Abstract
The core feature of separation anxiety is excessive distress when faced with actual or perceived separation from people to whom the individual has a strong emotional attachment. So far little is known about the neurobiological underpinnings of separation anxiety. Therefore, we investigated functional (amygdala responsiveness and functional connectivity during threat-related emotion processing) and structural (grey matter volume) imaging markers associated with separation anxiety as measured with the Relationship Scale Questionnaire in a large sample of healthy adults from the Münster Neuroimaging Cohort (N = 320). We used a robust emotional face-matching task and acquired high-resolution structural images for morphometric analyses using voxel-based morphometry. The main results were positive associations of separation anxiety scores with amygdala reactivity to emotional faces as well as increased amygdala grey matter volumes. A functional connectivity analysis revealed positive associations between separation anxiety and functional coupling of the amygdala with areas involved in visual processes and attention, including several occipital and somatosensory areas. Taken together, the results suggest a higher emotional involvement in subjects with separation anxiety while watching negative facial expressions, and potentially secondary neuro-structural adaptive processes. These results could help to understand and treat (adult) separation anxiety.
Keywords: adult separation anxiety, fMRI, voxel-based morphometry, amygdala
INTRODUCTION
The core feature of separation anxiety is excessive distress when faced with actual or perceived separation from people to whom the individual has a strong emotional attachment. Moreover, people with separation anxiety strongly worry about being alone and abandoned. In diagnostic classification systems such as the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV-TR, separation anxiety (disorder) was described primarily as a childhood disorder requiring an onset before the age of 18 as criterion. This age of onset requirement has recently been dropped in the DSM V, hence separation anxiety turned to a diagnosable disorder for adults. This is supported by several empirical studies, which argued that adult separation anxiety is more common than suggested by the DSM-IV-TR and might constitute a clinical category on its own (Ollendick et al., 1993; Manicavasagar et al., 1997; Cyranowski et al., 2002; Shear et al., 2006; Bögels et al., 2013). In addition, the clinical importance of separation anxiety as a risk factor for mental disorders has often been underestimated. Separation anxiety has been discussed to create a strong vulnerability for a number of affective and anxiety disorders, and clinicians should be more sensitive to the presence of separation anxiety (Manicavasagar et al., 1998; Lewinsohn et al., 2008; Silove et al., 2010).
So far little is known about the neurobiological underpinnings of adult separation anxiety and to the best of our knowledge, there is no study that investigated adult separation anxiety with neuroimaging techniques. However, one study reported that for healthy adolescents, increased amygdala activity was highly related to separation anxiety and concerns about being separated from parents and family (Killgore and Yurgelun-Todd, 2005).
Behavioral studies indicate that children with separation-anxiety disorder show negative emotional hyper-reactivity, deficits in emotion regulation, were found to interpret ambiguous situations as more threatening and seek more help from others instead of solving problems by themselves than healthy children (Dadds et al., 1996; Bögels and Zigterman, 2000; Carthy et al., 2009). In contrast to other subcategories of anxiety disorders, subjects with separation anxiety disorder in history recorded more severe symptoms of depression, anxiety and stress in adulthood (Silove et al., 2010).
However, the generation of hypotheses regarding functional and/or structural aberrations in adult subjects with high levels of separation anxiety is limited by the lack of pre-existing data. Nevertheless, there is some evidence that separation anxiety shares common features with other domains of anxiety (Bögels et al., 2013). Hence, we suggest that a common element of separation anxiety might be a hyperresponsiveness to negative social signals (faces). Up to now, hyperactivity or reactivity in a limbic circuit with the amygdala as a key structure has been observed during negative emotional processing in patients with social anxiety disorder (Stein et al., 2002; Straube et al., 2005; Phan et al., 2006), specific phobia (Schienle et al., 2005; Straube et al., 2006; Schweckendiek et al., 2011), panic disorder (van den Heuvel et al., 2005; Pfleiderer et al., 2007), and post-traumatic stress disorder (Shin et al., 2005; Francati et al., 2007) as well as in healthy but high-anxious subjects (Vrticka et al., 2008; Pejic et al., 2011; Sehlmeyer et al., 2011; Laeger et al., 2012; Abraham et al., 2013) and healthy subjects with a history of childhood maltreatment (Dannlowski et al., 2013; Dannlowski et al., 2012).
However, to understand the complex function of the amygdala in the context of emotion processing, its functional interplay with other brain areas should be taken into account. Functional connectivity (Friston, 1994) is one possibility to identify networks of brain regions showing patterns of co-activation throughout the time course of a task. During emotion processing, the amygdala was reported to show tight functional coupling to several prefrontal, temporal and occipital regions, as well as to hippocampal and thalamic areas, which are suggested as important for several neurocognitive domains, including emotion regulation, associative learning processes, stimulus evaluation, visceral responses and attentional processing (Banks et al., 2007; Robinson et al., 2010; Bzdok et al., 2012), see Davis and Whalen (2001) for a review. Regarding subjects with anxiety disorder, increased functional connectivity of the amygdala to prefrontal and occipital areas have been reported, which was implicated to be associated with dysfunctional emotion regulation and increased vigilance and attentional processes for anxiety-relevant stimuli (McClure et al., 2007; Kim et al., 2011; Strawn et al., 2012).
Regarding structural abnormalities, the literature on other anxiety disorders is less consistent with few studies, however, suggesting reduced amygdala volumes in paediatric patients with anxiety disorder (Milham et al., 2005) and adult patients suffering from panic disorder (Hayano et al., 2009).
In this study, we sought to uncover functional (amygdala responsiveness to emotional faces) and structural (grey matter volume) imaging markers associated with adult separation anxiety in a large sample of healthy subjects, carefully screened for psychiatric conditions. We hypothesized that healthy adults reporting higher degrees of separation anxiety would show amygdala hyper-responsiveness to negative social stimuli including an abnormal functional coupling of amygdala and sensory visual areas. Regarding structural aberrations, we speculated that higher separation anxiety could be associated with decreased amygdala grey matter volumes. We further hypothesized that these associations are independent from general measures of (unspecific) anxiety traits.
METHODS
Participants
The complete data set comprised 320 right-handed healthy volunteers. Data were collected in the context of a large ongoing study (Münster Neuroimaging Cohort) investigating the neurobiology of emotional processes. For all analyses, 14 subjects had to be excluded due to anatomical abnormalities leaving 306 subjects (154 women, mean age: 38.3, s.d. = 11.5 years; 152 men, mean age: 36.9; s.d. = 11.2 years). Participants were recruited by public notices and newspaper announcements. All subjects had no history of psychiatric illness, according to the Structured Clinical Interview for DSM-IV (SCID)-Interview (Wittchen et al., 1997), had no neurological conditions, were free of psychotropic medication, had normal or corrected-to-normal vision, and had adequate knowledge of German and cognitive abilities [verbal IQ >80; multiple-choice vocabulary intelligence test MWT-B (Lehrl, 2005)].
Subjects were screened for imaging safety concerns, and informed, written consent was obtained following the Declaration of Helsinki (World Medical Association, 1991). The experimental procedure was approved by the ethics committee of the Medical Faculty at the University of Münster. Handedness was defined by the Handedness Questionnaire (Raczkowski et al., 1974). For detailed sample characteristics, see Table 1.
Table 1.
Sociodemographic questionnaire and behavioral data of study participants
| Age | 37.6 ± 11.4 (18–59) |
|---|---|
| Years of education | 15.3 ± 2.2 (9–21) |
| Sex (M/F) | 152/154 |
| Verbal intelligence (MWT-B) | 116.7 ± 12.3 (92–145) |
| STAI-T | 31.8 ± 6.6 (20–54) |
| HAMA | 0.7 ± 1.3 (0–8) |
| BDI | 1.9 ± 2.5 (0–13) |
| RSQ separation anxiety | 24 ± 6.2 (12–64) |
| Percentage of correct shapes | 97.7 ± 1.1 (82–100) |
| Percentage of correct faces | 98.0 ± 1.1 (69–100) |
| Mean RT shapes (ms) | 866.3 ± 146.0 (451–1497) |
| Mean RT faces (ms) | 1051.2 ± 207.3 (642–2367) |
n = 306 representing the final sample included in the morphometry analysis; mean ± SE (range). M, male; F, female; STAI-T, State-Trait Anxiety Inventory-Trait version; HAMA, Hamilton Anxiety Rating Scale; BDI, Beck Depression Inventory; RSQ, Relationship Scales Questionnaire; MWT-B, Mehrfachwahl-Wortschatz-Intelligenztest (multiple choice vocabulary test); RT, reaction time.
Questionnaire measures
The Relationship Scales Questionnaire (RSQ; Griffin and Bartholomew, 1994; Steffanowski et al., 2001) was applied to assess separation anxiety. RSQ scores have shown temporal stability in longitudinal studies with adults so that they appear to measure stable traits of personality in adulthood (Scharfe and Bartholomew, 1994; Scharfe and Cole, 2006). Inter-item analysis of the separation anxiety scale showed acceptable internal consistency estimates of reliability in the whole sample (Cronbach’s α = 0.75). The questionnaire items were rated on a 1 (not at all like me) to 5 (very much like me) scale. Subjects had to indicate the extent to which they believe each of the statements best describes their feelings about close relationships. Ten items measured separation anxiety (e.g. ‘I worry about being abandoned’, ‘I want to merge completely with another person’, ‘I worry about being alone’).
In order to control for effects of unspecific trait anxiety, the State-Trait Anxiety Inventory (STAI-trait version; Spielberger et al., 1970) was administered as self-evaluation questionnaire. Additionally, the Hamilton Rating Scale of Anxiety (HAMA; Hamilton, 1959; Maier et al., 1988) was conducted by a clinical interviewer as an objective anxiety measure. The Beck Depression Inventory (BDI; Beck and Steer, 1987; Hautzinger et al., 1994) was used to assess the presence of depressive symptoms. RSQ scores were positively associated with STAI-trait scores (r = .29) and BDI scores (r = .15), but not with HAMA scores (r = .08).
Stimulus materials and procedure
A robust paradigm for eliciting amygdala responsiveness that has been used in several previous imaging studies was applied as experimental fMRI paradigm (Hariri et al., 2002; Dannlowski et al., 2011, 2012; Domschke et al., 2012). A set of negative (angry and fearful) faces was used. The paradigm consisted of five blocks of a sensorimotor control task alternating with four blocks of a face-processing task. During the face-processing task, participants viewed a trio of faces and selected one of the two faces (bottom) that was identical to the target face (top). Each face-processing block consisted of six images, balanced for target gender. During the sensorimotor control blocks, the participants viewed trios of geometric shapes (circles and ellipses) and selected one of the two shapes (bottom) that was identical to the target shape (top). Each sensorimotor control block consisted of six shape trios. All blocks were preceded by an instruction (‘Match faces’ or ‘Match shapes’ in German) that lasted 2 s. In the face-processing blocks, each of the six face trios was presented for 4 s with a variable inter-stimulus interval of 1.5–5.5 s (mean, 3.5 s), for a total block length of 47 s. In the sensorimotor control blocks, each of the six shape trios was presented for 4 s with a fixed inter-stimulus interval of 1.5 s, for a total block length of 35 s. The total task time was 363 s. Participant performance (accuracy and reaction time) was recorded.
fMRI data acquisition and analysis
T2* functional data were acquired using a 3 T scanner (Gyroscan Intera 3T, Philips Medical Systems, Best, NL), using a single-shot echoplanar sequence, with parameters selected to minimize distortion in the region of central interest, while retaining adequate a signal-to-noise ratio (S/N) and T2* sensitivity. Volumes consisting of 34 slices were acquired (matrix 64 × 64, resolution 3.6 mm × 3.6 mm × 3.6 mm; TR = 2.1 s, TE = 30 ms, FA = 90°). The slices were tilted 25° from the AC/PC line in order to minimize drop out artifacts in the mediotemporal and orbitofrontal region.
The paradigm presentation was projected to the rear end of the scanner (Sharp XG-PC10XE with additional HF shielding). During the experiment, subjects lay supine in the MRI scanner with the response box in their right hand. The head position was stabilized with a vacuum head cushion.
Data were analyzed using statistical parametric mapping software (SPM8, Welcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Functional data were pre-processed, including realignment using a set of six rigid-body transformations determined for each image, and normalization of each participant's functional images to the Montreal Neurological Institute International Consortium (MNI) for Brain Mapping template. Images were smoothed with a Gaussian kernel of 8-mm full-width at half-maximum (FWHM).
Twenty-four further subjects had to be excluded from the fMRI analyses due to excessive head movement (exclusion criterion 3 mm/3°) and/or due to technical problems with the functional sequence, leaving 282 subjects for functional data analyses.
The onsets and durations of the experimental conditions (faces and shapes) were modelled using a canonical hemodynamic response function in the context of the general linear model, and the model was corrected for serial correlations. A high-pass filter of 128 s was used to remove low-frequency noise. Movement parameters were entered as nuisance regressors. For each subject, one contrast image, contrasting negative faces with the shapes baseline, was generated in each individual first-level analysis. Then, second-level-group analyses were performed, regressing separation anxiety scores on brain activation to emotional faces.
At first, in order to address our hypotheses regarding amygdala responsiveness, region of interest (ROI) analyses of the bilateral amygdala were performed. The mask for bilateral amygdala was created by means of the WFU PickAtlas (Maldjian et al., 2003) by dilating the defined mask according to the AAL-Atlas (Tzourio-Mazoyer et al., 2002) by 1 mm. In addition, the anatomy toolbox (Eickhoff et al., 2006, 2005) was applied to evaluate the affected amygdala substructures. For exploratory reasons, an additional whole-brain analysis, with a cluster threshold of k = 20 voxels, was conducted. For both, amygdala ROI and whole-brain analyses, rigorous family-wise error (FWE) correction were applied on the voxel level, with a corrected statistical threshold of P < .05.
Second, we explored whether other variables influenced or confounded the association of neural activation and separation anxiety. Therefore, for each subject, the resulting contrast values of the resulting peak voxel from the second-level analysis were extracted and further analyzed by using SPSS Statistics 21 (IBM, Armonk, New York). We performed a linear multiple regression predicting amygdala responsiveness by separation anxiety scores, age, gender, total education time (in years), verbal intelligence, STAI scores, HAMA scores and BDI scores. To cover for multicollinearity, we additionally performed a linear regression model appertaining a collinearity analysis, regressing the STAI-T scores onto amygdala activations and in a second step regressing the RSQ scores to the residuals of the first model.
Third, we performed a functional connectivity analysis to characterize alterations associated with separation anxiety scores in the functional coupling between the amygdala and other brain areas. The methods for functional connectivity analyses of the amygdala have been described previously (Dannlowski et al., 2009). Briefly, for each subject, the signal time course of the entire right amygdala (defined as ‘seed’ region) was extracted and then entered into a new first-level model of the same subject predicting brain activity by the amygdala time series. The experimental conditions were entered as nuisance regressors. These resulting contrast images now represent functional connectivity maps of the amygdala, corrected for the experimental conditions (i.e. co-activation by the task). On the basis of these images, we performed a second-level whole-brain regression analysis on amygdala functional connectivity with separation anxiety scores as predictor, again using a cluster threshold of k = 20 voxels and a statistical threshold of P < 0.05, FWE corrected for the entire brain.
The anatomical labelling was performed by means of the AAL-Toolbox (Tzourio-Mazoyer et al., 2002), and the Brodmann areas were identified with the Talairach Daemon atlas (http://www.talairach.org). The sub-structural amygdala labelling was performed by means of the anatomy toolbox (Eickhoff et al., 2006, 2005).
Voxel-based morphometry acquisition and analysis
T1-weighted high-resolution anatomical images were acquired with a 3D fast gradient echo sequence (‘Turbo Field Echo’, TFE), TR = 7.4 ms, TE = 3.4 ms, FA = 9°, two signal averages, inversion prepulse every 814.5 ms, acquired over a field of view of 256 (FH) × 204 (AP) × 160 (RL) mm, phase encoding in AP and RL direction, reconstructed to cubic voxels of 0.5 mm × 0.5 mm × 0.5 mm. As described in our earlier work (Baune et al., 2012a,b), the voxel-based morphometry 8-toolbox (VBM8-toolbox) (http://dbm.neuro.unijena.de/vbm) was used for preprocessing the structural images with default parameters. Images were bias-corrected, tissue-classified, and normalized to MNI-space using linear (12-parameter affine) and non-linear transformations, within a unified model (Ashburner and Friston, 2005) including Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL)-normalization. Grey matter segments were modulated only by the non-linear components in order to preserve actual GM values locally (modulated GM volumes). As mentioned earlier, 14 subjects showing anatomical abnormalities or strong artifacts were identified and excluded. The remaining n = 306 images were clear of such problems. The modulated grey matter images were smoothed with a Gaussian kernel of 8 mm FWHW. Group statistics were calculated using exactly the same analysis strategy as described earlier for functional data by using SPM8, including a ROI analysis of the bilateral amygdala and a whole-brain approach. Again, to test for multiple comparisons, a rigorous FWE correction of P < .05 was applied. The resulting contrast values of the peak voxel of significant clusters from these second-level analyses were extracted for each subject for further analyses regressing out potential confounders, as already described earlier for the functional data.
RESULTS
Behavioral performance in the fMRI experiment
The mean accuracy rate in the shape condition was 97.7% (s.d. = 1.1%). The mean accuracy rate for the face condition was 98.0% (s.d. = 1.1%). The average reaction times for whole group were 866.3 ms (s.d. = 146 ms) for shapes and 1051.2 ms (s.d. = 207 ms) for faces. According to paired t-tests, correct responses between both conditions were not significant (P = 0.09), whereas there has been significant shorter reaction times to shapes than to faces (P < 0.001). Separation anxiety was not significantly correlated with reaction time or response hit rate controlling for age (P > 0.19).
fMRI activation and connectivity analysis
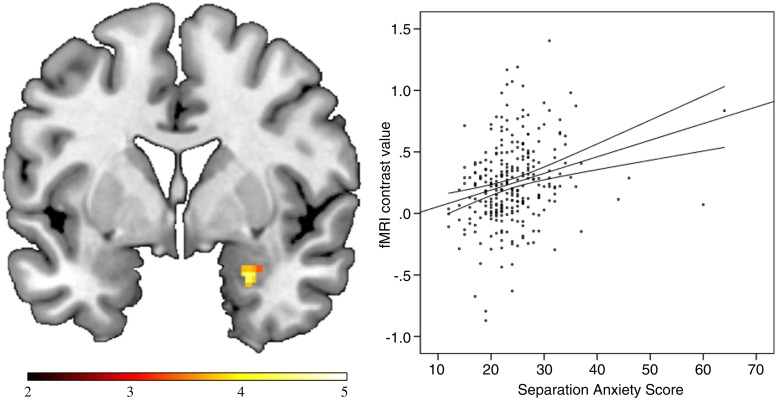
The regression analysis indicated a strong positive association of right amygdala responsiveness and separation anxiety scores (x = 26, y = 2, z = −24; t(280) = 4.44, PFWE-corrected = 0.001; r = 0.26, cluster size k = 33). When using the anatomy toolbox, a virtually identical cluster (x = 24, y = 0, z = −24; t(280) = 4.40, PFWE-corrected = 0.001, k = 28) within the basolateral parts of the amygdala was found (Figure 1). Excluding outliers (subjects with values more than 4 s.d. above the mean) did not affect the significance of these results (r = 0.27; P < 0.001). The subsequent multiple regression analysis predicting the mean activation of this significant cluster by separation anxiety score, age, gender, total education time, verbal intelligence, STAI score, HAMA score and BDI score, confirmed the strong association between separation anxiety and amygdala responsiveness, which remained nearly unchanged (β = 0.23, t(274) = 3.69, P < 0.001). Also a semi-partial correlation analysis between RSQ scores and amygdala function controlling for the STAI yielded significant semi-partial correlations of rp = 0.24 (P < 0.001). The collinearity analysis yielded highly tolerable values [tolerance > 0.68; variance inflation factor (VIF) < 1.54], which mean that multicollinearity did not inflate the variances of the parameter estimates. Thus, the association of separation anxiety and amygdala responsiveness to emotional faces was not significantly influenced by general measures of anxiety, depression level or sociodemographic factors, and separation anxiety decisively contributed to the explanation of variance of amygdala responses beyond the effects of other variables like trait anxiety, A non-parametric correlation (Spearman’s rho) between right amygdala responsiveness and separation anxiety scores yielded similar values ( = 0.28; P < 0.001).
= 0.28; P < 0.001).
Fig. 1.
Separation Anxiety is positively associated with right-amygdala responsiveness to negative faces. Left: Coronar view (y = 2) depicting association of separation anxiety scores and amygdale responsiveness to negative faces. Colour-bar, t-value. Right: Scatter plot depicting the positive correlation of the peak voxel contrast values (left panel) and the separation anxiety scores (r = 0.26, P < 0.001). Contour lines, mean confidence interval. fMRI, functional magnetic resonance imaging.
The whole-brain analysis indicated that no other brain area showed any significant association with separation anxiety scores in this task at this rigorous threshold.
The functional connectivity analysis revealed a significant positive correlation between separation anxiety scores and functional connectivity between the right amygdala and several occipital areas including the lingual gyrus, the middle occipital gyrus, the cuneus extending to the superior occipital gyrus, the postcentral area, the supplementory motor area and the precuneus. No negative correlations between separation anxiety scores and functional coupling of the amygdala with other brain areas were observed. For details, see Table 2.
Table 2.
Results of whole-brain functional connectivity regression analysis of separation anxiety scores on right amygdala functional connectivity at PFWE-corrected < 0.05, k = 20 voxels
| Anatomical region | BA | Cluster size (k) | P-value (FWE-corrected) | x | y | z | Side | t-value |
|---|---|---|---|---|---|---|---|---|
| Lingual gyrus | 30, 18 | 50 | <0.001 | 18 | −70 | 4 | R | 6.30 |
| Calcarine gyrus | 0.001 | 10 | −60 | 4 | R | 5.75 | ||
| Postcentral gyrus | 2, 3, 40, 1 | 53 | <0.001 | 48 | −36 | 62 | R | 6.26 |
| Supplementary motor area | 6 | 24 | <0.001 | 4 | 18 | 66 | R | 6.11 |
| Middle occipital gyrus | 19 | 22 | <0.001 | −32 | −84 | 34 | L | 5.92 |
| Postcentral gyrus | 3 | 23 | 0.001 | 54 | −16 | 54 | R | 5.82 |
| 0.002 | 44 | −20 | 60 | R | 5.55 | |||
| Cuneus, superior occipital gyrus | 7, 31, 19 | 41 | 0.002 | 18 | −78 | 30 | R | 5.60 |
| Precuneus | 7 | 22 | 0.003 | −6 | −48 | 52 | L | 5.50 |
BA, Brodmann area; R, right, L, left.
Voxel-based morphometry
The analysis of the bilateral amygdala revealed a significant positive association of amygdala grey matter volume and separation anxiety scores in the right amygdala (x = 33, y = 2, z = 14; t(304) = 3.76, PFWE-corrected = 0.008; r = 0.21, k = 29) as well as a small cluster in the left amygdala (x = −24, y = −2, z = 12; t(304) = 3.24, PFWE-corrected = 0.042; r = 0.18, k = 4) within the superficial parts of the amygdala labelled by using the anatomy toolbox. Excluding subjects with values >4 s.d. above mean does not decisively affect the significance of the structural results (r = 0.19; P < 0.001). The subsequent multiple regression analysis predicting the mean grey matter volume of the significant cluster in the right amygdala by separation anxiety score, age, gender, total education time, verbal intelligence, STAI score, HAMA score and BDI score, the association between separation anxiety and amygdala volume remained significant, albeit slightly weaker (β = .014, t(298) = 2.44, P = 0.015). The semi-partial analysis between RSQ scores and amygdala volume controlling for STAI scores also yielded significant semi-partial correlations (rp = 0.2; P = 0.001) as well as the non-parametric correlation between RSQ scores and amygdala volume ( = .19; P = .017). The collinearity analysis yielded tolerable values (tolerance > 0.63; VIF < 1.57). Again, our whole-brain analysis yielded no other brain area revealing an association of separation anxiety scores and grey matter structure outside the amygdala using our corrected statistical threshold. Bivariate correlation analyses between functional and structural data yielded no significant correlations (all P > 0.367), controlling for age.
= .19; P = .017). The collinearity analysis yielded tolerable values (tolerance > 0.63; VIF < 1.57). Again, our whole-brain analysis yielded no other brain area revealing an association of separation anxiety scores and grey matter structure outside the amygdala using our corrected statistical threshold. Bivariate correlation analyses between functional and structural data yielded no significant correlations (all P > 0.367), controlling for age.
DISCUSSION
To our knowledge, this is the first study investigating neural correlates of separation anxiety in adult subjects with neuroimaging methods. In line with our hypothesis, the main result of this study was a stronger reactivity to negative faces in subjects with higher separation anxiety. Additionally, the functional connectivity analysis revealed a positive association between separation anxiety and the functional coupling of the amygdala to occipital, somatosensory and supplementary motor areas.
Amygdala hyperactivity to threatening faces has been observed in subjects with higher trait anxiety (Stein et al., 2007), subclinical anxious subjects (Blackmon et al., 2011; Laeger et al., 2012), as well as in patients with anxiety disorders (Etkin and Wager, 2007; Klumpp et al., 2010). Corresponding to these findings, we found a strong reactivity to negative faces also in subjects with higher scores of separation anxiety. This apparent similarity may emerge due to the fact that separation anxiety shares common neurobiological features with other types of anxiety.
In social life, facial expressions are important cues for the evaluation of social contexts, and it is assumed that facial expressions of joy, sadness or threat act as a discriminative stimulus that an aversive or appetitive reaction may follow (Adolphs, 1999, 2001; Hooker et al., 2006). Presumed that for high separation-anxious people it is more important to assess, explain and predict other peoples’ intentions because of an exaggerated anxiety to get abandoned or forsaken, the hyper-reactivity of the amygdala could be associated with an automatically increased rapid emotional reaction due to higher individual relevance of facial, in particular to negative facial expressions that may hint a cue for the threat of leaving.
Our functional connectivity results, including the association between separation anxiety and functional coupling of the amygdala to occipital and somatosensory areas, seem to support the notion of an increased interplay between the amygdala and areas modulating attention and emotional salience, and in turn an increased attention load for social cues in subjects with separation anxiety. These areas have in common, that they are related to vigilance and emotional attention processing, especially when they are self-related (Straube and Miltner, 2011; Vuilleumier, 2005). The limbic and visual systems are extensively interconnected, and recent research has begun to reveal the neural processes by which attention and visual processes can be modulated by stimuli with a high individual affective significance. For this modulation, the amygdala seems to have a crucial hub function and projects readily top-down signals on sensory (including extrastriate and striate visual areas) pathways, which in turn influence the representation and emotional value, of (in particular threat-related) emotional events (Pessoa and Adolphs, 2010; Tamietto and de Gelder, 2010; Vuilleumier, 2005). This top-down modulation is assumed to generate saliency signals that modulate perceptual and motor processes to regulate adaptive behavior appropriately (Said et al., 2011; Pourtois et al., 2013). These networks have been recently confirmed, demonstrating that the functional connectivity between amygdala and sensory perceptual areas is modulated by vigilance for threatening facial features (Miyahara et al., 2013).
In contrast to our hypothesis, our morphometric analysis yielded a positive association between separation anxiety and amygdala volume that also seems to contrast previous reports regarding decreased amygdala volume in anxiety disorders (Milham et al., 2005; Hayano et al., 2009). Hence, complementary to the discussed functional findings, it is imaginable that the increased amygdala volume may not necessarily reflect a deficit in subjects with separation anxiety but potentially a compensatory structural process in our—psychiatrically healthy—study sample. A recently published study reported a positive association of amygdala volume with the size and complexity of social networks in adult humans (Bickart et al., 2011). This link between social network size and amygdala volume is further supported by neuroanatomical studies in non-human primates (Lewis and Barton, 2006). Therefore, the increased amygdala volume could result from a relative greater social network size of people with high separation anxiety given their distress resulting from being alone. However, these assumptions remain speculative and need further research.
Taken together, the positive association of separation anxiety and amygdala volume, hyper-reactivity and functional coupling between amygdala and occipital and somatosensory areas to emotional faces might be driven by a higher involvement, more detailed processing and correspondingly, an increased attentiveness particularly to social stimuli such as emotional faces. However, this interpretation remains speculative given that our paradigm was not suited for differentiating between social and non-social stimuli or different emotion types, and thus should be taken with care. Future studies should investigate samples with pathological forms of adult separation anxiety and compare these findings with other forms of anxiety disorders in order to provide neurobiologically informed arguments for or against the postulation of a distinct illness category in adults.
LIMITATIONS
Due to the fact that this is the first study that investigated separation anxiety with neuroimaging methods, conclusions and interpretations must be treated with caution and some methodological limitations should be acknowledged. First, we applied a frequently used face-matching paradigm for eliciting amygdala responsiveness that only displayed negative facial expressions. The study design did not allow separating effects of the response to faces in general and the response to (specific) threat-related facial expressions. It would be insightful whether the reported associations are specific to negative faces, or if they also apply to emotional stimuli in general. Future studies should address this limitation using different stimuli contrasting social threat with non-social threat. Further, the task demanded implicit emotion processing using faces of strangers rather than explicit emotion processing including facial stimuli from persons with close personal relationships to the participants. Potentially stronger results might be expectable for faces of people who play significant roles in the participants’ lives. In addition, we did not administer a measure of subclinical social anxiety besides the SCID ruling out a clinical diagnosis. Hence, potentially confounding effects by subclinical social anxiety should be addressed in future studies.
Second, the reported correlations were generally rather low possibly due to the fact that a healthy sample was used. If the assumption of a continuum of separation anxiety is right, even stronger results should also emerge in pathological forms of separation anxiety, e.g. in children with separation anxiety disorder or psychiatric patients suffering from disorders such as some personality disorders in which separation anxiety constitutes a diagnostic criterion. However, we used a questionnaire not providing cutoff scores for pathological forms of separation anxiety, probably due to the fact that adult separation anxiety disorder is currently not included in clinical classification systems. Therefore, it remains to be clarified whether our findings also apply for subjects suffering pathological forms of adult separation anxiety.
Supplementary Material
Acknowledgments
The authors thank Ahmad Hariri for providing the fMRI paradigm. The author further thanks Nina Nagelmann for her skillful technical support during the fMRI sessions.
This work was supported by grants of the Innovative Medizinische Forschung [IMF DA120309 to U.D., IMF DA211012 to U.D.]
REFERENCES
- Abraham A, Kaufmann C, Redlich R, et al. Self-referential and anxiety-relevant information processing in subclinical social anxiety: an fMRI study. Brain Imaging and Behavior. 2013;7:35–48. doi: 10.1007/s11682-012-9188-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Social cognition and the human brain. Trends in Cognitive Sciences. 1999;3:469–79. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–9. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Konrad C, Grotegerd D, et al. Interleukin-6 gene (IL-6): a possible role in brain morphology in the healthy adult brain. Journal of Neuroinflammation. 2012a;9:125–34. doi: 10.1186/1742-2094-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baune BT, Konrad C, Grotegerd D, et al. Tumor necrosis factor gene variation predicts hippocampus volume in healthy individuals. Biological Psychiatry. 2012b;72:655–62. doi: 10.1016/j.biopsych.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventory: Manual. San Antonio: The Psychological Corporation, Harcourt Brace Jovanovich; 1987. [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nature Neuroscience. 2011;14:163–4. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmon K, Barr WB, Carlson C, et al. Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiatry Research. 2011;194:296–303. doi: 10.1016/j.pscychresns.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human Brain Mapping. 2012;34:3247–66. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögels SM, Knappe S, Clark LA. Adult separation anxiety disorder in DSM-5. Clinical Psychology Review. 2013;33:663–74. doi: 10.1016/j.cpr.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Bögels SM, Zigterman D. Dysfunctional cognitions in children with social phobia, separation anxiety disorder, and generalized anxiety disorder. Journal of Abnormal Child Psychology. 2000;28:205–11. doi: 10.1023/a:1005179032470. [DOI] [PubMed] [Google Scholar]
- Carthy T, Horesh N, Apter A, Gross JJ. Patterns of emotional reactivity and regulation in children with anxiety disorders. Journal of Psychopathology and Behavioral Assessment. 2009;32:23–36. [Google Scholar]
- Cyranowski JM, Shear MK, Rucci P, et al. Adult separation anxiety: psychometric properties of a new structured clinical interview. Journal of Psychiatric Research. 2002;36:77–86. doi: 10.1016/s0022-3956(01)00051-6. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Barrett PM, Rapee RM, Ryan S. Family process and child anxiety and aggression: an observational analysis. Journal of Abnormal Child Psychology. 1996;24:715–34. doi: 10.1007/BF01664736. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Franke F, et al. Neuropeptide-S (NPS) receptor genotype modulates basolateral amygdala responsiveness to aversive stimuli. Neuropsychopharmacology. 2011;36:1879–85. doi: 10.1038/npp.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Huber F, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapping. 2013;34:2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, et al. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. The International Journal of Neuropsychopharmacology. 2009;12:11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71:286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Domschke K, Baune BT, Havlik L, et al. Catechol-O-methyltransferase gene variation: impact on amygdala response to aversive stimuli. Neuroimage. 2012;60:2222–9. doi: 10.1016/j.neuroimage.2012.02.039. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–82. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Reviews and overviews functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francati V, Vermetten E, Bremner JD. Funtional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depression Anxiety. 2007;24:202–218. doi: 10.1002/da.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Human Brain Mapping. 1994;2:56–78. [Google Scholar]
- Griffin DW, Bartholomew K. The metaphysics of measurement: the case of adult attachment. In: Bartholomew K, Perlman D, editors. Advances in Personal Relationships, Vol.5: Attachment Processes in Adulthood. London: Jessica Kingsley; 1994. pp. 17–52. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Britsh Journal of Medical Psychology. 1959;3:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M, Worall H, Keller F. Beck Depressions-Inventar (BDI). Testhandbuch. Bern: Hans Huber; 1994. [Google Scholar]
- Hayano F, Nakamura M, Asami T, et al. Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry and Clinical Neurosciences. 2009;63:266–76. doi: 10.1111/j.1440-1819.2009.01960.x. [DOI] [PubMed] [Google Scholar]
- Hooker CI, Germine LT, Knight RT, D’Esposito M. Amygdala response to facial expressions reflects emotional learning. The Journal of Neuroscience. 2006;26:8915–22. doi: 10.1523/JNEUROSCI.3048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16:1671–5. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research. 2011;223:403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala reactivity to faces at varying intensities of threat in generalized social phobia: an event-related functional MRI study. Psychiatry Research. 2010;183:167–9. doi: 10.1016/j.pscychresns.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger I, Dobel C, Dannlowski U, et al. Amygdala responsiveness to emotional words is modulated by subclinical anxiety and depression. Behavioural Brain Research. 2012;233:508–16. doi: 10.1016/j.bbr.2012.05.036. [DOI] [PubMed] [Google Scholar]
- Lehrl S. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. Balingen: Spitta Verlag; 2005. [Google Scholar]
- Lewinsohn PM, Holm-Denoma JM, Small JW, Seely JR, Joiner TE. Separation anxiety disorder in childhood as a risk factor for future mental illness. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:548–55. doi: 10.1097/CHI.0b013e31816765e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KP, Barton RA. Amygdala size and hypothalamus size predict social play frequency in nonhuman primates: a comparative analysis using independent contrasts. Journal of Comparative Psychology. 2006;120:31–7. doi: 10.1037/0735-7036.120.1.31. [DOI] [PubMed] [Google Scholar]
- Maier W, Buller R, Phillips ML, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of Affective Disorders. 1988;14:61–8. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–39. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manicavasagar V, Silove D, Hadzi-Pavlovic D. Subpopulations of early separation anxiety: relevance to risk of adult anxiety disorders. Journal of Affective Disorders. 1998;48:181–90. doi: 10.1016/s0165-0327(97)00170-5. [DOI] [PubMed] [Google Scholar]
- Manicavasagar V, Silove DM, Curtis J. Separation anxiety in adulthood: a phenomenological investigation. Comprehensive Psychiatry. 1997;38:274–82. doi: 10.1016/s0010-440x(97)90060-2. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biological Psychiatry. 2005;57:961–6. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Harada T, Ruffman T, Sadato N, Iidaka T. Functional connectivity between amygdala and facial regions involved in recognition of facial threat. Social Cognitive and Affective Neuroscience. 2013;8:181–9. doi: 10.1093/scan/nsr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollendick TH, Lease CA, Cooper C. Separation anxiety in young adults: a preliminary examination. Journal of Anxiety Disorders. 1993;7:293–305. [Google Scholar]
- Pejic T, Hermann A, Vaitl D, Stark R. Social anxiety modulates amygdala activation during social conditioning. Social Cognitive and Affective Neuroscience. 2011;8:267–76. doi: 10.1093/scan/nsr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nature Reviews Neuroscience. 2010;11:773–82. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleiderer B, Zinkirciran S, Arolt V, Heindel W, Deckert J, Domschke K. fMRI amygdala activation during a spontaneous panic attack in a patient with panic disorder. The World Journal of Biological Psychiatry. 2007;8:269–72. doi: 10.1080/15622970701216673. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59:424–9. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schettino A, Vuilleumier P. Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biological Psychology. 2013;92:492–512. doi: 10.1016/j.biopsycho.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat JW, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12:43–7. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Human Brain Mapping. 2010;31:173–84. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said CP, Haxby JV, Todorov AA. Brain systems for assessing the affective value of faces. Philosophical Transactions of the Royal Society. 2011;366:1660–70. doi: 10.1098/rstb.2010.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfe E, Bartholomew K. Reliability and stability of adult attachment patterns. Personal Relationships. 1994;1:23–43. [Google Scholar]
- Scharfe E, Cole V. Stability and change of attachment representations during emerging adulthood: an examination of mediators and moderators of change. Personal Relationships. 2006;13:363–74. [Google Scholar]
- Schienle A, Schäfer A, Walter B, Stark R, Vaitl D. Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neuroscience Letters. 2005;388:1–6. doi: 10.1016/j.neulet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Schweckendiek J, Klucken T, Merz CJ, et al. Weaving the (neuronal) web: fear learning in spider phobia. NeuroImage. 2011;54:681–8. doi: 10.1016/j.neuroimage.2010.07.049. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Dannlowski U, Schöning S, et al. Neural correlates of trait anxiety in fear extinction. Psychological Medicine. 2011;41:789–98. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- Shear MK, Jin R, Ruscio AM, Ph D, Walters EE, Kessler RC. Prevalence and correlates of estimated DSM-IV child and comorbidity survey replication. American Journal of Psychiatry. 2006;163:1074–83. doi: 10.1176/appi.ajp.163.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Silove DM, Marnane CL, Wagner R, Manicavasagar VL, Rees S. The prevalence and correlates of adult separation anxiety disorder in an anxiety clinic. BMC Psychiatry. 2010;10:21. doi: 10.1186/1471-244X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R. State-Trait Anxiety Inventory, Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologist Press; 1970. [Google Scholar]
- Steffanowski A, Oppl M, Meyerberg J, Schmidt J, Wittmann WW, Nübling R. Psychometrische Überprüfung einer deutschsprachigen version des relationship scales questionaire (RSQ) In: Bassler M, editor. Störungsspezifische Therapieansätze in der stationären Psychotherapie. Giessen: Psychosozial Verlag; 2001. pp. 320–42. [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry. 2007;164:318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel H-J, Miltner WHR. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–8. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel H-J, Miltner WHR. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biological Psychiatry. 2006;59:162–70. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Straube T, Miltner WHR. NeuroImage Attention to aversive emotion and specific activation of the right insula and right somatosensory cortex. Neuroimage. 2011;54:2534–38. doi: 10.1016/j.neuroimage.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Bitter SM, Weber WA, et al. Neurocircuitry of generalized anxiety disorder in adolescents: a pilot functional neuroimaging and functional connectivity study. Depression and Anxiety. 2012;29:939–47. doi: 10.1002/da.21961. [DOI] [PubMed] [Google Scholar]
- Tamietto M, de Gelder B. Neural bases of the non-conscious perception of emotional signals. Nature Reviews Neuroscience. 2010;11:697–709. doi: 10.1038/nrn2889. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Archives of General Psychiatry. 2005;62:922–33. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- Vrticka P, Andersson F, Grandjean D, Sander D, Vuilleumier P. Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS One. 2008;3:e2868. doi: 10.1371/journal.pone.0002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Wittchen H-U, Wunderlich U, Gruschwitz S, Zaudig M. Strukturiertes Klinisches Interview für DSM-IV. Goettingen: Hogrefe; 1997. [Google Scholar]
- World Medical Association. (1991). Declaration of Helsinki. Law, Medicine and Health Care. 1991;19:264–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.