Abstract
Social decision making is guided by the ability to intuitively judge personal attributes, including analysis of facial features to infer the trustworthiness of others. Although the neural basis for trustworthiness evaluation is well characterized in adults, less is known about its development during adolescence. We used event-related functional magnetic resonance imaging to examine age-related changes in neural activation and functional connectivity during the evaluation of trust in faces in a sample of adolescent females. During scanning, participants viewed masked presentations of faces and rated their trustworthiness. Parametric modeling of trust ratings revealed enhanced activation in amygdala and insula to untrustworthy faces, effects which peaked during mid-adolescence. Analysis of amygdala functional connectivity demonstrated enhanced amygdala–insula coupling during the evaluation of untrustworthy faces. This boost in connectivity was attenuated during mid-adolescence, suggesting a functional transition within face-processing circuits. Together, these findings underscore adolescence as a period of reorganization in neural circuits underlying socioemotional behavior.
Keywords: trust, adolescence, amygdala, insula, face processing
INTRODUCTION
Evaluating the trustworthiness of others is a key component of social behavior. Given relatively little information, such as facial appearance, individuals make rapid inferences about the traits of others (e.g. Locher et al., 1993; Bar et al., 2006; Todorov et al., 2009) that shape social interactions and guide decision making. These thin slice judgments (Ambady and Rosenthal, 1992) have been shown to impact outcomes ranging from mate selection to electoral success (Olivola and Todorov, 2010). As such, characterizing the mechanisms underlying these rapid evaluations is important for understanding how social behavior unfolds.
Adolescence is a critical developmental period concomitant with profound changes in social behavior. This period is characterized by increased levels of peer influence, exploratory behavior, impulsivity and reward seeking (Steinberg et al., 2008), with some potentially negative behavioral consequences (Eaton et al., 2012). Throughout adolescent development, emerging theory and evidence support that social factors play an increasingly important role in guiding decision making (Crone and Dahl, 2012) and that peer acceptance becomes an increasingly salient factor guiding behavior. As part of this changing social landscape, the ability to evaluate trust in others and develop social relationships based on trust and reciprocity becomes more important, particularly as increasing autonomy becomes further established by late adolescence. For example, interpersonal trust has been shown to impact decision making among peers, including drug use (Hundleby and Mercer, 1987) and use of contraceptives (Bauman and Berman, 2005). Given the possible health and socioemotional effects of these risky decisions, it is vital to understand the social evaluation of trust during adolescence.
Changes in socioemotional behaviors in adolescence have been explained in terms of the development of distinct neural systems in a number of neurobiological models (Nelson et al., 2005; Casey et al., 2008; Steinberg, 2008; Ernst and Fudge, 2009). Despite differing in complexity, these models commonly incorporate an affective limbic system and a regulatory prefrontal system (although the specific focus of models differ, see Burnett et al., 2011 for a comparison and review). Generally, pubertal hormone changes and brain development in the limbic forebrain are thought to mature before prefrontal systems involved in cognitive and self-regulatory processing, resulting in a period of increased risk-taking, changes in social behavior and vulnerability to affective disorders. Although this ‘standard model’ is parsimonious and characterizes predominant changes in neural function, the extent to which the concept of frontal immaturity explains the complexities of adolescent development is an open question (Pfeifer and Allen, 2012). For instance, it has been proposed that changes in other emotional and social processing regions during puberty also play a prominent role in adolescent behavior and vulnerability (for review, see Crone and Dahl, 2012).
Faces engage distributed neural regions that are proposed to underlie behavioral changes during adolescence. Rapid social-evaluative processing of untrustworthy faces in adults has been shown to selectively activate limbic and sensory brain areas, including the amygdala, insula and fusiform gyrus (Winston et al., 2002; Engell et al., 2007; Said et al., 2009). Administration of testosterone further increases amygdala responsivity to untrustworthy faces (Bos et al., 2012). Research on healthy aging has demonstrated that elderly adults (55–80 years) exhibit attenuated anterior insula responses to untrustworthy faces and rate them as being more approachable and trustworthy compared with younger adults (23–46 years; Castle et al., 2012). To our knowledge, no neuroimaging work has investigated comparable evaluative processing of trust in adolescence (but see van den Bos et al., 2011; Fett et al., 2013 for work on the development of social reciprocity). In adolescence, social networks have increasing influence over decisions and behaviors. The distributed neural circuitry associated with trait judgments such as trustworthiness may therefore demonstrate development-specific changes in this age range.
Studies examining the perception of fearful emotional expressions have shown increased amygdala activation in the middle years of adolescence (Monk et al., 2003; Guyer et al., 2008; Hare et al., 2008). Given that amygdala responses in adults track the valence content (i.e. hedonic value) of faces rather than individual trait judgments (such as trustworthiness) per se (Todorov and Engell, 2008), it is plausible that developmental changes in amygdala reactivity to untrustworthy faces parallel those observed to fearful expressions, as both types of faces contain negative content and serve as markers of social threat.
Here, we investigated neural activation underlying the evaluation of trust in faces using functional magnetic resonance imaging (fMRI) across adolescence. Unlike prior studies investigating fearful faces in adolescence, we used brief presentations of faces both to capture rapid face-processing mechanisms that contribute to ‘thin slice judgments’ of others and to reduce the potential contributions of voluntary regulation strategies from unduly influencing results. Neural responses along a dimension of trustworthiness are thus tracked using backward masking to present images just above the threshold of perceptual awareness. Based upon neuroimaging evidence in adults, we focused on regions implicated in the evaluation of trust—namely the amygdala, insula, posterior superior temporal sulcus (pSTS), and fusiform gyrus. Given evidence of non-linear activation profiles as a function of age across adolescence (Casey et al., 2008), we predicted that activity within these face-processing regions should be the greatest to untrustworthy faces in mid-adolescence. Evidence supporting this hypothesis stands to extend core findings in adolescence by examining the evaluation of social threat, rather than faces generally or specific emotional expressions (e.g. Monk et al., 2003; Guyer et al., 2008).
A secondary goal of this work was to investigate trust-related changes in functional coupling across adolescence. The brain regions implicated in the evaluation of trust in faces have been proposed as nodes within a distributed face-processing network (Haxby et al., 2000), wherein interactions between the fusiform gyrus, pSTS, amygdala, and insula are thought to mediate processing of emotional properties of faces. In this model, the fusiform gyrus and pSTS process the visual features of faces, whereas the amygdala and insula confer additional emotional information. Functional imaging studies in adults (e.g. Morris et al., 1998; Fairhall and Ishai, 2007) have shown coupled activation between visual cortical areas and the amygdala during processing of fearful facial expressions. Further, increases in visual cortical activity are attenuated in individuals with amygdala lesions (Vuilleumier et al., 2004), highlighting the potential role of feedback from the amygdala in mediating the activity of face-processing regions. The development of functional connectivity underlying emotional face processing across adolescence has received little attention. It is possible that puberty will increase the salience of emotional information in face processing, thus shifting patterns of functional connectivity within face-processing regions (Scherf et al., 2012). Findings supporting this hypothesis would begin to shed light on how socioemotional processing matures at the circuit level, adding novel information to an understudied topic in the developmental neuroscience literature.
METHODS
Participants
Participants provided written informed consent to take part in a study approved by the Duke University Institutional Review Board. The sample consisted of 43 healthy right-handed female volunteers (20 White, 17 Black, 3 Asian, 3 other; mean age 14.7 years, age range 10–20 years). Parental consent was also obtained for all participants as part of a larger investigation and parental assent was obtained from a parent or guardian of children under age 18. All participants (or their guardians) received monetary compensation for completion of the study at a rate of $20/h. Monetary compensation was split between the parent and the adolescent, and the adolescent received an additional prize bag as an incentive for hard work throughout the protocol (i.e. a Silly Band for every hour on task).
Materials and methods
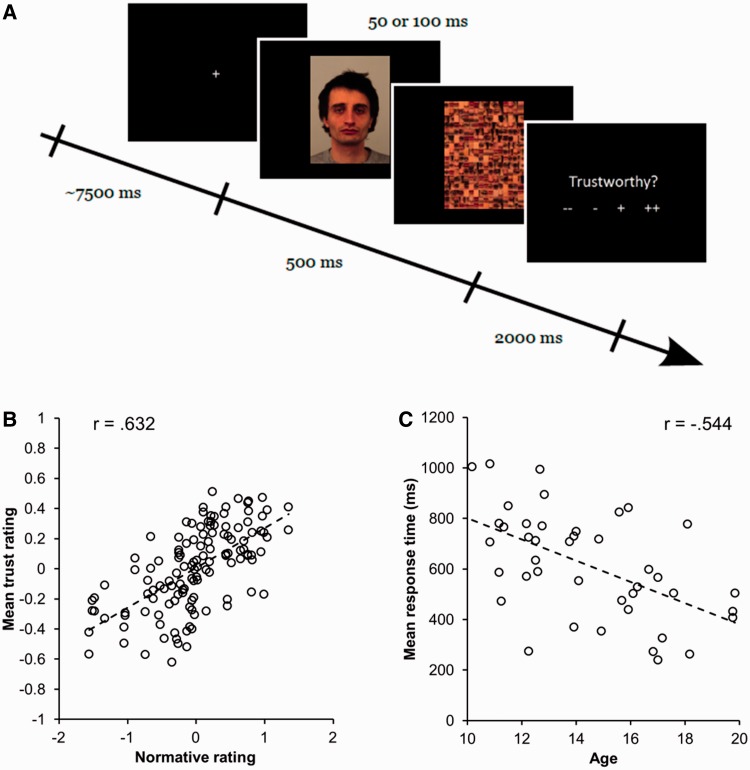
Stimuli consisted of direct-gaze facial images from the Karolinska face database (Lundqvist et al., 1998) rated on a number of trait dimensions, including trustworthiness, and visual masks constructed from components of faces generated using the FaceGen software (Version 3.1, http://facegen.com; Oosterhof and Todorov, 2008). Trials consisted of three phases: presentation of a face, a visual mask and the trustworthiness rating period (Figure 1A). On each trial following the presentation of faces and masks, participants were presented with the word ‘Trustworthy?’ and four symbols (‘− −’, ‘−’, ‘+’ and ‘+ +’) prompting for a response ranging from low to high trustworthiness. To clarify the concept of trustworthiness, participants were instructed as follows: ‘You will be asked how trustworthy you think these faces are. Trustworthy means how much you can trust this person. For example, someone you trust is someone you could tell a secret’. In order to isolate hemodynamic responses to individual trials, the 2 s response phase was followed by an inter-trial interval following a Poisson distribution with a mean duration of 7.5 s.
Fig. 1.
Experimental paradigm and behavioral results. (A) Trial structure for event-related task. Following a jittered inter-trial interval, participants are presented with a backward-masked face for 50 or 100 ms. Subsequently, they are asked to rate the trustworthiness of the face using a four-point Likert scale. (B) Scatterplot of group-averaged ratings of trustworthiness against adult database norms for the stimuli used. Adolescents show a strong positive correlation with the adult norms. (C) Scatterplot of changes in response time with age. Individuals show decreases in response time to make trustworthiness judgments with age. Pearson correlation coefficients are overlaid on scatterplots.
The presentation of facial images alternated in blocks of 34 trials in which face stimuli were visible for either 50 or 100 ms. The length of mask presentation was adjusted accordingly to ensure that the combined duration of the face and mask was 500 ms. All face stimuli were presented once at each duration in a pseudo-randomized order. The first block of trials always consisted of 100 ms presentations, and subsequent blocks were counterbalanced across participants. This order was selected to ensure that participants became acclimated to the task before the 50 ms block, given that very brief presentation times could confuse participants during the initial trial block as those images were barely visible.
Stimulus presentation and behavioral response acquisition were conducted using Presentation® software (Version 0.81, www.neurobs.com). Participants viewed stimuli projected to mirrors aligned with an LCD screen upon which images were projected from a stimulus control computer. Responses were made using a button box connected to the stimulus control computer via a serial cable. Through communication with the serial port, the software logged behavioral ratings and response latencies for each trial.
fMRI acquisition
Scanning was performed on a 3 Tesla General Electric MR 750 system with 50 mT/m gradients and an eight-channel head coil for parallel imaging (General Electric, Waukesha, WI, USA). High-resolution images were acquired using a 3D fast SPGR BRAVO pulse sequence (repetition time = 7.58 ms; echo time = 2.936 ms; image matrix = 2562; voxel size = 1 × 1 × 1 mm) for coregistration with the functional data. These structural images were aligned in the near-axial plane defined by the anterior and posterior commissures. Whole-brain functional images were acquired using a sensitivity-encoded (SENSE) spiral-in pulse sequence along the axial plane (repetition time = 2000 ms; echo time = 30 ms; image matrix = 64 × 128; α = 70°; voxel size = 3.8 × 3.8 × 3.8 mm; 34 contiguous slices). Four runs of 134 images were collected for the functional task. The first five images of each run were excluded in order to ensure that analysis was conducted on data acquired after the magnet reached a steady state.
Preprocessing
Preprocessing and statistical analysis of fMRI data were performed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Imaging Neuroscience). Functional images were spatially realigned to correct for motion artifacts, coregistered to high resolution anatomical scans, normalized to Montreal Neurologic Institute (MNI) space using high-dimensional warping implemented in the voxel-based morphometry (VBM) toolbox (http://dbm.neuro.uni-jena.de/vbm.html), smoothed using a 4 mm full-width half-maximum Gaussian kernel, and filtered using a 128 s high-pass temporal filter.
Data analysis
General linear models were constructed with separate regressors for the presentation of facial images and subsequent motor behavior during the response period. Regressors were created by convolving the canonical hemodynamic response implemented in SPM with box-car functions of 500 ms for masked facial images and the length of response times in the case of motor responses. To examine changes in functional activation related to perceived trustworthiness, a parametric regressor tracking behavioral ratings on a trial-by-trial basis was creating using first- (linear) and second- (non-linear) order polynomial functions. These regressors were orthogonalized with respect to the modeled responses for face presentations, such that the corresponding parameter estimates reflect changes in activity related to trustworthiness independent of the average response to all faces within a voxel. Motion parameters for translation (in the x, y and z dimensions) and rotation (roll, pitch and yaw) were entered as nuisance regressors for each run. In general, head motion was minimal; the average within-run root mean square displacement was 0.202 mm (s.d. = 0.183 mm). Models were collapsed across the two presentation times, as differences in trust ratings across durations did not vary as a function of age (see Results).
Contrasts for the average response to all faces and modulation by trust were created for each subject, treating run as a fixed effect. Separate random-effects group level analyses were conducted for each contrast using a multiple regression approach. In order to test our hypotheses, the models included terms for the average response, first-order changes with age to assess incremental changes across adolescence and second-order changes with age to assess peaks in mid-adolescence.
In addition to changes in neural activation, developmental alterations in functional connectivity with the amygdala were examined as an a priori region of interest (ROI). Functional connectivity maps were generated by correlating the mean parameter estimates of individual trials from an anatomically defined bilateral amygdala mask (Tzourio-Mazoyer et al., 2002) to all other voxels in the brain (Rissman et al., 2004). This single-trial model isolates task-related activation from an implicitly modeled baseline, which can subsequently be used to assess functional connectivity. Correlations were performed separately for faces rated as being low or high in trustworthiness, based on a median split of the participants’ own responses, using Pearson’s correlation coefficient. This approach ensures that trials varying from neutral to extreme values of trustworthiness (either low or high) are captured in each connectivity map. Following standardization using the Fisher transform, the connectivity map for high trustworthy faces was subtracted from that of low trustworthy faces. This contrast reveals how functional coupling across multiple trials differs between psychological contexts of low and high trust. These statistical maps were used as input for a group-level multiple-regression model testing average, first-order and second-order changes with age. One participant was excluded from this analysis because of insufficient variability in trustworthiness ratings. If amygdala coupling with face-processing regions changes during adolescence, either the first- or second-order term should be significantly different than zero, depending on whether the changes are incremental or peak in mid-adolescence.
Statistical inference was made using statistical thresholds corrected for multiple comparisons by estimating the false positive rate using Monte Carlo simulation (Forman et al., 1995). Alpha levels were estimated separately for whole-brain exploratory analyses in addition to bilateral ROIs drawn from the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002) implemented in Wake Forest University PickAtlas (Maldjian et al., 2003, 2004). A mask for the pSTS was created by excluding portions of superior temporal gyrus mask anterior to the slice y = −32 mm. Running simulations over 1000 iterations yielded different extent thresholds for inferences drawn from the whole brain (P < 0.001, k = 63), fusiform gyrus (P < 0.05, k = 27), pSTS (P < 0.05, k = 20), amygdala (P < 0.05, k = 1) and insula (P < 0.05, k = 20) necessary to produce a false positive rate of α = 0.05 (using the AlphaSim tool). To confirm that age-related effects were not driven by outliers, we estimated bootstrap means and standard errors by resampling 10 000 random draws of subjects.
RESULTS
Behavioral results
Analysis of on-line trustworthiness ratings demonstrated that adolescents rate the trustworthiness of rapidly presented faces in a manner consistent with that of adults. Within-subject correlations between on-line and normative ratings (Oosterhof and Todorov, 2008) were highly significant (MR = 0.237, s.e. = 0.02, P < 0.001; Figure 1B) and did not vary linearly with age (R = 0.097, P = 0.54). However, they did exhibit a non-linear trend (R = 0.29, P = 0.051) wherein ratings for mid-adolescents were less correlated with the adult norms. Ratings made by participants age 13–15 were nonetheless highly positively correlated with norms (MR = 0.209, s.e. = 0.03, P < 0.001), suggesting this group of participants still rated in a manner consistent with norms, albeit at a somewhat noisier level. Correlations of mean trustworthiness ratings failed to reveal any changes with age (all Ps > 0.51), ruling out systematic bias in the evaluation of faces (e.g. lower ratings of trust throughout adolescence). Reaction time data showed a linear relationship with age (R = −0.544, P < 0.001; Figure 1C), with older individuals responding faster. Non-linear covariation between reaction time and age was not significant (R = 0.05, P = 0.75).
Analysis of differences in ratings for 50 vs 100 ms presentations revealed no significant changes as a function of age. Comparisons of mean trustworthiness ratings across stimulus durations revealed 50 ms presentations were rated as more trustworthy than 100 ms presentations (M = 0.067, s.e. = 0.025, P < 0.05), which did not vary as a function of age (all Ps > 0.66). Correlations between on-line and normative ratings were significant at both 50 ms (MR = 0.208, s.e. = 0.02, P < 0.001) and 100 ms (MR = 0.276, s.e. = 0.02, P < 0.001) durations, which did not vary with age (all Ps > 0.09).
fMRI results
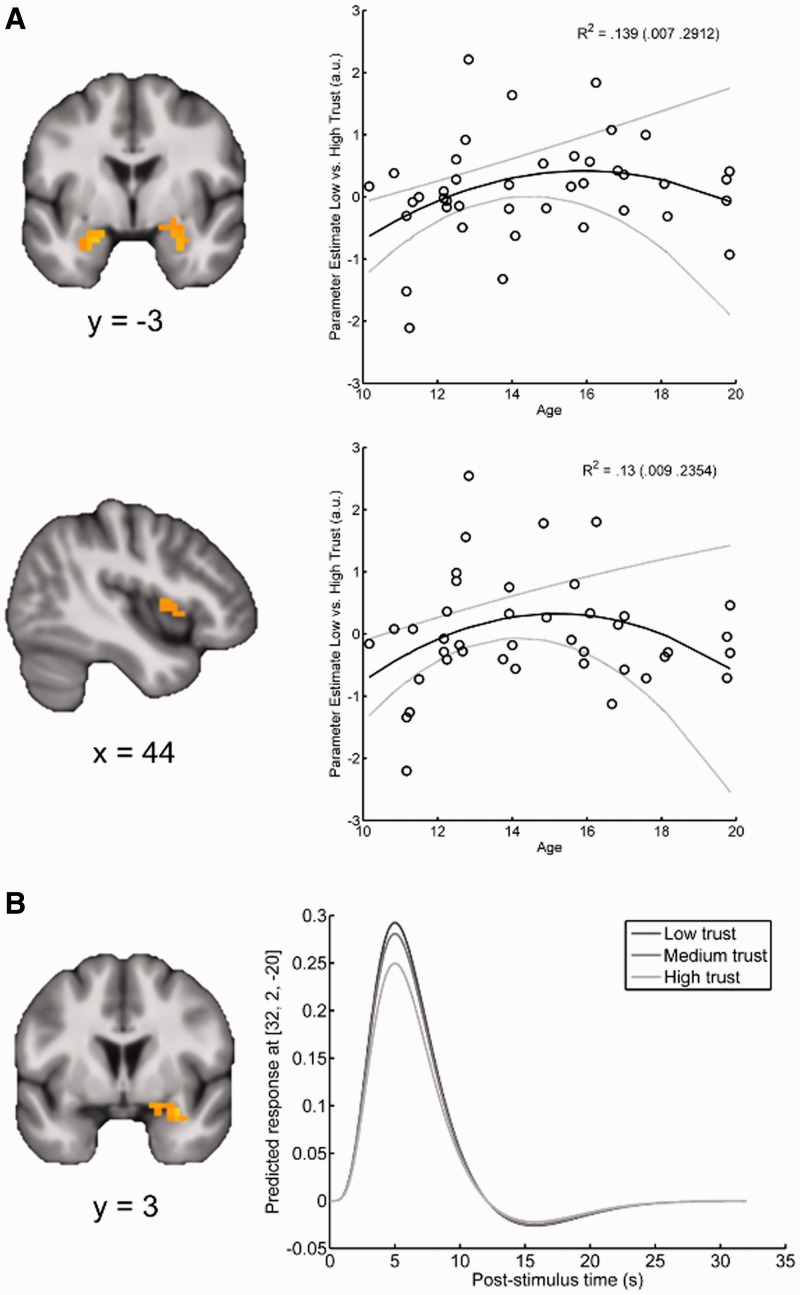
As no age-related behavioral effects were found as a function of stimulus duration, here we present results collapsed across both presentation times. The group-level multiple regression analyses with linear age, non-linear age and a constant term predicting linear parametric modulation by trust revealed several significant effects. Confirming our primary hypothesis, neural activation within bilateral amygdala (T = 2.51/2.33; XYZMNI = −21, −2, −23/29, 2, −27; k = 10/20) and the right anterior insula (T = 2.25; XYZMNI = 40, 21, 7; k = 25) showed greater linear modulation by untrustworthy faces in mid-adolescents relative to younger and older individuals (Figure 2A). Consistent with previous research in adult populations, a cluster of increased neural activation to untrustworthy faces was observed in the right amygdala (T = 2.61; XYZMNI = 32, 2, −20; k = 11; Figure 2B) independent of age. No clusters within the fusiform gyrus, pSTS, or additional regions from exploratory whole-brain analysis were significantly modulated by untrustworthy faces. No regions exhibited non-linear profiles of trustworthiness modulation—either as a main effect or an interaction with age.
Fig. 2.
Neural activation to untrustworthy stimuli during adolescence. (A) The amygdala (top) and insula (bottom) exhibit a peak modulation by untrustworthy faces during mid-adolescence. Parameter estimates reflect the direction of the linear relationship between hemodynamic activation and on-line ratings (positive values reflect increased activity for faces judged untrustworthy). Scatterplots reflect mean response in all significant voxels for illustrative purposes. Bootstrap means (95% CI) of R2 for the full model are displayed in the scatter plot. Solid black (gray) lines depict bootstrap means (95% CI) of model predictions. (B) Amygdala responses parametrically increase with untrustworthiness independent of age. Contrasts are thresholded at a voxel-wise level of P < 0.05 corrected for multiple comparisons and overlaid on the group mean anatomical image.
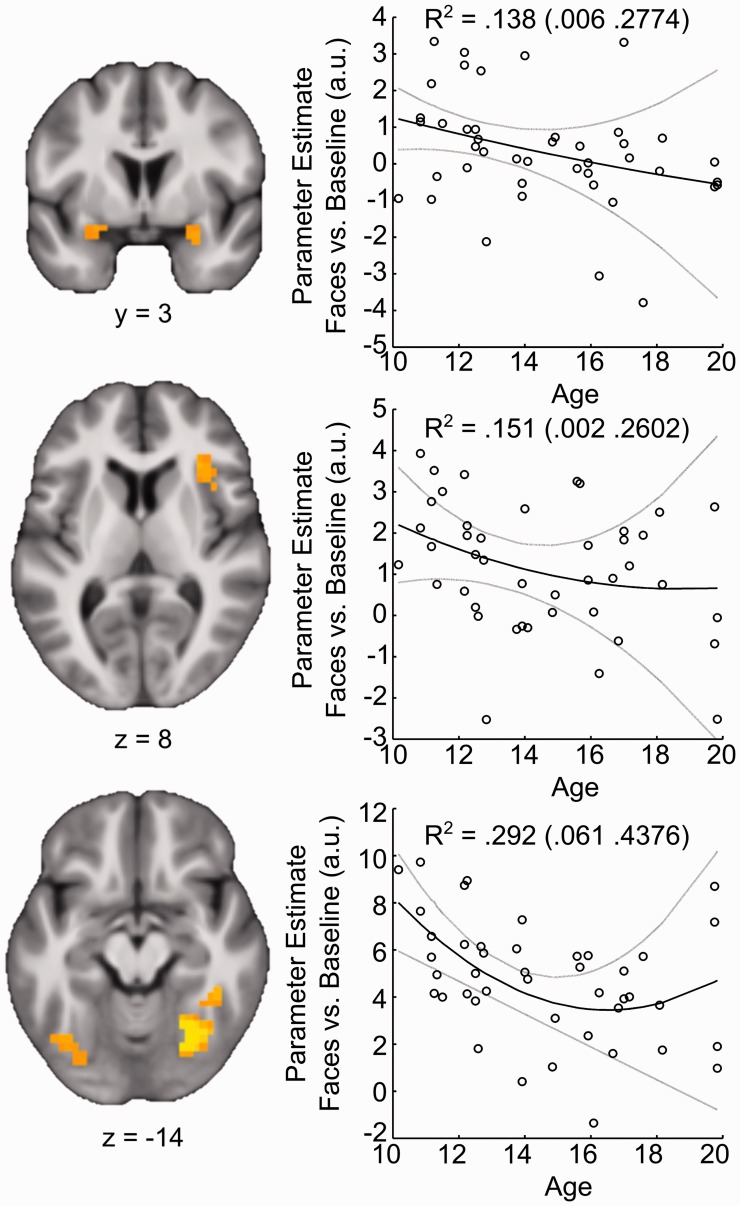
In addition to modulation along a dimension of perceived trust, multiple regions exhibited age-related changes during the processing of faces, irrespective of self-reported trustworthiness. These effects were identified in the group-level model predicting responses to all faces, controlling for the first- and second-order parametric modulators (see Methods). Clusters in bilateral amygdala (T = 2.02/T = 2.02; XYZMNI = −25, 2, −16/29, 2, −20; k = 6/6), right anterior insula (T = 2.22; XYZMNI = 36, 17, 11; k = 21) and bilateral fusiform gyrus (T = 2.43/3.97; XYZMNI = −40, −66, −20/21, −63, −12; k = 27/87) all showed linear decreases in general face-related activity through adolescence (Figure 3). Beyond linear changes, a cluster within the right fusiform gyrus (T = 3.46; XYZMNI = 25, 59, −12; k = 65) exhibited a second-order relationship with age, in which activity showed a specific decline in mid-adolescence. To ensure that these effects were not due to time on task (which decreased with age), post hoc correlations were run with individual differences in reaction time. Changes in mean activity within these regions failed to show significant relationships with reaction time (all Ps > 0.42).
Fig. 3.
Regions exhibiting decreases in activation to face stimuli during adolescence. Parameter estimates reflect the amplitude of response to faces vs baseline. Contrasts are thresholded at a voxel-wise level of P < 0.05 corrected for multiple comparisons and overlaid on the group mean anatomical image. Scatterplots reflect mean response in all significant voxels for illustrative purposes. Bootstrap means (95% CI) of R2 for the full model are displayed in the scatter plot. Solid black (gray) lines depict bootstrap means (95% CI) of model predictions.
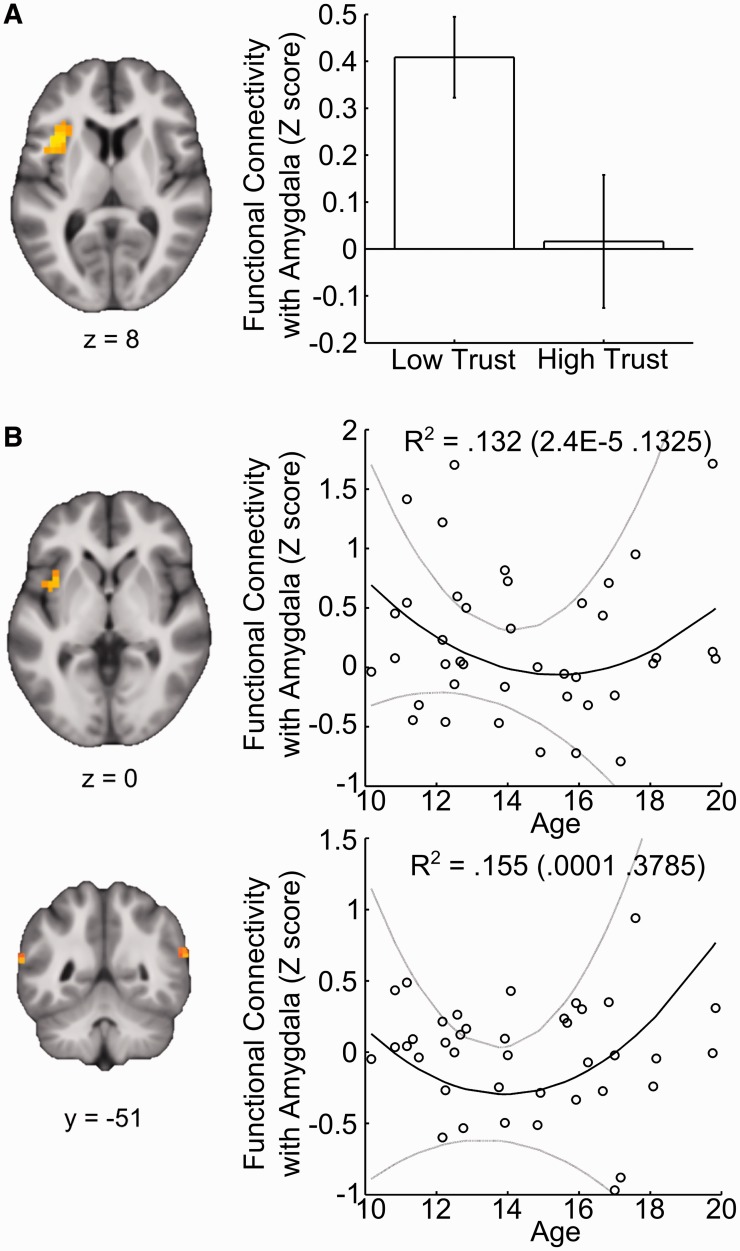
Several regions implicated in processing social stimuli demonstrated changes in amygdala connectivity through adolescence. However, functional connectivity analysis revealed enhanced coupling between the amygdala and the left anterior insula during untrustworthy relative to trustworthy trials (T = 3.24; XYZMNI = −36, 13, 7; k = 53) independent of age (Figure 4). In contrast, examining changes in functional connectivity with age revealed a partially overlapping region of left insula (T = 2.77; XYZMNI = −36, 6, 3; k = 29) that became relatively uncorrelated during middle- compared with early- or late-adolescence. Clusters in bilateral pSTS (T = 3.02/T = 2.62; XYZMNI = −40, −40, 18/63, −51, 18; k = 39/21) exhibited a similar drop-off in amygdala connectivity during mid-adolescence, with increased levels in older individuals. Analyses of amygdala connectivity within fusiform gyrus and whole brain masks did not reveal any significant effects. Together, these findings indicate an attenuation of cortical–amygdala coupling during mid-adolescence in select regions implicated in socioemotional processing.
Fig. 4.
Changes in amygdala connectivity during the presentation of faces rated as low vs high in trustworthiness. (A) Within left anterior insula, functional connectivity with the amygdala is greater during the presentation of untrustworthy relative to trustworthy faces—independent of age. (B) Clusters within the insula and pSTS exhibit age-dependent changes in the modulation of functional connectivity with the amygdala by face trustworthiness. Standardized measures reflect the difference in correlation for faces rated low vs high on trustworthiness. In left anterior insula and bilateral pSTS coupling with the amygdala increases during presentation of untrustworthy faces in early and late adolescence. Scatterplots reflect mean response in all significant voxels for illustrative purposes. Bootstrap means (95% CI) of R2 for the full model are displayed in the scatter plot. Solid black (gray) lines depict bootstrap means (95% CI) of model predictions.
DISCUSSION
Our results reveal multiple developmental changes in neural systems engaged during the evaluation of trustworthiness in faces. Although the right amygdala was consistently more active to untrustworthy faces across all age ranges—paralleling data in adults (e.g. Winston et al., 2002; Engell et al., 2007; Mende-Siedlecki et al., 2013), regions within insular cortex and amygdala exhibited activation profiles that varied significantly with age, with peak changes occurring in mid-adolescence. This increased limbic activity to untrustworthy faces in mid-adolescence is consistent with neurobiological models of adolescent development (Nelson et al., 2005; Casey et al., 2008; Steinberg, 2008; Ernst and Fudge, 2009), highlighting this as a key period of putative neurological and behavioral changes. Although the underlying causes for these changes remain to be fully elucidated, contributing factors could include functional sensitization by gonadal steroids, social influences that alter motivation for making valenced judgments of others (e.g. enhanced motivation for perceiving social threat), or the acquisition of new patterns of social behavior (Nelson et al., 2005). Beyond increases in overall activation, levels of amygdala connectivity within insula and pSTS were attenuated specifically during mid-adolescence. Together, these findings demonstrate that although limbic brain regions show a distinct period of sensitivity during mid-adolescence, their function in distributed networks continues to change during late adolescence.
The insula exhibited a developmental trajectory with increased reactivity to untrustworthy faces during mid-adolescence. The anterior insula is consistently implicated in decision making under uncertainty (Critchley et al., 2001a; Paulus et al., 2003; Huettel et al., 2005), thought to reflect ‘gut feelings’ that shape behavior (Bechara, 2001; Critchley et al., 2001b). Insula activity during the anticipation of uncertain outcomes is enhanced in adolescents relative to adults (Van Leijenhorst et al., 2010), supporting models of heightened limbic forebrain activity underlying increased risk-taking in adolescence (e.g. Casey et al., 2008). In this light, it is possible that the observed insula activation reflects heightened somatovisceral states associated with untrustworthy faces that should be evaluated in future work linking trustworthiness ratings and visceral responses.
The amygdala was most active in response to untrustworthy faces during mid-adolescence, corroborating evidence that puberty shifts the functional and structural properties of this brain region. Amygdala reactivity to emotional expressions has been shown to increase from late childhood (age 10) to early adolescence (age 13; Moore et al., 2012). Additionally, linear increases in amygdala gray matter volume during this time period have been revealed by both brain volumetry (Schumann et al., 2004) and morphometry (Neufang et al., 2009), although this structural change is predominant in males (Giedd et al., 1996).
Our finding of enhanced amygdala activity to untrustworthy faces during mid-adolescence extends similar results in studies examining responses to faces with fearful expressions (Monk et al., 2003; Hare et al., 2008) and of racial outgroup members (Telzer et al., 2013), suggesting enhanced responsivity to social threat stimuli during this critical period. Furthermore, the present results demonstrate that rapid social evaluation, presumably involving minimal regulatory processing, elicits enhanced amygdala activation in mid-adolescence but not late-adolescence. This finding raises the possibility that the processing of threatening stimuli (in the absence of top-down regulation) is sufficient to explain non-linear developmental trajectories observed during adolescence (e.g. Hare et al., 2008).
Functional coupling between the amygdala and cortical face-processing regions, specifically insula and pSTS, was attenuated during mid-adolescence. These functional connectivity decreases coincided with increased levels of activation to untrustworthy faces in the amygdala. This combination of increased reactivity and diminished circuit level function observed in the amygdala during mid-adolescence could be explained by several different factors. One possibility is the influence of hormones on amygdala function. Synaptic reorganization could explain decreases in activation through the elimination of unused synapses and development of long range connections. Alternatively, increases in white matter volume or myelination which continue throughout adolescence may contribute to the observed pattern of connectivity. Understanding how these mechanisms contribute to developmental changes in neural function remains an active field of inquiry (for review, see Paus et al., 2008).
Given the neuromodulatory role of the amygdala in emotional processing, and the evaluation of faces in particular, increases in functional coupling late in adolescence could explain developmental improvements in processing emotional expressions that take place through adolescence (Thomas et al., 2007). More specifically, rapid evaluation of facial stimuli by the amygdala (Whalen et al., 1998) may facilitate recognition of emotional expressions through interactions with pSTS and insula (Haxby et al., 2000; Adolphs, 2002) which become more functionally coupled later in development (Supekar et al., 2009). The protracted development of face-processing circuits, as opposed to peak reactivity in mid-adolescence, could explain later changes in emotion recognition. Although our findings generally support this hypothesis, future studies examining the development of amygdala connectivity are necessary, as our paradigm focused on social evaluation rather than affect recognition.
The present findings offer novel insights into models of brain maturation in adolescence. Although the non-linear developmental trajectory of amygdala and insula activation in response to untrustworthy faces is somewhat consistent with dual-system models of adolescent development, peak responsivity in mid-adolescence was observed in the absence of explicit regulation or prefrontal engagement. Further, connectivity within the face-processing network transitioned during mid-adolescence, with increases taking place in the late teenage years. Together, these findings support models focusing on changes in socioemotional processing during mid-adolescence (e.g. Crone and Dahl, 2012; Scherf et al., 2012).
Critically, the design of this study probed mechanisms of rapid, affectively laden, intuitive judgments while engaging minimal explicit regulatory processing. It is possible that other paradigms that more robustly engage prefrontal cortex, such as go–nogo or cognitive reappraisal, may be better suited to test dual-systems models. For instance, increased prefrontal activation during cognitive reappraisal across adolescence has been observed (McRae et al., 2012), although the extent to which this increase is necessary for mediating amygdala reactivity remains unclear. Future work independently manipulating emotional content and the use of regulatory strategies will be critical in determining if age-related enhancement in prefrontal control is necessary in explaining increased affective responsivity during mid-adolescence.
Analysis of neural responses to faces independent of perceived trust revealed decreases in activation across adolescence in bilateral amygdala, insula and fusiform gyrus. Additionally, post hoc correlational analyses with reaction times ruled out the possibility that these changes were related to task demands. The gradual linear decreases bear similarity to developmental reductions in gray matter volume in primary visual cortex (Gogtay et al., 2004). Such gray matter reductions could contribute to less activation to visual stimuli in general, under the assumption that gray matter volume and fMRI activation are linearly related. Regardless, the underlying cause and functional significance of these activation changes remain to be fully characterized.
There are some methodological limitations to this study. First, as examining sex differences was outside the purview of this study, the population of individuals studied was exclusively female. Although this fact obviates sex differences in the onset of puberty, making the time-course of developmental changes less variable, it precludes generalizing results to males. Second, although increasing levels of pubertal hormones are thought to contribute to behavioral and functional changes during adolescence, future work directly linking the observed effects with gonadal hormone levels and/or pubertal status is necessary. Additionally, the face stimuli used in this study consisted of adult faces. It is possible that differences in relationships with adults during adolescence could contribute to the observed effects. This is most likely a consideration for responses modeled to all faces, as covariates for subjective trust were modeled to be orthogonal to effects that are consistent across all stimuli. Finally, this study was cross-sectional rather than longitudinal. For these reasons, it will be important to replicate the findings reported here in independent samples.
In summary, we have characterized the protracted changes in the neural correlates of trustworthiness evaluations in adolescence. The main findings of increased sensitivity within limbic regions to untrustworthy faces and diminished connectivity between the amygdala and cortical socioemotional processing regions highlight mid-adolescence as a critical developmental period in shaping the architecture of neural systems underlying social behavior. This study also importantly contributes to the theoretical debate regarding large-scale models of brain development in adolescence. Our findings resonate with models emphasizing the key role of social and emotional changes in mid-adolescence over the ‘standard model’ that emphasizes a gradual shift from subcortical to cortical processing concomitant with the late maturation of regulatory control circuits in the prefrontal cortex. Finally, this study provides a framework for understanding the dysregulation of this circuit in mental health disorders associated with disrupted social and emotional processing, many of which emerge in adolescence.
Acknowledgments
This work was supported by a Duke Institute for Brain Sciences Research Incubator Award and the National Institutes of Health grant RC1 MH088678.
REFERENCES
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12(2):169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Ambady N, Rosenthal R. Thin slices of expressive behavior as predictors of interpersonal consequences—a metaanalysis. Psychological Bulletin. 1992;111(2):256–74. [Google Scholar]
- Bar M, Neta M, Linz H. Very first impressions. Emotion. 2006;6(2):269–78. doi: 10.1037/1528-3542.6.2.269. [DOI] [PubMed] [Google Scholar]
- Bauman LJ, Berman R. Adolescent relationships and condom use: trust, love and commitment. Aids and Behavior. 2005;9(2):211–22. doi: 10.1007/s10461-005-3902-2. [DOI] [PubMed] [Google Scholar]
- Bechara A. Neurobiology of decision-making: risk and reward. Seminars in Clinical Neuropsychiatry. 2001;6(3):205–16. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- Bos PA, Hermans EJ, Ramsey NF, van Honk J. The neural mechanisms by which testosterone acts on interpersonal trust. Neuroimage. 2012;61(3):730–7. doi: 10.1016/j.neuroimage.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ. The social brain in adolescence: evidence from functional magnetic resonance imaging and behavioural studies. Neuroscience and Biobehavioral Reviews. 2011;35(8):1654–64. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E, Eisenberger NI, Seeman TE, et al. Neural and behavioral bases of age differences in perceptions of trust. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(51):20848–52. doi: 10.1073/pnas.1218518109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001a;29(2):537–45. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neuroanatomical basis for first- and second-order representations of bodily states. Nature Neuroscience. 2001b;4(2):207–12. doi: 10.1038/84048. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–50. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, et al. Youth risk behavior surveillance—United States, 2011. Morbidity and Mortality Weekly Report. Surveillance Summaries. 2012;61(4):1–162. [PubMed] [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. Journal of Cognitive Neuroscience. 2007;19(9):1508–19. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neuroscience and Biobehavioral Reviews. 2009;33(3):367–82. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex. 2007;17(10):2400–6. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- Fett AK, Gromann PM, Giampietro V, Shergill SS, Krabbendam L. Default distrust? An fMRI investigation of the neural development of trust and cooperation. Social Cognitive and Affective Neuroscience. 2013;9(4):395–402. doi: 10.1093/scan/nss144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. The Journal of Comparative Neurology. 1996;366(2):223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, et al. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008;20(9):1565–82. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63(10):927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. The Journal of Neuroscience. 2005;25(13):3304–11. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundleby JD, Mercer GW. Family and friends as social environments and their relationship to young adolescents use of alcohol, tobacco, and marijuana. Journal of Marriage and the Family. 1987;49(1):151–64. [Google Scholar]
- Locher P, Unger R, Sociedade P, Wahl J. At first glance: accessibility of the physical attractiveness stereotype. Sex Roles. 1993;28(11–12):729–43. [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. (1998). The Karolinska directed emotional faces [Database of standardized facial images]. Stockholm, Sweden: Psychology Section, Department of Clinical Neuroscience, Karolinska Hospital, S-171–76. [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21(1):450–5. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, et al. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Said CP, Todorov A. The social evaluation of faces: a meta-analysis of functional neuroimaging studies. Social Cognitive and Affective Neuroscience. 2013;8(3):285–99. doi: 10.1093/scan/nsr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–8. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Moore WE, III, Pfeifer JH, Masten CL, Mazziotta JC, Iacoboni M, Dapretto M. Facing puberty: associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience. 2012;7(1):35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–74. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cerebral Cortex. 2009;19(2):464–73. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Olivola CY, Todorov A. Elected in 100 milliseconds: appearance-based trait inferences and voting. Journal of Nonverbal Behavior. 2010;34(2):83–110. [Google Scholar]
- Oosterhof NN, Todorov A. The functional basis of face evaluation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(32):11087–92. doi: 10.1073/pnas.0805664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–48. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences. 2012;16(6):322–9. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–63. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Said CP, Baron SG, Todorov A. Nonlinear amygdala response to face trustworthiness: contributions of high and low spatial frequency information. Journal of Cognitive Neuroscience. 2009;21(3):519–28. doi: 10.1162/jocn.2009.21041. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, Dahl RE. Facing changes and changing faces in adolescence: a new model for investigating adolescent-specific interactions between pubertal, brain and behavioral development. Developmental Cognitive Neuroscience. 2012;2(2):199–219. doi: 10.1016/j.dcn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. The Journal of Neuroscience. 2004;24(28):6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology. 2008;44(6):1764–78. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biology. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Humphreys KL, Shapiro M, Tottenham N. Amygdala sensitivity to race is not present in childhood but emerges over adolescence. Journal of Cognitive Neuroscience. 2013;25(2):234–44. doi: 10.1162/jocn_a_00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, De Bellis MD, Graham R, LaBar KS. Development of emotional facial recognition in late childhood and adolescence. Developmental Science. 2007;10(5):547–58. doi: 10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Todorov A, Engell AD. The role of the amygdala in implicit evaluation of emotionally neutral faces. Social Cognitive and Affective Neuroscience. 2008;3(4):303–12. doi: 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Pakrashi M, Oosterhof NN. Evaluating faces on trustworthiness after minimal time exposure. Social Cognition. 2009;27(6):813–33. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van den Bos W, van Dijk E, Westenberg M, Rombouts SA, Crone EA. Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychological Science. 2011;22(1):60–70. doi: 10.1177/0956797610391102. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20(1):61–9. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7(11):1271–8. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The Journal of Neuroscience. 1998;18(1):411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5(3):277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]