segregating cognitive and affective components. Nature Neuroscience. 2001;4(3):237–8. doi: 10.1038/85076. [DOI] [PubMed] [Google Scholar]
- Goel V, Vartanian O. Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set-shift problems. Cerebral Cortex. 2005;15(8):1170–7. doi: 10.1093/cercor/bhh217. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Gough HG. A creative personality scale for the Adjective Check List. Journal of Personality and Social Psychology. 1979;37(8):1398. [Google Scholar]
- Graziano WG, Ward D. Probing the Big Five in adolescence: personality andadjustment during a developmental transition. Journal of Personality. 1992;60(2):425–39. doi: 10.1111/j.1467-6494.1992.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Gremel C, Costa R. Premotor cortex is critical for goal-directed actions. Frontiers in Computational Neuroscience. 2013;7:110. doi: 10.3389/fncom.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hill KT, Miller LM. Auditory attentional control and selection during cocktail party listening. Cerebral Cortex. 2010;20(3):583–90. doi: 10.1093/cercor/bhp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseinifar J, Siedkalan MM, Zirak SR, et al. An investigation of the relation between creativity and five factors of personality in students. Procedia—Social and Behavioral Sciences. 2011;30:2037–41. [Google Scholar]
- Hu X, Erb M, Ackermann H, Martin JA, Grodd W, Reiterer SM. Voxel-based morphometry studies of personality: issue of statistical model specification—effect of nuisance covariates. Neuroimage. 2011;54(3):1994–2005. doi: 10.1016/j.neuroimage.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Hwang WY, Chen N, Dung J, Yang Y. Multiple representation skills and creativity effects on mathematical problem solving using a multimedia whiteboard system. Educational Technology and Society. 2007;10(2):191. [Google Scholar]
- Jung RE, Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behavioral and Brain Sciences. 2007;30(2):135–54. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Jung RE, Mead BS, Carrasco J, Flores RA. The structure of creative cognition in the human brain. Frontiers in Human Neuroscience. 2013;7:330. doi: 10.3389/fnhum.2013.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Segall JM, Jeremy Bockholt H, et al. Neuroanatomy of creativity. Human Brain Mapping. 2010;31(3):398–409. doi: 10.1002/hbm.20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Beeman M, Bowden EM, Haberman J, et al. Neural activity when people solve verbal problems with insight. PLoS Biology. 2004;2(4):e97. doi: 10.1371/journal.pbio.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Maguire RP, Kurki T, Brück A, Rinne JO. Mapping brain structure and personality in late adulthood. Neuroimage. 2005;24(2):315–22. doi: 10.1016/j.neuroimage.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience. 2011;12(4):231–42. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- King LA, Walker LM, Broyles SJ. Creativity and the five-factor model. Journal of Research in Personality. 1996;30(2):189–203. [Google Scholar]
- Knoch D, Gianotti LRR, Pascual-Leone A, et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. The Journal of Neuroscience. 2006;26(24):6469–72. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Chen K, Womer F, et al. Sex differences of gray matter morphology in cortico-limbic-striatal neural system in major depressive disorder. Journal of Psychiatric Research. 2013;47(6):733–9. doi: 10.1016/j.jpsychires.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa EH, Sato JR, Lacerda SS, et al. Meditation training increases brain efficiency in an attention task. Neuroimage. 2012;59(1):745–9. doi: 10.1016/j.neuroimage.2011.06.088. [DOI] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32(1):477–84. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger S, Rutter B, Stark R, Windmann S, Hermann C, Abraham A. Using a shoe as a plant pot: neural correlates of passive conceptual expansion. Brain Research. 2012;1430:52–61. doi: 10.1016/j.brainres.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Lee K, Ogunfowora B, Ashton MC. Personality traits beyond the Big Five: are they within the HEXACO space? Journal of Personality. 2005;73(5):1437–63. doi: 10.1111/j.1467-6494.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- Lin C, Wang M. The Creativity Assessment Packet. Taipei, Taiwan: Psychological Publishing; 1994. [Google Scholar]
- Liu M-J, Shih W-L, Ma L-Y. Are children with Asperger syndrome creative in divergent thinking and feeling? A brief report. Research in Autism Spectrum Disorders. 2011;5(1):294–8. [Google Scholar]
- Ma HH. The effect size of variables associated with creativity: a meta-analysis. Creativity Research Journal. 2009;21(1):30–42. [Google Scholar]
- Maker CJ, Schiever SW. Teaching Models in Education of the Gifted. Austin, TX: PRO-ED, Incorporated; 2005. [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Current Opinion in Neurobiology. 2001;11(2):194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- McCrae RR. Creativity, divergent thinking, and openness to experience. Journal of Personality and Social Psychology. 1987;52(6):1258. [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience & Biobehavioral Reviews. 2008;32(3):525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL. 2009. Cognitive processes associated with creativity scale development and validation. (Doctoral dissertation, Ball State University) [Google Scholar]
- Miller GF, Tal IR. Schizotypy versus openness and intelligence as predictors of creativity. Schizophrenia Research. 2007;93(1–3):317–24. doi: 10.1016/j.schres.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Moore DW, Bhadelia RA, Billings RL, et al. Hemispheric connectivity and the visual–spatial divergent-thinking component of creativity. Brain and Cognition. 2009;70(3):267–72. doi: 10.1016/j.bandc.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2002;52(8):785–94. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- Oldham GR, Cummings A. Employee creativity: personal and contextual factors at work. Academy of Management Journal. 1996;39(3):607–34. [Google Scholar]
- Ortet G, Ibáñez MI, Moya J, Villa H, Viruela A, Mezquita L. Assessing the five factors of personality in adolescents the junior version of the Spanish NEO-PI-R. Assessment. 2012;19(1):114–30. doi: 10.1177/1073191111410166. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York, NY, US: Oxford University Press; 2002. pp. 31–50. [Google Scholar]
- Piffer D. Can creativity be measured? An attempt to clarify the notion of creativity and general directions for future research. Thinking Skills and Creativity. 2012;7(3):258–64. [Google Scholar]
- Plassmann H, O’Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. The Journal of Neuroscience. 2010;30(32):10799–808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Qiu J, Li H, Jou J, et al. Neural correlates of the “Aha” experiences: evidence from an fMRI study of insight problem solving. Cortex. 2010;46(3):397–403. doi: 10.1016/j.cortex.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Section 4: The Advanced Progressive Matrices. San Antonio, TX: Harcourt Assessment; 1998. [Google Scholar]
- Rhodes M. An analysis of creativity. The Phi Delta Kappan. 1961;42(7):305–10. [Google Scholar]
- Rolls ET, Browning AS, Inoue K, Hernadi I. Novel visual stimuli activate a population of neurons in the primate orbitofrontal cortex. Neurobiology of Learning and Memory. 2005;84:111–23. doi: 10.1016/j.nlm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. The Journal of Neuroscience. 2008;28(33):8338–43. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runco MA. Creativity: Theories and Themes: Research, Development, and Practice. Amsterdam: Elsevier Academic Press; 2007. [Google Scholar]
- Satzinger JW, Garfield MJ, Nagasundaram M. The creative process: the effects of group memory on individual idea generation. Journal of Management Information Systems. 1999;15(4):143–60. [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47(5):633–6. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Reviews Neuroscience. 2009;10(12):885–92. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DL, Mitchell AD, Lahna DL, et al. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. NeuroImage. 2010;50(4):1392–401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobe ER, Ross NM, Fleck JI. Influence of handedness and bilateral eye movements on creativity. Brain and Cognition. 2009;71(3):204–14. doi: 10.1016/j.bandc.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Silver M, Montana G, Nichols TE. False positives in neuroimaging genetics using voxel-based morphometry data. NeuroImage. 2011;54(2):992–1000. doi: 10.1016/j.neuroimage.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldz S, Vaillant GE. The Big Five personality traits and the life course: a 45-year longitudinal study. Journal of Research in Personality. 1999;33(2):208–32. [Google Scholar]
- Sternberg RJ. Handbook of Creativity. New York: Cambridge University Press; 1999. [Google Scholar]
- Sternberg RJ, Lubart TI. Investing in creativity. Psychological Inquiry. 1993;4(3):229–32. [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, et al. Training of working memory impacts structural connectivity. The Journal of Neuroscience. 2010a;30(9):3297–303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Hashizume H, et al. Failing to deactivate: the association between brain activity during a working memory task and creativity. NeuroImage. 2011;55(2):681–7. doi: 10.1016/j.neuroimage.2010.11.052. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, et al. A voxel-based morphometry study of gray and white matter correlates of a need for uniqueness. NeuroImage. 2012;63(3):1119–26. doi: 10.1016/j.neuroimage.2012.08.037. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, et al. Regional gray matter volume of dopaminergic system associate with creativity: evidence from voxel-based morphometry. NeuroImage. 2010b;51(2):578–85. doi: 10.1016/j.neuroimage.2010.02.078. [DOI] [PubMed] [Google Scholar]
- Taki Y, Thyreau B, Kinomura S, et al. A longitudinal study of the relationship between personality traits and the annual rate of volume changes in regional gray matter in healthy adults. Human Brain Mapping. 2013;34(12):3347–53. doi: 10.1002/hbm.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin E, Kircaali-Iftar G. Comparison of the effectiveness and efficiency of two response prompting procedures delivered by sibling tutors. Education and Training in Mental Retardation and Developmental Disabilities. 2002;37(3):283–99. [Google Scholar]
- Tsuchida A, Doll BB, Fellows LK. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. The Journal of Neuroscience. 2010;30(50):16868–75. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. AFNI 3dDeconvolve Documentation. Medical College of Wisconsin; 2000. Simultaneous inference for fMRI data. [cited; Available from: http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf. [Google Scholar]
- Westerhausen R, Moosmann M, Alho K, et al. Identification of attention and cognitive control networks in a parametric auditory fMRI study. Neuropsychologia. 2010;48(7):2075–81. doi: 10.1016/j.neuropsychologia.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Whitney C, Jefferies E, Kircher T. Heterogeneity of the left temporal lobe in semantic representation and control: priming multiple versus single meanings of ambiguous words. Cerebral Cortex. 2011;21(4):831–44. doi: 10.1093/cercor/bhq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams FE. Models for encouraging creativity in the classroom by integrating cognitive–affective behaviors. Educational Technology. 1969;9(12):7–13. [Google Scholar]
- Williams FE. Creativity Assessment Packet (CAP): manual. Buffalo, New York: DOK Publishers; 1980. [Google Scholar]
- Williams FE. Creativity Assessment Packet Examiner's Manual. Austin, TX: PRO-ED; 1993. [Google Scholar]
- Woodman RW. Creativity as a construct in personality theory. The Journal of Creative Behavior. 1981;15(1):43–66. [Google Scholar]
- Woodward TS, Cairo TA, Ruff CC, Takane Y, Hunter MA, Ngan ETC. Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience. 2006;139(1):317–25. doi: 10.1016/j.neuroscience.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Yang F-C, Chou K-H, Fuh J-L, et al. Altered gray matter volume in the frontal pain modulation network in patients with cluster headache. Pain. 2013;154(6):801–7. doi: 10.1016/j.pain.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Zeng L, Salvendy G, Zhang M. Factor structure of web site creativity. Computers in Human Behavior. 2009;25(2):568–77. [Google Scholar]
- Zou L, Ding G, Abutalebi J, Shu H, Peng D. Structural plasticity of the left caudate in bimodal bilinguals. Cortex. 2012;48(9):1197–206. doi: 10.1016/j.cortex.2011.05.022. [DOI] [PubMed] [Google Scholar]