Abstract
Gaze and arrow cues automatically orient visual attention, even when they have no predictive value, but the neural circuitry by which they direct attention is not clear. Recent evidence has indicated that the ventral frontoparietal attention network is primarily engaged by breaches of a viewer’s cue-related expectations. Accordingly, we hypothesized that to the extent that non-predictive gaze and arrow cues automatically engender expectations with regard to cue location, they should activate the ventral attention network when they cue attention invalidly. Using event-related fMRI, we found that invalid gaze but not arrow cues activated the ventral attention network, specifically in the area of the right temporal parietal junction (TPJ), as well as nodes along the dorsal attention network associated with a redirection of attention to the correct target location. In additional whole-brain analyses, facilitation of behavioral response time by valid gaze cues was linearly associated with the degree of activation in the right TPJ. We conclude from our findings that gaze direction elicits potent expectations in humans with regard to an actor’s intention that engage attention networks if not differently from, at least more robustly than, arrow cues.
Keywords: gaze, arrow, visual attention, spatial orienting, intention
INTRODUCTION
There is abundant research evidence that a person’s direction of gaze automatically shifts a viewer’s attention to the gazed-at location (Friesen and Kingstone, 1998; Driver et al., 1999; Langton and Bruce, 1999; Ristic et al., 2002; Friesen et al., 2004). Experimental studies of the visual orienting effects of gaze direction have typically been conducted with a modified version of the classic visuospatial cuing paradigm developed by Posner (1980). In the standard task, a transient peripheral cue, such as a brief change in luminance, occurs momentarily to the left or right of a central point of fixation, and the viewer’s task is to report the location of a subsequent target. When valid, the cue accurately indicates the location of the forthcoming target. When invalid, the cue indicates the location opposite the target. At short cue-target intervals, even when the cue does not predict the target location, the viewer is quicker to detect validly than invalidly cued targets. Faster response times to validly cued targets have been taken as evidence that transient luminance cues automatically orient visuospatial attention prior to target onset. On invalid trials, the target’s appearance in the uncued location requires the viewer to reorient attention to that location, increasing response time.
In the Posner-like gaze-cuing task, a face with the eyes directed either to the left or right is presented centrally and, as in the standard task, the viewer responds by indicating on which side a subsequent target appears. Viewers respond more quickly to targets that are in the direction of the gaze cue than those in the location opposite the gaze cue. The attention-orienting effect of gaze shifts has been viewed as automatic or reflexive because it is rapid, typically occurring within 100–200 ms of the cue, and because it occurs even when the viewer knows that the gaze cues are not predictive or are counterpredictive of the forthcoming target’s location.
Initial evidence that centrally presented gaze direction cues, which require perceptual analysis and interpretation, orient visuospatial attention reflexively led to the proposal that gaze cuing has a special social–biological status in human evolution (Friesen and Kingstone, 1998; Langton et al., 2000). This possibility, however, has been put into question by evidence that non-predictive, centrally presented symbolic cues, namely arrows, whose significance is culturally rather than biologically determined, also elicit reflexive orienting effects (Ristic et al., 2002; Tipples, 2002). Some studies that have compared the effects of counterpredictive gaze and arrows cues have shown that automatic orienting to gaze direction is more resistant to top-down, volitional control (such as when a viewer orients attention in the direction of a gaze cue despite knowing that it is counterpredictive) and are thus ‘more reflexive’ than orienting responses to arrows (Driver et al., 1999; Friesen et al., 2004, 2007). Nevertheless, other studies have demonstrated that arrows are as effective as gaze in overriding volitional control and orienting attention automatically (Tipples, 2008; Kuhn and Kingstone, 2009).
Neuroimaging research on visuospatial orienting has aimed to differentiate the neural systems underlying goal-directed, top-down control of visual attention, such as when a viewer volitionally orients in the direction of a predictive arrow cue, and stimulus-driven, bottom-up control of visual attention, such as when a viewer’s attention is automatically drawn to an abrupt luminance change or a feature singleton. This large body of research has produced evidence of two partially separable frontoparietal attention networks (Corbetta and Shulman, 2002; Corbetta et al., 2008). The dorsal frontoparietal network includes the superior parietal lobule, intraparietal sulcus and frontal eye fields. The dorsal network is bilateral and is most strongly associated with volitional attentional shifts, but is also activated by stimulus-driven shifts of attention. The ventral frontoparietal network includes the temporal parietal junction (posterior superior temporal, supramarginal and angular gyrus) and inferior frontal cortex. The ventral network is right-lateralized and is activated when a salient visual stimulus appears in an unexpected location, suggesting that the ventral network orients attention in a stimulus-driven manner (Corbetta et al., 2000; Corbetta and Shulman, 2002). Recent evidence, however, has indicated that the ventral frontoparietal network is engaged less by stimulus salience than by the relevance of the stimulus to the ongoing task, and particularly breaches of the viewer’s task-based expectations (Kincade et al., 2005; Indovina and Macaluso, 2007 see Corbetta et al., 2008, for a review). For example, Kincade et al. (2005) demonstrated that the ventral system, and particularly the temporal parietal junction (TPJ), was engaged when target location was invalidly cued by predictive arrows, requiring a reorienting response, but was not activated when target location was invalidly cued by salient but non-predictive color singletons, despite the fact that the task-irrelevant color singletons oriented attention reflexively, as indicated by participants’ response times. Accordingly, Kincade et al. proposed that the ventral attentional system responds more specifically to a mismatch between expectation and sensory input.
Recent fMRI studies attempting to differentiate the neural bases of automatic orienting to uninformative gaze and arrow cues (Hietanen et al., 2006; Tipper et al., 2008; Sato et al., 2009; Greene et al., 2011) have reported varying degrees of overlap in brain activation during automatic orienting to gaze and arrow cues. When differences have been found, arrows have tended to activate components of the dorsal attention network (Hietanen et al., 2006; Sato et al., 2009), whereas gaze cues have tended to activate components of the ventral attention network (Tipper et al., 2008; Greene et al., 2011). The aforementioned studies investigated differences in orienting to non-predictive gaze and arrow cues and non-directional control stimuli without regard to cue validity. In contrast, Engel et al. (2010) used an event-related fMRI paradigm to compare responses to valid vs invalid cues. These authors found, in ROI analyses, that invalid arrow cues, compared with valid arrow cues, elicited significantly greater activation in the ventral reorienting attention network, including right TPJ and inferior frontal cortex. Comparisons of invalid and valid gaze cues revealed no such differences. These findings come as a surprise for at least two reasons. First, gaze is of enormous social significance as one the most reliable indications of a person’s direction of attention and, more generally, of his or her ‘intentional stance’ toward the object world. Accordingly, one would conjecture that gaze direction would be at least as potent as arrows in driving expectations with regard to target location in a cuing paradigm, and in activating the ventral reorienting system when those expectations are violated (Corbetta et al., 2008; see also Callejas et al., 2014). Second, prior research (Pelphrey et al., 2003, 2005) investigating neural responses to gaze direction outside of the context of a Posner-like cuing paradigm, has suggested that we tend to view gaze shifts as intentional and goal-directed, even when these shifts have no experiment-related predictive value. In these studies, participants viewed a virtual actor who on alternate trials either directed her gaze toward an object that appeared on the periphery or shifted her gaze toward empty space rather than the object. On control trials, the actor’s eyes did not move. Participants’ task was simply to indicate whether or not the actor’s eyes moved. In both studies, participants exhibited increased activation in the ventral attention system, most notably the posterior superior temporal sulcus, when the actor directed her gaze toward empty space. These findings are consistent with the proposal that the ventral reorienting system is activated in response to a violation of expectation, in this case that gaze should be directed not randomly, but in a rational, goal-directed manner (Dennett, 1987; Pelphrey et al., 2005).
In the present study, we implemented an event-related fMRI design and conducted whole-brain analyses to assess differences in orienting and reorienting to gaze and arrow cues, with a particular interest in whether these cues generate similar or different expectancies as indexed by the ventral attention reorienting system. For each cue type, attentional ‘orienting’ effects were examined by comparing brain activation in response to validly cued targets with those trials on which the target was preceded by a neutral, non-directional cue (valid > neutral). Attentional ‘reorienting’ effects were examined by comparing activation for invalidly cued targets with validly cued targets (invalid > valid). In addition, we examined relationships between facilitation of behavioral response time (invalid RT > valid RT) by gaze and arrow cues and the patterns of brain activation these cues elicited, reasoning that increased behavioral facilitation should be associated with brain evidence of more potent expectations in regard to target location.
Although this study was similar in design to that conducted by Engel et al. (2010), several methodological differences warrant mention. First, the same photorealistic face was used for all gaze trials in an effort to limit brain responses to the critical factor of cue type. Second, a face with closed eyes was used as neutral gaze cue to avoid directional cuing associated with direct or downward gaze (cf. Engel et al., 2010). Third, arrow cues were centered and double-shafted to preclude spatial (vs symbolic) cuing of attention. Fourth, to eliminate possible confounding effects of abrupt peripheral luminance changes (Friesen et al., 2005; Hietanen et al., 2006), targets were isoluminant with the background display on which they appeared.
METHODS
Participants
Participants were 20 healthy, right-handed males from 15 to 25 years of age (M = 20.2, SD = 3.2) who were screened for neurological and psychiatric conditions, were not using psychoactive medications and had normal or corrected-to-normal vision. Participants were recruited from among individuals who had served as controls in an autism research program at Boston University Medical Center (BUMC) and provided informed consent according to guidelines set by the BUMC Institutional Review Board.
Behavioral task
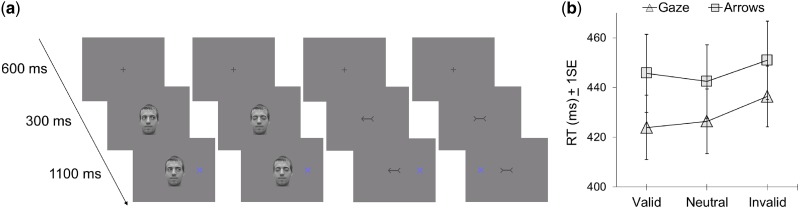
The fMRI behavioral task consisted of separate gaze and arrow cuing conditions, administered in consecutive runs and counterbalanced for order, with a brief break between them. Each condition included 160 trials: 40 valid, 40 invalid, 40 neutral and 40 null. The start of a trial was signaled by a fixation cross, which appeared at screen center for 600 ms, and was then replaced by a directional or non-directional (i.e., neutral) cuing stimulus. After 300 ms, a single target appeared to the left or right of the cue. The task was to identify the position of the target with a dominant-hand index finger response within the remaining 1100 ms of the trial. Responses were given on a button box on which two buttons were aligned horizontally so as to correspond to the left-right positions of the targets. On null trials, the fixation cross appeared for the entire 2000 ms trial duration. Trial order for each event-related run was optimized with OptSeq2.
Participants were instructed to maintain fixation at the location of the initial fixation cross for the duration of each trial, and were informed that cues were not predictive of target location. Stimuli were presented and responses recorded with Presentation 12.0 software. Stimuli were projected with an LCD projector onto a tangent screen positioned in front of the participant's forehead and viewed through a tilted mirror. Prior to imaging, participants completed a behavioral training session in a mock scanner (consisting of 24 trials each for the gaze and arrow conditions) to acclimatize them to the scanner environment and to ensure they understood the task.
Stimuli and trial sequences are illustrated in Figure 1. The initial fixation cross subtended 0.2° of visual angle horizontally and vertically. The cuing stimuli in the gaze condition were digital photographs of a male face with gaze directed to the right or left, or with eyes closed for neutral trials. The same face was used for every trial. The face subtended 4.9° horizontally and 7.6° vertically. The arrow cue consisted of a central shaft with a head and tail at either end, and was drawn to occupy the same space as the orbits of the eyes in the gaze condition. The vertices of the head and tail both pointed to the left or right on directional trials, and inward on neutral trials. The arrow stimuli subtended 3.1° horizontally and 1.3° vertically. Targets appeared at a horizontal distance of 6.4° to the left or right from the midpoint of the eyes or the shaft in the gaze and arrow conditions, respectively. Targets subtended 1.5° horizontally and vertically and were drawn in a blue hue isoluminant with the grayscale background on which all stimuli were presented.
Fig. 1.
(a) Examples of experimental stimuli: valid gaze trial; neutral gaze trial; invalid arrow trial; neutral arrow trial. A fixation cue appeared for 600 ms, followed by a directional or non-directional (neutral) cue. After 300 ms, the target appeared to the left or the right of the cue for the 1100 ms remaining in the trial, during which a response had to be given; (b) Means and standard errors of manual RT for identifying target location during fMRI as a function of cue type and validity.
Participants’ visual point of regard (POR) during the fMRI session was recorded remotely with an ASL 5000 LRO MRI-compatible eye tracker. The eye-tracking computer registered each new cue and subsequent target via a digital pulse programmed in Presentation. Cue ROIs were drawn to encompass 1° of visual angle to the left and right of the lateral edges of the central cuing stimuli (eyes, double-shafted arrow). Target ROIs extended laterally from the midpoint between the edge of the cue and target stimulus all the way to the edge of the screen. To count as a fixation, POR had to be maintained for at least five continuous data samples (∼80–85 ms at a sample rate of 60 Hz) within an area of 1° of visual angle. Eye-tracking data were successfully collected for 16 of the 20 participants. These data were collected to confirm that participant’s fixated cues as instructed and to assess the possibility that participants overtly attended to the target stimuli.
MRI methods
Data acquisition
All imaging data were collected using a six-channel SENSE receiver coil on a 3-T Philips Intera scanner. fMRI parameters were as follows: SE-EPI; TR = 2 s; TE = 28 ms; flip angle = 90°; FOV = 230 × 230 mm; 44 gapless axial slices aligned parallel to the intercommisural plane and collected in interleaved order; slice thickness = 3.5 mm; matrix = 128 × 128; imaging resolution = 1.8 × 1.8 × 3.5. Structural MRI parameters were: 3D MP-RAGE imaging; TR/TE/TI = 7.2/3.4/885 ms; flip angle = 8°; FOV = 230 × 230; 100–120 gapless 1.5 mm axial slices aligned parallel to the intercommisural plane; matrix = 256 × 256; image resolution = 0.9 × 0.9 × 1.5 mm.
Data analysis
The first three volumes of each run were discarded to allow the MR signal to reach steady state. Pre-processing and statistical analysis of functional imaging data were conducted with FMRI Expert Analysis Tool (FEAT) Version 5.98, part of FMRIB’s Software Library (www.fmrib.ox.ac.uk/fsl). Pre-processing included motion correction with MCFLIRT (Jenkinson, 2002); slice-timing correction with Fourier-space time-series phase shifting; non-brain removal with BET (Smith, 2002); spatial smoothing with a Gaussian kernel of full-width half-maximum of 5 mm; mean-based intensity normalization of all volumes by the same factor; high-pass temporal filtering (Gaussian-weighted least-squares straight-line fitting) with a cutoff of 50 s. Functional data were registered to the high-resolution structural scan using six-parameter rigid-body transformation, and then normalized to the MNI-152 2 mm standard space template using 12-parameter affine registration via FLIRT (Jenkinson, 2001, 2002). Registration from the high-resolution structural scan to standard space was then refined using FNIRT non-linear registration (Andersson, 2007a, b). Time-series statistical analyses were carried out for gaze and arrow runs separately (excluding trials with errors, non-responses, or outlying RT) for each participant using FMRIB’s Improved Linear Model with local autocorrelation correction (Woolrich et al., 2001). fMRI responses for each stimulus category (neutral, valid, invalid) within each run were modeled with a gamma variate function (with a mean lag of 6 s and SD of 3 s) and its temporal derivative, and then statistical maps of the contrasts of interest (valid > neutral, invalid > valid) were generated with a first-level fixed effects analyses for each cue type for each subject.
Group-level statistical analyses were carried out using FEAT FLAME 1 (Woolrich et al., 2004). Subject-level fixed effect data were first entered into four group-level mixed effects analyses of mean activation for each contrast for each cue. Then, direct comparisons between each cue type were conducted in paired-samples (gaze > arrow, arrow > gaze) mixed-model analyses for each contrast. For all group analyses, Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.0 and a corrected, brain-wide cluster significance threshold of P < 0.05 (Worsley, 2001). In analyses in which no effects were observed, the Z value was raised to 2.3 or lowered to 1.7, at P < 0.05, corrected, to assess the possibility of higher amplitude activation over fewer voxels or lower amplitude activation over more voxels, and in only one case produced a positive result, as reported below.
To assess the relationship between behavioral cuing effects and brain activation, each participant’s mean RT difference between valid and invalid trials (invalid − valid RT), excluding trials with errors, non-responses or outlying RT, was entered as a covariate into the whole brain, mixed effects analyses of the valid > neutral and invalid > valid contrasts for gaze and arrows. RT differences were demeaned, and the same brain-wide cluster threshold (Z > 2.0, P < 0.05) as in the main analyses was used. Whole-brain evidence of correlation between BOLD response and invalid − valid RT was further examined by assessing associations between response benefit (neutral − valid RT) and response cost (invalid − neutral RT) in ROIs centered on the peak voxel of correlation. ROIs were spheres 16 mm in diameter constructed using FSL tools and analyzed for mean percent signal changed with FSL fslmaths. Correlations with RT were then conducted with SPSS 20.
RESULTS
Behavioral results
Statistical analyses of behavioral data were conducted with SPSS 20 and were conducted for accurate responses only, excluding errors, non-responses and outlier responses. Errors were made on 0.7 and 0.4% of gaze and arrow trials, respectively. Non-responses comprised 0.5% of gaze trials and of arrow trials. A response was considered an outlier if reaction time (RT) was greater than 2 SDs above a participant’s mean RT for a given condition. Outlier responses occurred on 5.0 and 4.6% of gaze and arrow trials, respectively, and did not differ in frequency between cue types, F (1, 19) = 0.5, P = 0.48. After excluding these trials, accuracy was above 90% for all participants in all six conditions defined by cue type (gaze, arrow) and validity (valid, invalid, neutral), and mean accuracy was 93.8% for gaze trials and 94.4% for arrow trials. A 2 × 3 ANOVA showed no main effects of cue type, F (1, 19) = 0.7, P = 0.41, or cue validity, F (2, 38) = 0.5, P = 0.62, on accuracy, nor any interaction between these factors, F (2, 38) = 0.1, P = 0.87.
RT analyses for accurate responses are presented in Table 1 and Figure 1b. A 2 × 3 ANOVA revealed a main effect of cue validity, F (2, 38) = 3.9, P = 0.03. Pairwise comparisons showed a significant RT difference between valid and invalid trials (P = 0.04) and between neutral and invalid trials (P = 0.02), but not between valid and neutral trials (P = 0.92). There was a marginally significant effect of cue type, F (1, 19) = 3.7, P = 0.07, reflecting faster RT in the gaze relative to the arrow condition. There was no cue × validity interaction, F (2, 38) = 0.7, P = 0.50. When only valid and invalid trials were considered, there was a marginal effect of cue type, F (1, 19) = 3.7, P = 0.07, a significant effect of cue validity, F (1, 19) = 4.6, P = 0.05, and no interaction effect, F (1, 19) = 1.0, P = 0.35.
Table 1.
RTs for gaze and arrow cuing
| Validity |
M (SD) |
|
|---|---|---|
| Gaze | Arrow | |
| Valid | 423.9 (57.9) | 445.7 (70.0) |
| Neutral | 426.4 (58.0) | 442.4 (65.9) |
| Invalid | 436.5 (54.9) | 451.0 (70.3) |
Note: Mean and SD (in ms) of manual RT for identifying target location during fMRI as a function of cue type (gaze, arrow) and validity (valid, neutral, invalid).
Analyses of the eye movement data showed that mean number of cue fixations per trial did not differ between gaze (M = 1.5) and arrow (M = 1.4) trials, F (1, 14) = 0.5, P = 0.48. Participants fixated the targets infrequently, and the mean number of target fixations per trial did not differ between gaze (M = 0.08) and arrow (M = 0.12) conditions, F (1, 14) = 0.3, P = 0.60. In both analyses, there were no effects of validity, nor were there cue × validity interactions.
fMRI results
Orienting
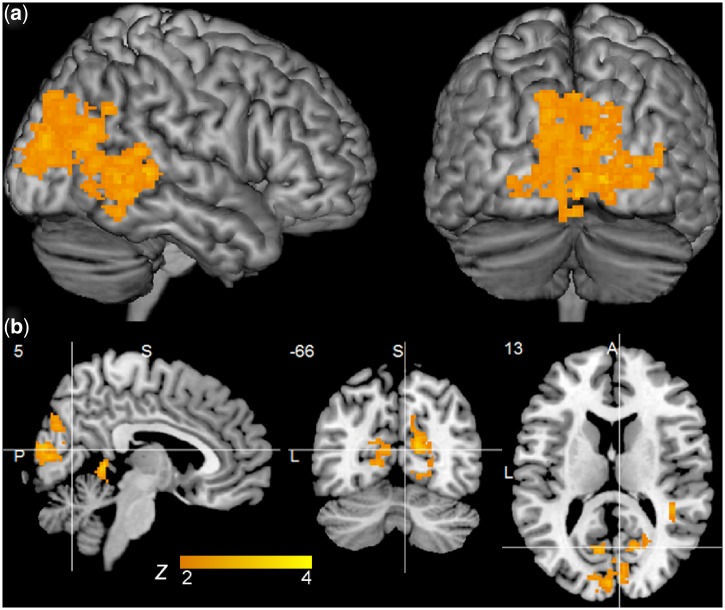
Contrasts between validly cued and neutral trials (valid > neutral) yielded no effects for the gaze condition, but revealed a large cluster of activation in medial occipital and right lateral occipital cortex in the arrow condition (Table 2, Figure 2). Direct comparisons between cues (Table 3) revealed no areas of increased activity for gaze relative to arrow orienting, but showed three clusters of increased activation for arrow relative to gaze orienting in whole-brain cluster analyses thresholded at Z = 1.7, corrected at P < 0.05: (i) a bilateral cluster extending from the lateral occipital cortices through the precuneus to the precentral gyri; (ii) a cluster extending from the right occipital cortex through occipital–temporal fusiform cortex; and (iii) a cluster in the right cerebellum.
Table 2.
Whole-brain, cluster-thresholded analyses of gaze and arrow orienting and reorienting
| Brain region | L/R | Volume | −log10(P) | Center of gravity |
Maximum Z-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Gaze orienting | |||||||
| None | |||||||
| Arrow orienting | |||||||
| Intracalcarine cortex | R | 21 032 | 8.1 | 5 | −66 | 13 | 3.8 |
| Gaze reorienting | |||||||
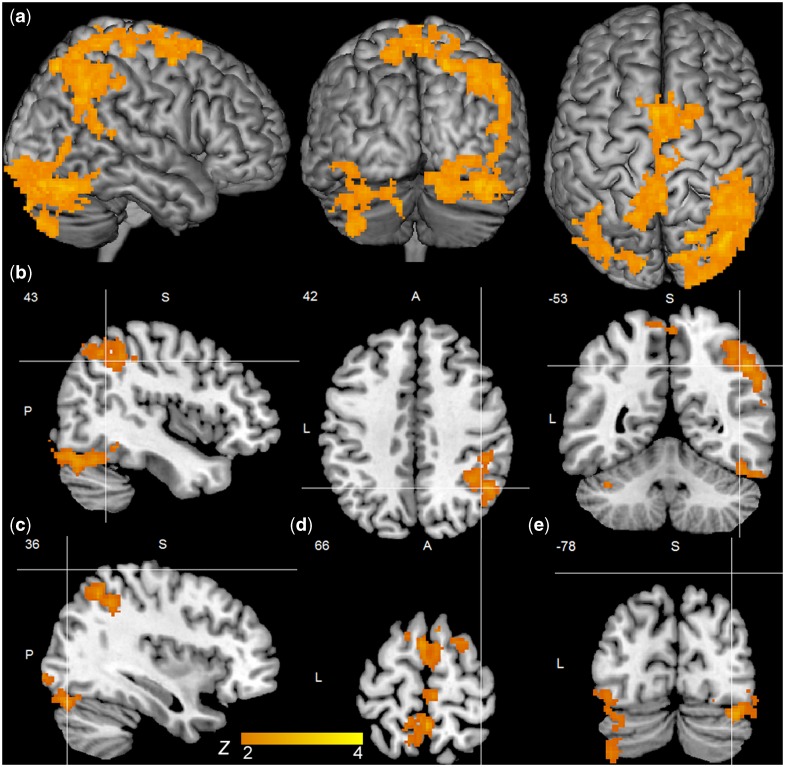
| Angular gyrus | R | 10 296 | 3.8 | 43 | −53 | 42 | 3.5 |
| Lateral occipital cortex | R | 9992 | 3.7 | 36 | −77 | −19 | 3.6 |
| Precentral gyrus | 8472 | 3.1 | 0 | −22 | 66 | 3.7 | |
| Cerebellum (Left crus I) | L | 4712 | 1.4 | −35 | −78 | −31 | 3.0 |
| Arrow reorienting | |||||||
| None | |||||||
Note: Center of gravity for clusters thresholded at Z = 2.0, P < 0.05 [−log10(P) > 1.3] for gaze and arrow orienting (minimum significant cluster size = 4096 and 4256 voxels, respectively) and gaze and arrow reorienting (minimum significant cluster size = 4600 and 4112 voxels, respectively). Volumes in 1-mm voxels and coordinates in MNI space.
Fig. 2.
Activation for orienting (valid > neutral) to arrow cues. (a) Clusters thresholded at Z = 2.0, P < 0.05 for arrow orienting; (b) Sagittal (x = 5), coronal (y = −66) and axial (z = 13) views at cluster center of gravity.
Table 3.
Whole-brain, cluster-thresholded analyses of gaze vs arrow orienting and reorienting
| Brain region | L/R | Volume | −log10(P) | Center of gravity |
Maximum Z-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Gaze > arrow orienting | |||||||
| None | |||||||
| Arrow > gaze orienting | |||||||
| Precuneous cortex | R | 12 896 | 2.7 | 6 | −64 | 50 | 3.1 |
| Lateral occipital cortex | R | 11 752 | 2.4 | 45 | −64 | −12 | 3.0 |
| Cerebellum (VIIIb) | R | 8472 | 1.5 | 19 | −53 | −58 | 3.4 |
| Gaze > arrow reorienting | |||||||
| Lateral occipital cortex | R | 8504 | 3.4 | 36 | −77 | −25 | 3.8 |
| Precentral gyrus | R | 8048 | 3.2 | 2 | −28 | 67 | 3.5 |
| Angular gyrus | R | 7944 | 3.2 | 42 | −56 | 38 | 3.6 |
| Arrow > gaze reorienting | |||||||
| None | |||||||
Note: Center of gravity for clusters thresholded at Z = 1.7, P < 0.05 [−log10(P) > 1.3] for gaze vs arrow orienting (minimum significant cluster size = 7888 voxels) and for clusters thresholded at Z = 2.0, P < 0.05 [−log10(P) > 1.3] for gaze vs arrow reorienting (minimum significant cluster size = 4192 voxels). Volumes in 1-mm voxels and coordinates in MNI space. Peak activations within significant clusters are reported in Supplementary Table S1.
Reorienting
Contrasts between invalidly and validly cued trials (invalid > valid) yielded four clusters of activation for gaze cues: (i) a cluster extending from right superior lateral occipital cortex to the angular and supramarginal gyri and posterior superior temporal sulcus; (ii) a cluster comprising mainly right lateral occipital and occipital fusiform cortex; (iii) a bilateral cluster extending from the precuneus through the superior parietal lobe to superior frontal cortex and the area of the frontal eye fields; and (iv) a cluster of activation in the left cerebellum (Table 2, Figure 3). Parallel contrasts between invalidly and validly cued trials demonstrated no effects for arrow cues. Comparisons of reorienting for gaze relative to arrow cues (Table 3) showed a pattern of results highly similar to those for gaze trials alone, except that there was no evidence of differential activation in the left cerebellum. There were no areas of increased activity for reorienting to arrow compared to gaze cues.
Fig. 3.
Activation for reorienting (invalid > valid) to gaze cues. (a) Clusters thresholded at Z = 2.0, P < 0.05 for gaze reorienting; (b) sagittal (x = 43), axial (z = 42) and coronal (y = −53) views of cluster centered in the angular gyrus; (c) sagittal view (x = 36) of cluster centered in the lateral occipital cortex; (d) axial view (z = 66) of cluster centered in the precentral gyrus, and (e) coronal view (y = −78) of cluster centered in the left cerebellum.
Correlation between behavior and brain activation
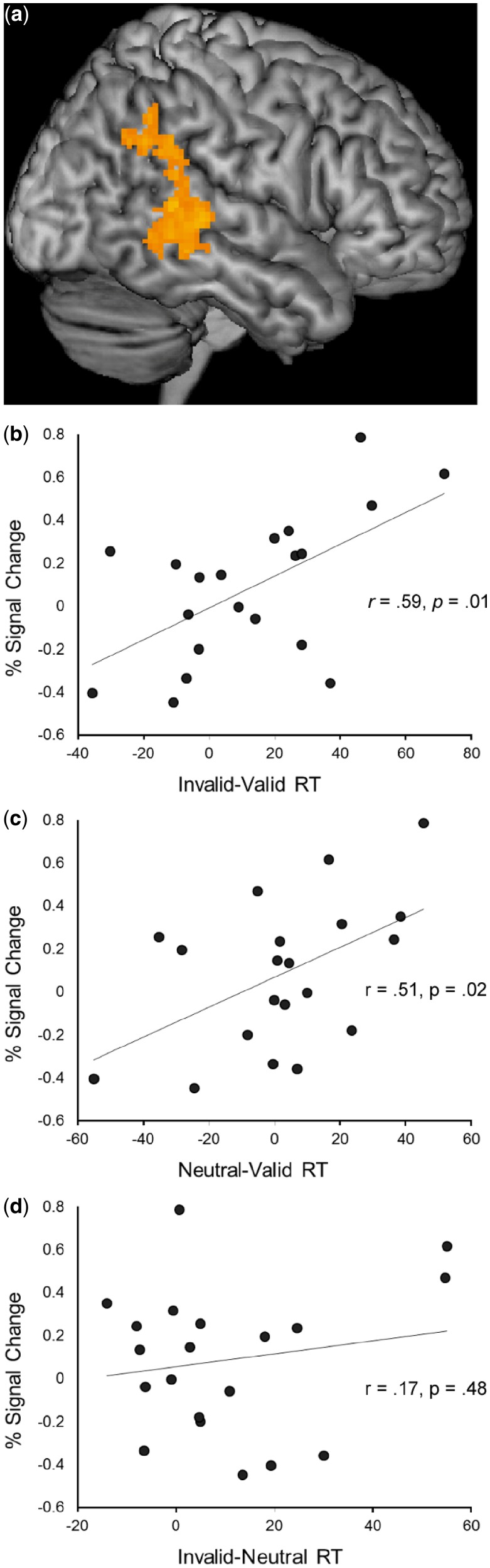
Whole-brain analyses in which the effect of cue validity on behavioral response time (invalid − valid RT) was covaried with hemodynamic response specific to orienting (valid > neutral trials) and reorienting (invalid > valid trials) revealed a cluster of association centered in right supramarginal gyrus (62,−41, 11), extending posteriorly to the angular gyrus and inferiorly to the superior temporal sulcus, for reorienting to gaze cues (Table 4, Figure 4). None of the remaining correlational analyses yielded significant results. The relationship between the reorienting effect of gaze on brain activation and behavioral facilitation of RT was further examined by partitioning the latter into the relative benefit (neutral − valid RT) and cost (invalid − neutral RT) effects of valid and invalid cues. Mean percent signal change during reorienting was measured within a 16 mm sphere centered on the peak voxel of association (54, −40, 6), and was then correlated with the RT variables. As can be seen in Figure 4, the association between increased activation in response to invalid gaze cues during reorienting and RT facilitation (r = 0.59, P = 0.01) was accounted for by the benefit in RT conferred by valid gaze cues (r = 0.51, P = 0.02), rather than the cost to RT resulting from invalid gaze cues (r = 0.17, P = 0.48).
Table 4.
Correlation between brain activation during reorienting to gaze cues and RT facilitation by gaze cues
| Brain region | L/R | Peak correlation |
Maximum Z-Value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Superior marginal gyrus/superior temporal sulcus | R | 54 | −40 | 6 | 3.8 |
| Superior marginal gyrus/posterior middle temporal gyrus | R | 72 | −30 | 2 | 3.1 |
| Superior marginal gyrus | R | 66 | −42 | 30 | 3.0 |
| Posterior middle temporal gyrus | R | 68 | −36 | 0 | 2.9 |
| Angular gyrus | R | 60 | −52 | 32 | 2.9 |
| Middle temporal gyrus/superior temporal sulcus | R | 62 | −42 | −4 | 2.9 |
A whole-brain, cluster-thresholded (Z = 2.0, P < 0.05) correlation between facilitation of behavioral response time (invalid − valid RT) and activation for reorienting (invalid − valid) to gaze cues yielded a cluster of 4728 voxels (P < 0.04) centered in the right supramarginal gyrus (62, −41, 11). Volume in 1-mm voxels and coordinates in MNI space.
Fig. 4.
(a) Whole-brain, cluster-thresholded (Z = 2.0, P < 0.05) correlation between facilitation of behavioral response time (invalid − valid RT) and activation for reorienting (invalid − valid) to gaze cues, centered in right supramarginal gyrus (62,−41, 11). Scatter plots of mean percent signal change within ROI centered at peak of association and (b) response facilitation (invalid − valid RT), (c) response benefit (neutral − valid RT) and (d) response cost (invalid − neutral RT).
DISCUSSION
We examined the neural correlates of automatic visuospatial orienting and reorienting to non-predictive gaze and arrows cues. We were most interested in the degree to which non-predictive gaze and arrow cues activate the ventral frontoparietal attention system, which has been shown in endogenous cuing tasks to respond to a violation of behaviorally relevant expectations, presumably initiating a reorientation to the uncued target location (Corbetta et al., 2008). We reasoned that if non-predictive gaze cues engaged attention differently from or more potently than arrow cues, this would be reflected in a difference in the activation of the ventral reorienting network. We had two main findings. First, although gaze and arrow cues were similar in their effects on behavioral response time, whole-brain analyses of invalid compared with valid cues showed that the ventral attention system, particularly nodes in the TPJ and inferior parietal cortex, was engaged when participants reoriented attention after invalid gaze cues, but not when they reoriented after invalid arrow cues. These differences held up when whole-brain effects of reorienting to gaze and arrows cues were directly compared. Second, in whole-brain correlational analyses, we found that facilitation of behavioral response time by gaze cues, which varied among individual participants, was linearly associated with TPJ activation during reorienting. Exploratory ROI analyses showed that this correlation was accounted for by the benefit to response time conferred by valid cues relative to neutral cues, rather than a cost associated with invalid cues. These findings suggest, in line with our argument above, that gaze direction may automatically elicit expectations with regard to an actor’s intention, and that TPJ activates to redirect attention as a function of the strength of these expectations. Consistent with this interpretation was our finding of corollary activation in the dorsal attention network in response to invalid gaze cues, extending from superior parietal cortex to the area of the frontal eye fields, and thought to reflect the reorienting of attention to the uncued target (Corbetta and Shulman, 2002).
Comparison of valid to neutral arrow trials showed activation bilaterally, primarily in cuneal and adjacent occipital cortex, but also extending anteriorly into parietal cortex. In contrast, comparison of valid gaze cues to non-directional neutral gaze cues revealed no activation specifically associated with gaze orienting. Direct comparisons of orienting to gaze and arrows largely mirrored these results, showing activation in right occipital–temporal fusiform cortex as well as bilateral activation extending from the lateral occipital cortices medially through superior parietal cortex to the precentral gyrus that was specific to arrow orienting, but no activation for gaze relative to arrow orienting. We propose that our findings might be accounted for by a greater automaticity of gaze orienting compared with arrow orienting, the latter of which may require increased top-down effort in cue analysis and localizing and maintaining attention in response to cue direction. This is not to exclude the possibility that gaze direction exerts its orienting effects via similar cortical circuitry as arrows, particularly the dorsal attention network, but to suggest that in its greater automaticity and subtlety, its effects may have been below the threshold of detection of our design. Supporting this interpretation were the shorter latencies found for RT to gaze than to arrow cues, which approached statistical significance.
Our main finding that reorienting to gaze but not arrow cues engaged the ventral attention network was the opposite of the findings reported by Engel et al. (2010), and requires consideration. The cuing stimuli used in the two studies differed in several respects. On one hand, we used the same stimulus face across all gaze cuing trials, rather than faces of varying identity, which was intended to amplify the effects of the critical directional information in the gaze condition. On the other hand, Engel et al. used arrows that were single-shafted and not centered, which may have biased attention spatially (Tipples, 2002), and consequently may have interacted with the symbolic cuing effects of arrows. These differences between studies were arguably reflected in Engel et al.’s finding, the opposite of ours, of shorter RTs to arrow than to gaze cues.
Although we did not find an interaction effect between cue (gaze, arrow) and validity (valid, invalid) in our RT analyses, inspection of the data shows that the validity effect of arrows cues was smaller and somewhat more variable amongst participants. However, if this were to account for the absence of significant activation of the ventral attention network during reorienting to arrows, we might have at least expected an association between behavioral facilitation and activation of the TPJ in the arrow condition comparable with the one we found for gaze cues, especially given the increased variability of behavioral facilitation. However, no correlation was found. Nonetheless, our failure to identify any neural signature specific to arrow reorienting leaves open the possibility that non-predictive arrows engender expectations that automatically reorient attention via similar mechanisms as non-predictive gaze cue, but at a level we were unable to detect in the current study design. Consistent with this suggestion are the findings from a recent study by Callejas et al. (2014) who directly compared predictive gaze cues to predictive arrow cues, which would presumably induce stronger expectations in the viewer regarding target location than non-predictive arrow cues. These authors found that TPJ was similarly activated by predictive gaze and arrow cues when they invalidly cued target location.
To our knowledge, this is the first study to use isoluminant targets to address possible confounding of reflexive orienting to non-predictive gaze and arrows by peripheral luminance changes (Friesen et al., 2005; Hietanen et al., 2006), which also engage attention automatically. Our use of isoluminant targets is the key difference between the stimuli and procedures in the present study and the numerous prior studies demonstrating that arrows orient attention reflexively. Although this methodological feature did not affect our results statistically, the validity effect for arrows, as noted, was smaller than for gaze. As is well known, the reflexive orienting effects of gaze and arrows are practically small, variable and require many trials to detect. Thus, the possibility that target features entailing a peripheral change of luminance interact with the central orienting effects of arrow cues seems worthy of further investigation.
In summary, gaze cues, even when non-predictive, appear to engender strong expectations of target location that, when violated, evoke patterns of activation in the ventral and dorsal attention networks similar to predictive arrow cues (Kincade et al., 2005; Indovina and Macaluso, 2007). Similar reorienting effects were not observed for arrow cues, which was not necessarily expected in that arrows and gaze share several properties as directional cues. Apart from gaze, arrows are the most ubiquitous signals of direction in the human world. Further, as human symbols that serve a communicative function, arrows convey intent, although not in as an immediate way as gaze. Accordingly, it seems highly plausible that the reflexive orienting effects of arrows would have been built on the same neural circuitry underlying reflexive orienting to gaze. In light of these considerations, and evidence to the contrary of our findings from Engel et al. (2010), we defer making a definitive conclusion with regard to whether non-predictive arrows orient and reorient attention via different neural mechanisms than non-predictive gaze cues. However, our activation findings suggest that to the extent that arrows engender expectations via past experience and learning, and orient attention automatically, they do so less robustly and reliably, and for that reason their behavioral effects may indeed be ‘less reflexive’ than gaze cues.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This research was supported by a National Institute of Mental Health award (K01 MH 073944) to R.M.J.
REFERENCES
- Andersson J, Jenkinson M, Smith S. 2007a. Non-linear optimisation. FMRIB technical report TR07JA1. University of Oxford, UK. Available: www.fmrib.ox.ac.uk/analysis/techrep. [Google Scholar]
- Andersson J, Jenkinson M, Smith S. 2007b. Non-linear registration. FMRIB technical report TR07JA2. University of Oxford, UK. Available: www.fmrib.ox.ac.uk/analysis/techrep. [Google Scholar]
- Callejas A, Shulman GL, Corbetta M. Dorsal and ventral attention systems underlie social and symbolic cueing. Journal of Cognitive Neuroscience. 2014;26:63–80. doi: 10.1162/jocn_a_00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–7. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dennett D. The Intentional Stance. Cambridge, MA: MIT Press/Bradford Books; 1987. [Google Scholar]
- Driver J, Davis G, Ricciardelli P. Gaze perception triggers reflexive visuospatial orienting. Visual Cognition. 1999;6:509–40. [Google Scholar]
- Engel AD, Nummenmaa L, Oosterhof NN, Henson RN, Haxby JV, Calder AJ. Differential activation of frontoparietal attention networks by social and symbolic spatial cues. Social Cognitive and Affective Neuroscience. 2010;5:432–40. doi: 10.1093/scan/nsq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review. 1998;5:490–5. [Google Scholar]
- Friesen CK, Moore C, Kingstone A. Does gaze direction really trigger a reflexive shift of spatial attention? Brain and Cognition. 2005;57:66–9. doi: 10.1016/j.bandc.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Friesen CK, Ristic J, Kingstone A. Attentional effects of counterpredictive gaze and arrow cues. Journal of Experimental Psychology: Human Perception and Performance. 2004;30:319–29. doi: 10.1037/0096-1523.30.2.319. [DOI] [PubMed] [Google Scholar]
- Greene DJ, Colich N, Iacoboni M, Zaidel E, Bookheimer SY, Dapretto M. Atypical neural networks for social orienting in autism spectrum disorders. Neuroimage. 2011;56:354–62. doi: 10.1016/j.neuroimage.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen JK, Nummenmaa L, Nyman MJ, Parkkola R, Hamalainen K. Automatic attention orienting by social symbolic cues activates different neural networks: an FMRI study. Neuroimage. 2006;33:406–13. doi: 10.1016/j.neuroimage.2006.06.048. [DOI] [PubMed] [Google Scholar]
- Indovina I, Macaluso E. Dissociation of stimulus relevance and saliency factors during shifts of visuospatial attention. Cerebral Cortex. 2007;17:1701–11. doi: 10.1093/cercor/bhl081. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-orienting of attention. The Journal of Neuroscience. 2005;25:4593–604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn G, Kingstone A. Look away! Eyes and arrows engage oculomotor responses automatically. Attention, Perception, & Psychophysics. 2009;71:314–27. doi: 10.3758/APP.71.2.314. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Bruce V. Reflexive visual orienting in response to the social attention of others. Visual Cognition. 1999;6:541–67. [Google Scholar]
- Langton SRH, Watt RJ, Bruce V. Do the eyes have it? Cues to the direction of social attention. Trends in Cognitive Sciences. 2000;4:50–9. doi: 10.1016/s1364-6613(99)01436-9. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–48. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: the influence of context. Neuropsychologia. 2003;41:156–70. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Ristic J, Friesen CK, Kingstone A. Are eyes special? It depends on how you look at it. Psychonomic Bulletin & Review. 2002;9:507–13. doi: 10.3758/bf03196306. [DOI] [PubMed] [Google Scholar]
- Ristic J, Wright A, Kingstone A. Attentional control and reflexive orienting to gaze and arrow cues. Psychonomic Bulletin & Review. 2007;14:964–9. doi: 10.3758/bf03194129. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Yoshikawa S. Commonalities in the neural mechanisms underlying automatic attention shifts by gaze, gestures, and symbols. Neuroimage. 2009;45:984–92. doi: 10.1016/j.neuroimage.2008.12.052. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper CM, Handy TC, Giesbrecht B, Kingstone A. Brain responses to biological relevance. Journal of Cognitive Neuroscience. 2008;20:879–91. doi: 10.1162/jocn.2008.20510. [DOI] [PubMed] [Google Scholar]
- Tipples J. Eye gaze is not unique: automatic orienting in response to uninformative arrows. Psychonomic Bulletin & Review. 2002;9:314–18. doi: 10.3758/bf03196287. [DOI] [PubMed] [Google Scholar]
- Tipples J. Orienting to counterpredictive gaze and arrow cues. Perception and Psychophysics. 2008;70:77–87. doi: 10.3758/pp.70.1.77. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modelling of fMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. Multi-level linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Cambridge: Oxford University Press; 2001. pp. 251–70. [Google Scholar]