Abstract
One of the most effective strategies for regulating emotional responses is cognitive reappraisal. While prior work has made great strides in characterizing reappraisal’s neural mechanisms and behavioral outcomes, the key issue of how regulation varies as a function of emotional intensity remains unaddressed. We compared the behavioral and neural correlates of reappraisal of high- and low-intensity emotional responses using functional magnetic resonance imaging (fMRI). We found that successful reappraisal of both high- and low-intensity emotions depends upon recruitment of dorsomedial (dmPFC) as well as left dorsolateral (dlPFC) and ventrolateral (vlPFC) prefrontal cortex. However, reappraisal of high-intensity emotions more strongly activated left dlPFC, and in addition, activated right lateral and dorsomedial PFC regions not recruited by low-intensity reappraisal. No brain regions were more strongly recruited during reappraisal of low when compared with high-intensity emotions. Taken together, these results suggest that reappraisal of high-intensity emotion requires greater cognitive resources as evidenced by quantitative and qualitative differences in prefrontal recruitment. These data have implications for understanding how and when specific PFC systems are needed to regulate different types of emotional responses.
Keywords: reappraisal, emotional intensity
INTRODUCTION
Emotions routinely present themselves as powerful and varied forces in our everyday lives. To make this point more concrete, imagine it is a typical weekday morning and you are stuck in bumper-to-bumper traffic. After what feels like an eternity, the car in front of you starts to creep forward and you release your now-cramped foot from the brake pedal. Just as you begin to accelerate, a sporty little convertible shooting up the shoulder of the road cuts abruptly into your lane, forcing you to slam on your brakes. Now imagine that in addition to cutting into your lane that convertible had knocked off your side mirror and sped off into the sunset. While both of these scenarios would elicit anger and frustration, the emotions felt in the second scenario would likely be much more intense. Importantly, in both instances, it may behoove you to regulate your feelings of anger (i.e. to prevent negative physiological consequences and/or a confrontation with the reckless convertible driver).
The example above raises an important question for the study of emotion regulation—do the strategies that work for managing life’s annoyances also work for major emotional issues? Or put another way, does the way in which you regulate and how successful you are at regulating differ as a function of the intensity of the response you experience? How we manage emotional pushes and pulls, both small and large, is a strong predictor of mental health and wellbeing and knowing which strategies to use in different situations may be critical for creating effective clinical treatments (Gross and Munoz, 1995; Gross and John, 2003; Taylor and Liberzon, 2007; Eftekhari et al., 2009; Kim and Cicchetti, 2009). Cognitive regulatory strategies such as reappraisal, where one reinterprets the meaning of a stimulus so as to alter its emotional impact, have been shown to be among the most adaptive means for regulating emotion. Reappraisal is flexible, and can be used to increase or decrease positive or negative affect, and appears to do so without the deleterious consequences for memory or physiological responding associated with other strategies such as emotional suppression (Gross and Levenson, 1997; Gross, 1998, 2001; Ochsner et al., 2012).
Yet, no studies to date have examined how emotional intensity impacts the neural bases of reappraisal. This is surprising, given that second to valence, intensity is perhaps the most critical dimension on which emotional experiences vary (Brehm, 1999; Russell, 2003). Prior work has shown that activation in brain regions such as the amygdala, nucleus accumbens and orbitofrontal cortex scales with affective intensity (Anderson et al., 2003; Cunningham et al., 2004; Phan et al., 2004; Colibazzi et al., 2010; Waugh et al., 2010; Williams et al., 2001). However, such findings have been obtained exclusively in the context of passive viewing, leaving open the question of how people self-regulate in the context of intense emotional experiences. In general, reappraisal is supported by dorsolateral, ventrolateral and dorsomedial prefrontal (dmPFC, vlPFC and dlPFC, respectively) cortical regions that are also known to underlie domain-general cognitive control processes (Duncan and Owen, 2000; Miller and Cohen, 2001). While meta-analytic data suggest that these effects are consistent across studies (Buhle et al., 2013), there is also evidence that factors such as a stimulus’ valence or whether one’s reappraisal goal is to increase or decrease emotion can yield different patterns of activation (Ochsner et al., 2012). To determine whether emotional intensity also may impact the neural bases of reappraisal, the present study directly compared reappraisal of low- and high-intensity emotional responses. In doing so, two specific hypotheses were tested.
The first was that regulation of low- and high-intensity emotion might share a common set of control-related brain regions that support core reappraisal processes. It was hypothesized that these brain regions were likely to include dmPFC and left-lateralized vlPFC and dlPFC for two reasons. First, these regions are known to support domain-general cognitive control processes including working memory, response selection and inhibition, and self-monitoring (Wager and Smith, 2003; Ridderinkhof et al., 2004; Simmonds et al., 2008; Rottschy et al., 2012). In the context of reappraisal, these may be critical for maintaining reappraisals and reappraisal goals in working memory, selecting appropriate reappraisals and reflecting on one’s emotional state so as to monitor how successfully one is regulating (Ochsner and Gross, 2005, 2008; Ochsner et al., 2012). Second, these are among the most consistently reported brain regions in the reappraisal literature to date providing further support for the notion that they are important for an array of reappraisal contexts (Diekhof et al., 2011; Buhle et al., 2013).
The second hypothesis was that some of the control systems supporting reappraisal of low- and high-intensity emotion might be different. These differences could manifest in one of two ways. On the one hand, the increased cognitive demands of reappraising high-intensity emotion might require greater reliance on the core set of control processes—and underlying systems—used to reappraise low-intensity stimuli. For example, regulating high-intensity stimuli may require greater working memory capacity (to generate additional reappraisals as needed) than low-intensity stimuli which may elicit enhanced recruitment of left dlPFC, a brain region strongly implicated in verbal working memory (Wager and Smith, 2003). On the other hand, regulating high- and low-intensity emotions might differ not only in the degree to which they recruit regions that support shared processes, but also regulating high-intensity emotions might recruit additional brain regions altogether. On this view, once emotions reach a certain intensity they place qualitatively rather than quantitatively different demands on control systems. If this is the case, we reasoned that such demands may be met by recruitment of right lateral PFC for two reasons. First, right dlPFC and vlPFC support the inhibition of prepotent responses (Konishi et al., 1999; Aron et al., 2004; Wager et al., 2005). In the context of reappraising a highly arousing stimulus, such inhibitory control may be critical for overriding one’s natural tendency to appraise a stimulus as negative. Second, right lateral PFC recruitment has been shown to scale with executive demands across a host of domains, suggesting that it may generally supply auxiliary cognitive resources when the control system is heavily taxed (Rottschy et al., 2012).
To test these two hypotheses, an experiment was conducted wherein participants either reappraised or responded naturally to emotional stimuli that evoked either high or low amounts of emotional intensity. Patterns of brain activation were then compared for reappraisal of high- and low-intensity emotional stimuli so as to identify core brain regions common to both types of reappraisal as well as those that differed. Brain regions that showed differential activity for reappraisal of high- and low-intensity emotion were further classified as showing one of two types of differences: (i) brain regions showing quantitative differences were those that were recruited for both types of reappraisal but more strongly for reappraisal of high-intensity emotion, while (ii) those showing qualitative differences were only recruited during reappraisal of high-intensity emotion.
METHODS
Participants
Thirty adults were recruited in compliance with the human subjects regulations of Columbia University to participate in this study (13 females, mean age = 21.97). Participants were paid $20/h for their participation. All participants were screened prior to participation and determined to be right-handed with no history of psychiatric or medical illnesses. Prior analyses that did not examine the role of emotional intensity on reappraisal’s neural correlates have been reported elsewhere (Wager et al., 2008; Denny et al., 2013).
Participant training procedures
Prior to scanning, participants were extensively trained on the experimental paradigm in accordance with well-validated procedures (Ochsner et al., 2004b). Participants were told that during the scanner task they would see a series of images preceded by instructional cues. The experimenter told participants that these cues would inform them about the type of stimuli they were to see and whether they were supposed to reappraise or respond naturally (‘look’ cue) to the stimuli. On reappraisal trials participants were told to, ‘re-interpret the possible antecedents, outcomes and/or reality of the events you see in such as way that your emotional response is decreased.’ For example, if one were reinterpreting being cut off by a sports car, one might imagine that the car’s driver was driving aggressively because he was rushing to get his pregnant wife to the hospital. This reinterpretation variant of reappraisal has been successfully used to reduce negative emotion in similar functional magnetic resonance imaging (fMRI) designs before (Ochsner et al., 2002, 2004b; Kim and Hamann, 2007; McRae et al., 2010). Participants practiced reappraising a set of images that were not used in the fMRI task while receiving feedback from an experimenter to ensure that they fully understood the instructions. Participants were asked to not look away from image stimuli or to distract themselves with irrelevant or positive thoughts during the task.
Task design
Participants completed 108 experimental trials inside the MRI scanner. Three variants of a basic reappraisal trial structure were used. The basic structure began with a 2-s instructional cue followed by a 4 s anticipatory interval during which a fixation cross was presented on the screen. In accordance with the cue presented, participants did one of the following: (i) look at the neutral image and respond naturally (look/neutral), (ii) look at the negative images and respond naturally (look/negative) or (iii) reappraise the negative image (reappraise/negative). There were 36 trials of each image/instruction type. The image was then presented for 8 s. Subsequent to image presentation, a fixation cross was presented for a jittered inter-stimulus interval (4–7 s) and then a rating screen appeared for 2.1 s. On the rating screen, participants were asked to rate how strongly negative they felt on a scale of 0–4 (0 = not negative at all, 4 = very negative). The trial concluded after the rating screen with a fixation cross that lasted 4–7 s (the duration was jittered). In addition to the 36 basic trials, participants also completed 36 ‘anticipation-only’ trials (trials that did not include image presentation) and 36 ‘stimulus-only’ trials (trials that did not include the 4-s anticipation period). Given that the focus of the present manuscript was on emotional responses to the affective images, ‘anticipation-only’ trials were not analyzed.
Which images were paired with which strategies (look vs reappraise) were counterbalanced across participants. Thus, 15 participants saw image set A with a look cue and image set B with a reappraise cue while the other 15 participants saw image set B with a look cue and image set A with a reappraise cue. Responses on look trials were used to classify negative images elicited high- or low-intensity affective responses. Look trials were used because participants were instructed to respond naturally on these trials. Participants rated stimuli in a highly consistent manner on; Cronbach’s α was 0.92 and 0.97, respectively, within the two sets of 15 participants. Mean ratings of emotional intensity were calculated for each stimulus based on these look ratings and a median split was subsequently performed on the mean ratings so as to classify each stimulus as eliciting high- or low-intensity emotional responses. Low- and high-intensity emotional stimuli did not differ in terms of properties such as luminance, visual complexity or whether they contained faces (Ps > 0.37). The 24 look/negative and 24 reappraise/negative trials were divided up so as to produce four categories of stimuli: look/low/negative, look/high/negative, reappraise/low/negative and reappraise/high/negative.
Imaging acquisition and analysis
Whole-brain fMRI data were acquired on a 1.5T GE Signa Twin Speed Excite HD scanner (GE Medical Systems). Anatomical SPGR (T1) images were acquired with 124 1.5 mm slices (TR of 19 ms, TE of 5 ms and field of view of 22 cm). Functional images were acquired with a T2*-sensitive EPI BOLD sequence. Twenty-four axial slices were collected with a TR of 2000 ms (TE of 40 ms, flip angle of 60°, field of view of 22 cm and 3.44 × 3.44 × 4.5 mm voxels). Stimuli were presented using E-Prime. Stimuli were displayed using an LCD projector and a back-projection screen mounted in the scanner suite. Participants made their responses using a five-finger-button-response unit with a molded hand brace (Avotec Inc. and Resonance Technologies).
Preprocessing was performed using FSL (FMRIB Center, University of Oxford) and SPM2 (Wellcome Department of Cognitive Neurology, UCL) using well-validated procedures (Wager et al., 2008). Functional images were slice-time and motion corrected using FSL. Structural T1-weighted images were coregistered to the first functional image for each subject using an iterative procedure of automated registration using mutual information coregistration in SPM2 and manual adjustment of the automated algorithm’s starting point by a trained analyst until the automated procedure provided satisfactory alignment. Structural images were normalized (spatially warped) to a standard template brain (the MNI avg15T1.img) using SPM2’s default options (7 × 8 × 7 non-linear basis functions) and the warping parameters were applied to functional images for each subject. Normalized functional images were interpolated to 3 × 3 × 3 mm voxels and spatially smoothed with a 6-mm Gaussian filter.
First- and second-level GLM analyses were performed in NeuroElf (neuroelf.net). The cue, anticipation, stimulus-viewing and response portions of each trial were modeled as boxcar regressors convolved with a canonical hemodynamic response function. Separate regressors were made for the different trial types so that neural responses associated with different instructions (look or reappraise), stimulus valence (neutral or negative) and intensity of emotional response (high or low) could be differentiated. Mean global signal was added as an additional covariate of no interest at the group level. All random-effects group-level analyses were thresholded using a peak and extent combination that controlled the family-wise error rate at α < 0.05, as calculated by AlphaSim, the Monte Carlo simulation method implemented in NeuroElf.
Two imaging analyses were conducted to identify brain regions associated with reappraisal of low- and high-intensity stimuli. First, to identify regions involved in reappraisal of both high- and low-intensity stimuli, a conjunction analysis was performed on the reappraise > look contrast for high-intensity stimuli and for low-intensity stimuli. Second, the reappraise > look contrast for high-intensity stimuli was directly compared with the same contrast for low-intensity stimuli ([reappraise/high > look/high] > [reappraise/low > look/low]). Brain regions identified in this contrast that also fell within a mask produced by the conjunction analysis were identified as showing quantitative differences as a function of emotional intensity (i.e. they were active for reappraisal of both low- and high-intensity emotions but to a greater degree for high-intensity emotions). Brain regions identified in the contrast between the high- and low-intensity reappraisal contrasts that fell outside of those regions identified in the conjunction analysis were characterized as showing qualitative differences as a function of stimulus intensity (i.e. identified in the high, but not low intensity, contrast).
To examine whether comparable results might be obtained using a continuous, rather than categorical approach, a follow-up parametric analysis was conducted. In this analysis, mean ratings of emotional intensity were calculated for each stimulus based on their average rating in the ‘look’ condition. These ratings were z-transformed and subsequently used as a parametric modulator for the reappraise/negative condition.
Because prior work has strongly suggested that reappraisal modulates activity in the amygdala, targeted analyses were completed to examine how stimulus intensity and strategy may impact amygdala modulation. A priori regions of interest in the left (−18, −3, −15) and right amygdala (30, −3, −15) were identified using data from a recent meta-analysis of 48 published neuroimaging studies of reappraisal (Buhle et al., 2013). From these regions of interest, parameter estimates were extracted for the conditions of interest and paired t-tests were completed to examine (i) whether reappraisal reduced amygdala activity for low- or high-intensity stimuli and (ii) whether the reappraisal-related decrease in the amygdala response differed for low- and high-intensity stimuli.
RESULTS
Behavioral results
Manipulation check for stimulus intensity
The split half method was effective, as indicated by the fact that negative stimuli classified as evoking high-intensity emotional responses elicited more negative affect on look trials (M = 3.06) than did stimuli classified as evoking low-intensity responses (low intensity M = 2.16, t(29) = 9.35, P < 0.001) or neutral stimuli (neutral M = 0.34, t(29) = 22.53, P < 0.001). Responses were also reported to be more negative for look/low/negative trials than look/neutral trials (t(29) = 17.15, P < 0.001). These findings together suggest that negative affect was very strong on look/high/negative trials, moderately strong on look/low/negative trials and weak on look/neutral trials.
Success of reappraisal of high- and low-intensity emotional responses
Negative affect ratings were subjected to a 2 (intensity: low and high) × 2 (strategy: look and reappraise) ANOVA. As expected, the ANOVA revealed significant main effects of both affective intensity (F(1,29) = 97.74, P < 0.001) and strategy (F(1,29) = 67.56, P < 0.001), with high-intensity trials eliciting more negative affect than low-intensity trials and reappraisal trials eliciting less negative affect than look trials. An interaction between strategy and affectivity intensity was observed (F(1,29) = 17.31, P < 0.001), due to greater decreases in negative affect for reappraisal on high-intensity trials than low-intensity trials. While the reappraisal-induced decrease in negative affect (reappraisal success) for high-intensity trials (M = 1.19, t(29) = 9.35, P < 10−8) was greater than the decrease for low-intensity trials (M = 0.74, t(29) = 5.99, P < 10−5), this may be at least partially attributable to likely to floor effects (affect can only be reduced to a certain point).
To account for differences in baseline (look condition) negative affect between high- and low-intensity trials, reappraisal success also was examined as a function of the percent decrease in negative affect observed on reappraisal trials when compared with look trials, ((look/negative) − (reappraise/negative))/(look/negative) × 100). This analysis showed that reappraisal resulted in significant proportional reductions in negative affect for both high- (M = 0.38, t(29) = 10.44, P < 10−10) and low-intensity trials (M = 0.30, t(29) = 5.73, P < 10−5), thus suggesting that it was an effective regulation strategy for the two conditions (Figure 1). Regulation success was marginally greater for high-intensity than low-intensity trials (t(29) = 1.87, P = 0.07).
Fig. 1.
Average negative affect ratings made in response to the four aversive trial types. Negative affect was significantly reduced (P < 0.001) by reappraisal in both intensity conditions. Percent decreases in negative affect due to reappraisal are noted.
Commonalities between reappraisal of high- and low-intensity emotional responses
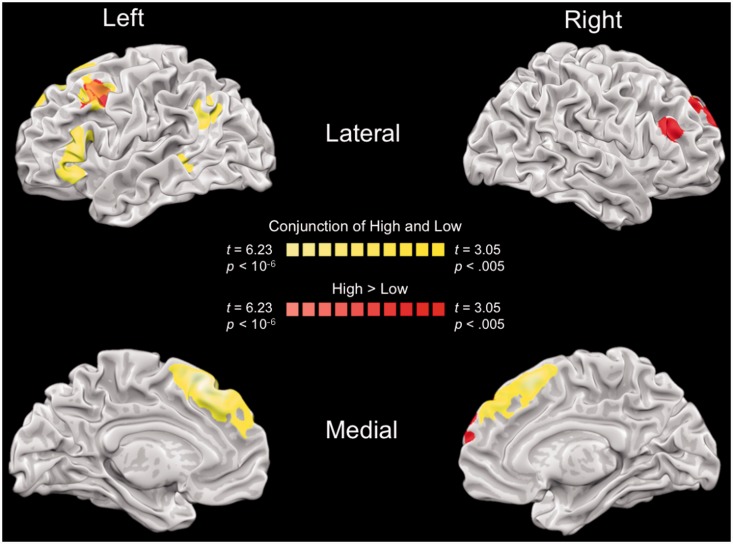
To identify the neural bases of reappraisal of high- and low-intensity emotional responses, we first contrasted activity on reappraise/high/negative trials to activity on look/high/negative trials (the high-intensity regulation contrast) and activity on reappraise/low/negative trials to activity on look/low/negative trials (the low-intensity regulation contrast). Brain regions identified in each of these contrasts are reported in Table 1. A conjunction analysis of these two contrasts revealed that reappraisal of high- and low-intensity emotional responses recruited overlapping brain regions in prefrontal and temporal cortices (Figure 2 and Table 1). Reappraisal of both types of responses elicited large activations in dorsomedial as well as left dorsolateral and ventrolateral PFC. This conjunction analysis captured the majority of voxels (2409/2259, 93.77%) activated during reappraisal of low-intensity images and comprises a set of regions commonly observed during reappraisal (Ochsner and Gross, 2005, 2008).
Table 1.
Reappraisal of low- and high-intensity emotion
| Coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Side | Number of Voxels | t | x | y | z |
| Reappraise/low>Look/low | ||||||
| Inferior frontal gyrus | Left | 521 | 7.34 | −48 | 28 | 14 |
| Superior and middle frontal gyrus; cingulate gyrus | Left | 1467 | 9.65 | −6 | 22 | 56 |
| Middle and superior temporal gyrus | Left | 198 | 5.64 | −57 | −41 | −4 |
| MInferior parietal lobule | Left | 223 | 5.09 | −48 | −56 | 35 |
| Reappraise/high>Look/high | ||||||
| Middle, inferior and superior frontal gyrus; anterior insula | Left | 6021 | 9.67 | −33 | 7 | 44 |
| Inferior and superior parietal lobule; inferior, middle and superior temporal gyrus | Left | 1809 | 7.12 | −48 | −68 | 47 |
| Superior temporal gyrus | Right | 238 | 5.44 | 57 | −53 | 20 |
| Cerebellum | Right | 157 | 4.50 | 27 | −68 | −37 |
| Conjunction | ||||||
| Inferior frontal gyrus | Left | 402 | 5.76 | −48 | 28 | 17 |
| Superior and middle frontal gyrus | Left | 1257 | 8.92 | −6 | 25 | 53 |
| Middle temporal gyrus | Left | 150 | 4.87 | −63 | −38 | −4 |
| Inferior parietal lobule | Left | 192 | 5.09 | −48 | −56 | 35 |
A version of this table including local maxima is provided in Supplementary Table S1 of the online supplement.
Fig. 2.
Brain regions that were similarly activated for reappraisal of high-intensity and low-intensity emotional responses (conjunction of ReappraiseHigh > LookHigh and ReappraiseLow > LookLow) are shown in yellow. Brain regions that were more active for reappraisal of high-intensity emotional responses than low-intensity emotional responses ([ReappraiseHigh > LookHigh]−[ReappraiseLow > LookLow]) are shown in red. High, high-intensity reappraisal contrast (ReappraiseHigh > LookHigh); Low, low-intensity reappraisal contrast (ReappraiseLow > LookLow).
Differential bases of reappraisal of high- and low-intensity emotional responses
Quantitative differences between reappraisal of high- and low-intensity emotional responses
To examine quantitative differences between the low- and high-intensity reappraisal conditions, we identified brain regions that (i) showed increased activity during reappraisal of both high- and low-intensity emotions (as determined by the previous conjunction analysis) and (ii) showed greater activity for one of the two reappraisal contrasts (e.g. (reappraise/high > look/high) > (reappraise/low > look/low)). Using these criteria, only one control related region in left dlPFC showed quantitative differences in activity for reappraisal of low- and high-intensity emotional responses (Figure 2 and Table 2). This single cluster in the left middle frontal gyrus (MFG) was recruited during regulation of both high- and low-intensity emotions, but to a greater extent during reappraisal of high-intensity emotions.
Table 2.
Differences between reappraisal of low- and high-intensity emotion
| Coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | Side | Number of Voxels | t | x | y | z |
| (Reappraise/high > Look/high) > (Reappraise/Low > Look/Low), masked by conjunction | ||||||
| Middle frontal gyrus | Left | 193 | 5.53 | −30 | 10 | 44 |
| (Reappraise/high > Look/high) > (Reappraise/Low > Look/Low), outside of conjunction | ||||||
| Middle and inferior frontal gyrus | Right | 154 | 4.55 | 45 | 34 | 23 |
| Superior and middle frontal gyrus | Right | 199 | 4.24 | 15 | 49 | 44 |
A version of this table including local maxima is provided in Supplementary Table S2 of the online supplement.
Qualitative differences between reappraisal of high- and low-intensity emotional responses
In addition to the cluster identified in left dlPFC, activation in dmPFC and right lateral PFC was stronger during reappraisal of high-intensity emotional stimuli than during reappraisal of low-intensity emotional stimuli (Figure 2 and Table 2). Follow-up t-tests revealed that these regions were strongly recruited during the reappraisal of high-intensity stimuli (Ps < 0.0005), but not low-intensity stimuli (Ps > 0.63). No brain regions showed greater activation for the low-intensity reappraisal contrast than the high-intensity reappraisal contrast. Follow-up regression analyses revealed that no brain regions showed a linear relationship with regulation success for high-intensity stimuli but that recruitment of the right MFG (and no other brain regions) showed a quadratic relationship with regulation success, such that very low and very high left MFG recruitment was associated with high regulation success (βMFGsquared = −0.71, t(29) = −2.19, P < 0.05). Taken together, these results suggest that (i) activation in dorsomedial and right lateral PFC reflects qualitative differences between reappraisal of high- and low-intensity stimuli and (ii) recruitment of right MFG in particular is associated with differential regulation success.
Parametric analysis of stimulus intensity during reappraisal
A parametric analysis examining how trial-by-trial variability in stimulus intensity predicted neural responses during reappraisal revealed no brain regions that survived FWE correction. However, at a less stringent extent threshold (P < 0.005, k = 20 voxels), a single lateral prefrontal (−36, 37, 17; 21 voxels) and parietal cluster (−33, −53, 59; 32 voxels) showed activation that scaled positively with stimulus intensity.
Amygdala response as a function of intensity and strategy
Reappraisal did not significantly reduce amygdala activity for high (left: mean decrease = 0.02, t(29) = 0.99, P = 0.33; right: mean decrease = 0.05, t(29) = 1.09, P = 0.29) or low-intensity stimuli relative to their respective look conditions (left: mean decrease = 0.004, t(29) = 0.10, P = 0.92; right: mean decrease = 0.01, t(29) = 0.20, P = 0.84). Additionally, the magnitude of the reappraisal-related amygdala decrease did not significantly differ between low- and high-intensity stimuli (left: mean difference = 0.02, t(29) = 0.60, P = 0.56; right: mean difference = 0.03, t(29) = 0.53, P = 0.60). Taken together, these data suggest that reappraisal did not significantly modulate the amygdala response for high- or low-intensity stimuli. Additionally, reappraisal success did not predict differential modulation of the amygdala on the left or right side for low- or high-intensity stimuli (Ps > 0.33).
DISCUSSION
This study is the first to directly compare cognitive reappraisal of high- and low-intensity emotional responses. Behaviorally, reappraisal effectively reduced negative affect associated with both high- and low-intensity emotional responses. At the neural level, similarities and differences were observed between the high- and low-intensity reappraisal conditions. On the one hand, both reappraisal conditions recruited left dorsolateral and ventrolateral PFC as well as dorsomedial PFC. On the other hand, reappraisal of high-intensity emotions was associated with (i) enhanced recruitment of left dorsolateral PFC, as well as (ii) recruitment of additional swaths of cortex in right lateral and dorsomedial prefrontal regions.
Commonalities between reappraisal of high- and low-intensity emotional responses
Cognitive reappraisal effectively reduced negative affect associated with high- and low-intensity emotional responses and employed partially overlapping prefrontal networks in doing so. Reappraisal of both high- and low-intensity emotion activated dorsomedial PFC, implicated in emotional awareness and self-monitoring (Amodio and Frith, 2006; Olsson and Ochsner, 2008), left dorsolateral PFC, implicated in working memory and selective attention (Wager and Smith, 2003; Wager et al., 2004), and left ventrolateral PFC, implicated in retrieving and selecting semantic information and responses (Thompson-Schill et al., 1997, 2005; Badre and Wagner, 2007). Taken together, the present data suggest that for both high- and low-intensity emotional situations reappraisal relies heavily on the ability to hold and manipulate reappraisals in working memory, to select relevant information from potential reappraisals and to monitor one’s emotional state (Ochsner et al., 2012).
Differential bases of reappraisal of high- and low-intensity emotional responses
Quantitative differences between reappraisal of high- and low-intensity emotional responses
Within the regions identified by the conjunction analysis, only a portion of left dlPFC showed greater activation during reappraisal of high-intensity emotional responses than during reappraisal of low-intensity emotional responses. Importantly, no brain regions showed greater activation for the low-intensity reappraisal contrast than the high-intensity reappraisal contrast. Prior work has shown that on ‘cold’ cognitive tasks, activity in left dlPFC is modulated by demands placed on selective attention and working memory (Wager and Smith, 2003; Nee et al., 2007), as well as by enhanced motivation to perform tasks successfully (Savine et al., 2010). In the context of the present results, these findings suggest that enhanced left dlPFC activity may reflect an increased motivation to reduce the unpleasant feelings associated with high-intensity negative affect as well as an increased need for cognitive resources that may serve that goal (e.g. control processes to draw attention away from the emotional features of stimuli).
Qualitative differences between reappraisal of high- and low-intensity emotional responses
A direct comparison between the reappraisal conditions revealed that right lateral and dorsomedial PFC were recruited during reappraisal of high-, but not low-, intensity emotional responses. There are at least two potential interpretations for this pattern of results. First, dmPFC and right lateral PFC may be recruited during reappraisal of high-, but not low-, intensity emotional stimuli simply because it is more difficult to reappraise highly arousing stimuli requires additional cognitive resources. This notion is supported by meta-analytic data suggesting that right lateral PFC activation scales with cognitive load on executive function tasks (Rottschy et al., 2012). Yet, the quadratic relationship between right dorsolateral prefrontal recruitment and regulatory success suggests that not all participants recruited such cognitive resources in the same way. It may be that individuals who find reappraisal to be easy only weakly recruit right lateral PFC, while individuals who find reappraising high-intensity stimuli to be difficult overcome this challenge by recruiting additional resources. Second, reappraisal of high-intensity stimuli may involve suppressing or inhibiting prepotent, aversive responses to emotional stimuli, so that one may replace that strong initial appraisal with a more tempered reappraisal. Right lateral PFC is particularly critical for such forms of inhibition on cognitive control tasks (Aron et al., 2004; Aron, 2007), providing further support for this possibility. While prior work has not strongly linked dmPFC with task difficulty or response inhibition, it may play a complementary role to right lateral PFC during regulation of high-intensity emotion. For example, enhanced dmPFC recruitment may reflect a greater need for monitoring reappraisal performance, or alternatively, an increase in attention toward one’s emotional state or those of the individuals depicted in the photographic stimuli that comes as a direct result of feeling more intense emotions (Ochsner et al., 2004a; Amodio and Frith, 2006; Olsson and Ochsner, 2008).
IMPLICATIONS FOR CONCEPTUALIZING THE NEURAL BASES OF REAPPRAISAL
Our results have two implications for how we conceptualize the neurocognitive processes that underlie emotion regulation. The first implication relates to how we maintain comparable levels of reappraisal success for high- and low-intensity emotional responses using shared processes. The results of the present study suggest that reappraisal produced significant drops in negative affect for both high and low emotions and that overlapping prefrontal regions are recruited during reappraisal of high- and low-intensity emotional responses. Recruitment of dorsal and left lateral prefrontal regions implicated in self-monitoring of one’s emotional experience (Ochsner et al., 2004a), working memory (Wager and Smith, 2003) and response inhibition and selection (Van Snellenberg, 2009) was observed for both reappraisal conditions, although the magnitude of activation in left dlPFC varied according to emotional intensity. Across reappraisal studies, activations have been observed in left LPFC and dmPFC more consistently than anywhere else, and also have been observed within studies that included more than one stimulus type or reappraisal goal (Ochsner et al., 2004b; Eippert et al., 2007; Kim and Hamann, 2007; van Reekum et al., 2007; Kanske et al., 2011). This suggests that reappraisal generally recruits medial and left-lateralized prefrontal control circuitry during reappraisal, regardless of the intensity of the emotion one is reappraising. This may have important ramifications for understanding what makes individuals more effective at reappraising. For example, developmental and clinical populations known to have reduced cognitive control capabilities might show proportional deficits in reappraisal ability across emotional situations that vary in their intensity. If such is the case, interventions to improve reappraisal of less intense emotions may also strengthen reappraisal capacity for high-intensity emotions.
The second implication concerns the need to account for key emotion-related stimulus variables in studies of emotion regulation. By manipulating emotional intensity, the present study identified ways in which patterns of neural activity can be impacted by stimulus factors and also offered potential explanations for between-study variability observed in the broader literature. To date, only a handful of studies have directly examined (i.e. within-study) how factors such as one’s regulatory goal (e.g. to increase or decrease emotion) or the nature of the emotional stimulus (e.g. positive vs negative) may impact the neural bases of reappraisal. That said, in looking across the literature, decreasing affect and regulating responses to negative stimuli tend to elicit more bilateral than left-lateralized prefrontal activation (Ochsner et al., 2012). Taken together with the present data, this suggests that while nearly all forms of reappraisal recruit left vlPFC and dlPFC along with dmPFC, more demanding regulatory situations (i.e. when emotional stimuli are particularly intense) require additional recruitment of right LPFC and dmPFC. One limitation of the present design was that emotional intensity and task difficulty were inextricably linked, making it impossible to determine which of the two was driving differences between the low- and high-intensity reappraisal condition. Future work may build on the results of the present study, however, by attempting to dissociate the effects of emotional intensity and task difficulty on the neural bases of emotion regulation.
CONCLUSIONS AND FUTURE DIRECTIONS
The aim of the present study was to examine the behavioral effects and neural bases of reappraisal of high- and low-intensity emotions. We found that reappraisal effectively regulated both high- and low-intensity negative affect and was associated with recruitment of dorsomedial and left LPFC regions associated with self-monitoring, working memory and response inhibition/selection. Importantly, reappraisal of high-intensity emotional responses was associated with increased activity in left dlPFC as well as in right LPFC and a more anterior portion of dmPFC. In contrast, reappraisal of low-intensity emotional responses did not recruit any brain regions not observed during the high-intensity reappraisal condition.
Future work should examine four points related to these findings. First, meta-analytic techniques ought to be applied to the rapidly expanding reappraisal literature to formally test how factors such as stimulus valence or intensity as well as regulatory goals impact prefrontal recruitment. Second, while the stimuli in the present study were effective at eliciting strong emotional responses, they are still likely to have elicited less affect than ‘real-life’ experiences. Follow-up studies are needed to assess whether reappraisal continues to be an effective strategy when regulating emotional responses to highly intense or self-relevant stimuli such as personal memories or social interactions—especially in clinical populations for whom such responses are most troubling. Third, given prior work suggesting that the amygdala (i) tracks with affective intensity and (ii) may mediate the relationship between prefrontal recruitment and decreases in negative affect during reappraisal, it would be worthwhile to examine whether this relationship is mediated by a stimulus’s intensity (Anderson et al., 2003; Cunningham et al., 2004; Wager et al., 2008). While no differences in reappraisal-related amygdala modulation were observed in the present study, such effects may not be easily revealed by subtraction analyses between conditions that are matched for intensity, and may be better suited for mediation. Finally, in the present study participants were instructed to reappraise both high- and low-intensity emotional stimuli as a means of self-regulating their emotions. Yet in real life, the regulatory strategy people choose may vary as a function of emotional intensity, available regulatory resources, or other variables (Urry and Gross, 2010; Opitz et al., 2012). Indeed, prior work suggests that when allowed to choose, individuals prefer to distract themselves upon encountering highly intense emotional stimuli and prefer to reappraise low-intensity stimuli (Sheppes et al., 2011). This raises the possibility that participants in the present study may have been less likely to follow instructions to reappraise high-intensity stimuli than low-intensity stimuli. However, post-experiment interviews with participants suggested that participants followed the instructions to reappraise on the majority of trials for both high- (mean = 86.36%) and low-intensity stimuli (mean = 87.12%). Taken together, this suggests that individuals can and will reappraise a variety of types of stimuli when instructed to do so, but also that when left to their own devices they may prefer certain regulatory strategies for certain stimuli. Thus, future work might consider comparing reappraisal and distraction for high- and low-intensity emotions as a means of examining whether the ‘match’ of a regulatory strategy to the emotion being regulated impacts neural recruitment and regulatory efficacy.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (grant number MH094056 awarded to JAS; R01 MH076137 awarded to KNO and TDW) and the National Science Foundation (grant number 0631637 awarded to KNO)
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Review. Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13(3):214–28. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Brehm JW. The intensity of emotion. 1999. Personality and Social Psychology Review. 1999;3(1):2–22. doi: 10.1207/s15327957pspr0301_1. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, et al. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht154. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colibazzi T, Posner J, Wang Z, et al. Neural systems subserving valence and arousal during the experience of induced emotions. Emotion. 2010;10(3):377–89. doi: 10.1037/a0018484. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. Journal of Cognitive Neuroscience. 2004;16(10):1717–29. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Denny BT, Ochsner KN, Weber J, Wager TD. Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nss148. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58(1):275–85. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23(10):475–83. doi: 10.1016/s0166-2236(00)01633-7. [Record as supplied by publisher] [DOI] [PubMed] [Google Scholar]
- Eftekhari A, Zoellner LA, Vigil SA. Patterns of emotion regulation and psychopathology. Anxiety, Stress & Coping. 2009;22(5):571–86. doi: 10.1080/10615800802179860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28(5):409–23. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74(1):224–37. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation in adulthood: timing is everything. Current Directions in Psychological Science. 2001;10(6):214–9. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychological. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Munoz RF. Emotion regulation and mental health. Clinical Psychological: Science and Practice. 1995;2:151–64. [Google Scholar]
- Kanske P, Heissler J, Schonfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex. 2011;21(6):1379–88. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kim J, Cicchetti D. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychology and Psychiatry. 2010;51(6):706–16. doi: 10.1111/j.1469-7610.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19(5):1–23. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–91. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience. 2010;22(2):248–62. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, John J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective & Behavioral Neuroscience. 2007;7(1):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Currents Directions in Psychological Science. 2008;17(1):153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow D, Hanelin J, Ramachandran T, Mackey S. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004a;16(10):1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004b;23(2):483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12(2):65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Opitz PC, Gross JJ, Urry HL. Selection, optimization, and compensation in the domain of emotion regulation: applications to adolescence, older age, and major depressive disorder. Social and Personality Psychology Compass. 2012;6(2):142–155. [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, et al. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60(1):830–46. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychological Review. 2003;110(1):145–72. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Savine AC, Beck SM, Edwards BG, Chiew KS, Braver TS. Enhancement of cognitive control by approach and avoidance motivational states. Cognition & Emotion. 2010;24(2):338–56. doi: 10.1080/02699930903381564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G, Scheibe S, Suri G, Gross JJ. Emotion-regulation choice. Psychological Science. 2011;22(11):1391–6. doi: 10.1177/0956797611418350. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–32. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends in Cognitive Sciences. 2007;11(10):413–8. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Current Opinion in Neurobiology. 2005;15(2):219–24. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences United States of America. 1997;94(26):14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Gross JJ. Emotion regulation in older age. Current Directions in Psychological Science. 2010;19(6):352–7. [Google Scholar]
- van Reekum CM, Johnstone T, Urry HL, et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36(3):1041–55. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Van Snellenberg JX. Cognitive and motivational functions of the human prefrontal cortex. In: Christensen A-L, Goldberg E, Bougakov D, editors. Luria’s Legacy in the 21st Century. Oxford, UK: Oxford University Press; 2009. pp. 30–61. [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22(4):1679–93. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(4):255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27(2):323–40. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Hamilton JP, Gotlib IH. The neural temporal dynamics of the intensity of emotional experience. Neuroimage. 2010;49(2):1699–707. doi: 10.1016/j.neuroimage.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14(5):1070–9. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.