Abstract
Alexithymia is a psychological construct that can be divided into a cognitive and affective dimension. The cognitive dimension is characterized by difficulties in identifying, verbalizing and analysing feelings. The affective dimension comprises reduced levels of emotional experience and imagination. Alexithymia is widely regarded to arise from an impairment of emotion regulation. This is the first functional magnetic resonance imaging (fMRI) study to critically evaluate this by investigating the neural correlates of emotion regulation as a function of alexithymia levels. The aim of the current study was to investigate the neural correlates underlying the two alexithymia dimensions during emotion perception and emotion regulation. Using fMRI, we scanned 51 healthy subjects while viewing, reappraising or suppressing negative emotional pictures. The results support the idea that cognitive alexithymia, but not affective alexithymia, is associated with lower activation in emotional attention and recognition networks during emotion perception. However, in contrast with several theories, no alexithymia-related differences were found during emotion regulation (neither reappraisal nor suppression). These findings suggest that alexithymia may result from an early emotion processing deficit rather than compromised frontal circuits subserving higher-order emotion regulation processes.
Keywords: alexithymia, emotion processing, emotion regulation, amygdala, neuroimaging
Alexithymia (‘no words for feelings’) is a psychological construct characterized by difficulties in identifying and describing one’s feelings, and in distinguishing them from bodily sensations of arousal. Individuals with high scores on alexithymia may further show a lack of imagination and an externally oriented thinking style with a lack of introspection (Sifneos, 1973; Vorst and Bermond, 2001). Alexithymia can be divided into an affective and a cognitive dimension (Vorst and Bermond, 2001). The cognitive alexithymia dimension comprises the subscales difficulties in identifying, analysing and verbalizing feelings, while the affective dimension consists of the subscales emotionalizing (the degree to which someone is emotionally aroused by emotional stimuli) and fantasizing (the degree to which someone is inclined to imagine). Based on these dimensions, Bermond proposed to distinguish two subtypes of alexithymia [Type I and Type II; (Bermond et al., 2007)]. Type I alexithymia is characterized by high scores on both dimensions (i.e. lower cognitive emotional processing capacities and lower emotional arousal). Type II is characterized by high scores on the cognitive dimension, but normal or even low scores on the affective dimension (i.e. lower cognitive emotional processing capacities, but normal or heightened emotional arousal). Recent studies have suggested that the two alexithymia dimensions might be differently related to the development of psychopathology (Moormann et al., 2008; Van der Meer et al., 2009). Therefore, it is of relevance to gain insight into the neural bases underlying these two dimensions.
Individuals with high scores on alexithymia experience difficulties in emotion processing. Furthermore, alexithymia is generally regarded to be an emotion regulation impairment (Taylor et al., 1997; Taylor and Bagby, 2004; Aleman, 2005; Wingbermühle et al., 2012). However, as far as we know, the neural correlates of emotion regulation in alexithymia through functional neuroimaging have not yet been investigated.
Emotion processing can be seen as a three-phase process with (i) the identification of the emotional significance of a stimulus, (ii) the generation of an affective state and (iii) emotion regulation (Phillips et al., 2003). Previous research has suggested that problems in emotion processing related to alexithymia may already occur during the first two phases of emotion processing. For example, individuals with high scores on alexithymia show deficits in the identification of facial expressions (Swart et al., 2009; Grynberg et al., 2012) and declined attention towards emotional stimuli (Mueller et al., 2006). Furthermore, studies have indicated that individuals with high scores on alexithymia differ in their physiological responses to emotional stimuli (Roedema and Simons, 1999; Bermond et al., 2010), which might reflect differences in the generation of affective states. Phillips and colleagues (2003) suggested that a ventral system, including the amygdala, insula, ventral striatum, ventral anterior cingulate cortex (ACC) and the ventral prefrontal cortex, is involved during the first two phases of emotion processing. Previous studies investigating alexithymia-related brain activation have reported activation differences in this system (Bermond et al., 2006; van der Velde et al., 2013). As far as we know, only one study examined the neural correlates of the two alexithymia dimensions separately through functional magnetic resonance imaging (fMRI) during emotion processing (Pouga et al., 2010), linking the cognitive dimension to lower amygdala activation and the Emotionalizing factor of the affective dimension to higher ACC and lower premotor activation. This indicates that the cognitive and affective alexithymia dimensions may be associated with separable neural correlates during the first two phases of emotion processing.
Emotion regulation, the third phase of emotion processing, is defined as the inhibition or modulation of the affective state and the emotional response (Phillips et al., 2003). Gross (1998) described reappraisal and suppression, two often applied and widely studied regulation strategies. Reappraisal is a strategy used to change the initial emotional response in such a way that it decreases (e.g. making a negative stimuli less negative), while suppression focuses on inhibiting emotional expressive behavior (e.g. not showing how you feel) (Gross, 1998). Aleman (2005) proposed that alexithymia would be related to difficulties in emotion regulation. Indeed, several studies suggested that individuals with alexithymia seem to apply more suppression, a less efficient strategy, while applying less reappraisal in comparison with non-alexithymic subjects (Swart et al., 2009; Kessler et al., 2010; Wingenfeld et al., 2011; Stasiewicz et al., 2012). However, others have failed to show this association (Geenen et al., 2012; Weiss et al., 2012). During reappraisal, activation in the ventromedial prefrontal cortex and in a dorsal system, including the inferior frontal gyrus, the dorsal ACC and the dorsal medial frontal cortex, increases [for meta-analysis, see (Diekhof et al., 2011)]. By increasing activation in these areas, amygdala activation is consequently reduced, resulting in lower negative affect. Research on suppression indicates increased activation in areas involved in inhibitory control, such as the dorsolateral prefrontal cortex (DLPFC), supplementary motor area, inferior parietal cortex and the precuneus (Goldin et al., 2008; Vanderhasselt et al., 2012). The increased activation in these areas probably results in the restricted facial expression during suppression (Vanderhasselt et al., 2012). Alexithymia-related activation differences during emotion regulation are not yet investigated through fMRI. However, an electroencephalography (EEG) study indicated that during reappraisal, P3 amplitudes in the DLPFC, fusiform gyrus and inferior temporal gyrus decrease in a low alexithymia group, but not in a high alexithymia group (Pollatos and Gramann, 2012). Furthermore, during suppression event-related potentials are negatively correlated to alexithymia (Walker et al., 2011). These findings suggest that alexithymia might be related to differential brain activation patterns during emotion regulation.
The current study is the first fMRI study on emotion regulation in alexithymia. We aimed to investigate neural correlates as a function of the cognitive and affective alexithymia dimensions during the different phases of emotion processing. To this extent, we presented 51 subjects differing in alexithymia scores with an emotion regulation task. The task consisted of an emotion perception part in which subjects viewed negative or neutral pictures, followed by an emotion regulation part where subjects were instructed to either attend to, reappraise or suppress the emotional content of the picture. We hypothesize that the two alexithymia dimensions are associated with separable neural correlates. We suggest that during the first two phases of emotion processing, the cognitive alexithymia dimension will be associated with decreased activation in brain areas involved in the identification of emotions (such as the amygdala) while the affective dimension is proposed to be associated with activation in areas involved in emotional awareness (such as the ACC). During reappraisal we expect to find lower activation in the dorsal system (e.g. DLPFC) associated with the cognitive dimension, as reappraisal can be seen as a cognitive task (Ochsner and Gross, 2005).
METHOD
Participants
Fifty-one healthy subjects were included from a multi-center (Groningen and Amsterdam) add-on study from the GROUP project [Genetic Risk & Outcome of Psychosis, (Korver et al., 2012)]. This sample did not overlap with previous fMRI studies from our group (Swart et al., 2011; Liemburg et al., 2012; Goerlich Dobre et al., 2014). Participants reported no presence or history of any neurological or psychiatric disorder, which was confirmed with a diagnostic interview. Furthermore, there were no psychotic or mood disorders present in the first-degree relatives of the participants. Demographic variables are presented in Table 1.
Table 1.
Mean scores of demographic data, questionnaire data and rating scores on the emotion regulation task and the associations with alexithymia
| Demographic or behavioral variable | Mean (SD) | Statistics Cognitive dimension | Statistics Emotionalizinga | Statistics Fantasizing | |||
|---|---|---|---|---|---|---|---|
| Gender (n male) | 28 | t = 1.28 | P = .21 | U = 177 | P = .006* | t = −.98 | P = .33 |
| Handedness (n right) | 44 | t = −.26 | P = .79 | U = 133 | P = .56 | t = .23 | P = .82 |
| Scanner site (n Groningen) | 24 | t = −.86 | P = .40 | U = 312 | P = .82 | t = −.26 | P = .80 |
| Age (years) | 37.1 (10.3) | r = −.16 | P = .25 | ρ = −.01 | P = .95 | r = .15 | P = .30 |
| Education (years) | 17.1 (0.8) | r = −.14 | P = .34 | ρ = −.06 | P = .70 | r = −.11 | P = .42 |
| ERQ | |||||||
| Reappraisal | 5.0 (1.0) | r = −.16 | P = .25 | ρ = .18 | P = .22 | r = .07 | P = .64 |
| Suppression | 2.8 (1.2) | r = .63 | P < .001** | ρ = .20 | P = .16 | r = −.19 | P = .18 |
| PANAS | |||||||
| Positive affect | 32.7 (6.0) | r = −.16 | P = .26 | ρ = −.07 | P = .62 | r = .16 | P = .28 |
| Negative affect | 13.1 (4.5) | r = −.09 | P = .53 | ρ = −.34 | P = .02* | r = .26 | P = .06 |
| Rating scores | |||||||
| Attend neutral | 1.1 (0.2) | ρ = .21 | P = .15 | ρ = −.12 | P = .41 | r = −.06 | P = .60 |
| Attend negative | 2.6 (0.5) | ρ = −.02 | P = .87 | ρ = −.28 | P = .04* | r = −.04 | P = .80 |
| Reappraise | 2.1 (0.6) | ρ = .02 | P = .90 | ρ = −.16 | P = .27 | r = −.12 | P = .62 |
| Suppress | 2.4 (0.6) | ρ = .06 | P = .65 | ρ = −.13 | P = .37 | r = −.08 | P = .81 |
aNon-parametric testing due to non-normality; *significant at P < .05, not surviving multiple comparison correction; **significant at P < .001 (controlled for multiple comparisons).
ERQ, Emotion regulation questionnaire; PANAS, Positive and negative symptom scale; SD, standard deviation.
Behavioral measurements
Diagnostic interviews
During the assessment of the GROUP study (max. 2 years prior to the fMRI scan), all participants were screened with a diagnostic interview. The SCAN interview [Schedules for the Clinical Assessment of Psychiatry (Wing et al., 1990)] was used to assess the current psychiatric state and psychiatric history of all the participants from the Groningen sample. For the sample from Amsterdam, the CASH [Comprehensive Assessment of Symptoms and History (Andreasen et al., 1992)] was applied for assessing diagnosis and psychopathology. When participants had been given a clinical diagnosis, they were excluded from the study. Prior to the fMRI session, participants were asked if there were any changes in their psychological well-being since the last GROUP assessment. If participants reported relevant changes in mood, psychotic symptoms or anxiety, they were excluded from the study.
Bermond–Vorst Alexithymia Questionnaire
The Bermond–Vorst Alexithymia Questionnaire (BVAQ) is a 40-item self-report scale used to assess alexithymia. The BVAQ consists of five subscales (eight items per scale), Identifying, Verbalizing, Analysing, Emotionalizing and Fantasizing as defined by Nemiah and Sifneos (1970). Answers were rated on a five-point Likert scale (1 = certainly does not apply to me, 5 = certainly does apply to me), with higher scores indicating more pronounced alexithymic characteristics. Previous studies have confirmed the five-factor structure of the BVAQ and have shown that the BVAQ has good psychometric properties (Zech et al., 1999; Berthoz et al., 2000; Vorst and Bermond, 2001).
Using the BVAQ, a second-order distinction can be made in which the subscales Emotionalizing and Fantasizing are grouped into an affective dimension, and the subscales Identifying, Verbalizing and Analysing feelings into a cognitive dimension of alexithymia. The validity of this two-factor structure has been demonstrated by factor analyses (Bailey and Henry, 2007; Bekker et al., 2007; Bermond et al., 2007). However, others failed to provide support for this two-factor structure (Bekker et al., 2007; Bagby et al., 2009). In the current sample, the existence of the cognitive dimension was supported by significant correlations between the three sub-factors of the cognitive dimension (r = .44 till .65; P ≤ .001). However, the two sub-factors of the affective dimension were not correlated to each other (r = .07; P = .62). Therefore, the Emotionalizing and Fantasizing sub-factors were treated as separate variables, in line with the approach of previous studies (Bekker et al., 2007; Goerlich-Dobre et al., 2014). In 2008, Deborde and colleagues developed cut-off scores based on the BVAQ-B. The BVAQ-B is a shorter version of the BVAQ, which is calculated by summing up the scores on items 21 through 40 (Zech et al., 1999). To give an indication of severity of alexithymia in our sample, BVAQ-B scores were calculated. A score of 53 or higher indicates high alexithymia, while scores of 43 or lower indicate low alexithymia (Deborde et al., 2008).
Emotion Regulation Questionnaire
The use of the emotion regulation strategies, reappraisal and suppression, was assessed through the Emotion Regulation Questionnaire (ERQ) (Gross and John, 2003). The ERQ consists of 10 items of which four examine suppression and six examine reappraisal. On a seven-point scale subjects had to rate to what extent a certain statement applied to them (strongly disagree till strongly agree). The ERQ has been proven to be a reliable and valid measure of emotion regulation (Gross and John, 2003). To get a clear view on the relationship between the two strategies, the total scores of both subscales were divided by the number of items per subscale.
Positive and Negative Affect Scale
The positive and negative affect scale (PANAS) (Watson et al., 1988) was used to measure the current affective state. The scale consists of 10 positive affect items (reflecting the extent to which a person feels enthusiastic, active and alert) and 10 negative affect items (reflecting distress, anger, fear and guilt). Participants rated on a five-point scale to what extent they experienced certain mood states. The PANAS has been proven to be a reliable and valid measure of positive and negative affect (Crawford and Henry, 2004).
Emotion regulation task
The emotion regulation task [adapted from (Ochsner and Gross, 2005)] consisted of four conditions, Attend Neutral, Attend Negative, Reappraise and Suppress. The stimuli were 66 negative (mean valence: 2.54, mean arousal: 5.83) and 22 neutral pictures (mean valence: 5.10, mean arousal: 3.26) from the International Affective Picture System. Each trial was constructed as follows. First, a picture appeared with the instruction ‘view’ (View condition, 2 s). After that, the word ‘view’ changed in one of the following instructions: ‘reappraise’, ‘suppress’ or ‘attend’ (Regulation condition, 4 s). Reappraise meant that the participants had to reappraise the picture in such a way that it became less emotionally disturbing. During suppression, subjects were instructed to refrain from expressing their emotions, in a way that bystanders would not be able to read their emotions by looking at their face. During attend, participants just had to look closely at the picture and not change the way they were feeling. The 22 neutral pictures were always paired with the ‘attend’ instruction. Negative pictures could be paired with either reappraise (22 pictures), suppress (22 pictures) or attend (22 pictures). Following regulation, a black screen appeared (2 s). Subsequently, participants were asked to rate how negative they were feeling on a four-point scale (1 = not negative at all; 4 = extremely negative) (3 s). After rating negative affect the word ‘relax’ appeared (4 s) allowing participants to relax, followed by a black screen (0.5 s) to alert participants the next trial was coming. A single trial lasted for 15.5 s. After 9 or 10 trials, a fixation cross appeared for 20 s. The trial order was randomized using a randomized block design.
Prior to the fMRI scan, a short training was given to teach the application of reappraisal and suppression strategies. During this training, participants practiced the different strategies on negative pictures by telling the researchers how they would apply the strategy.
Data acquisition
MRI data were acquired using two 3.0-Tesla whole body scanners (Philips Intera, Best, The Netherlands) located at the University Medical Center Groningen and at the Academic Medical Center in Amsterdam. Both systems were equipped with an 8-SENSE head coil and scan parameters were set identical. The functional images were acquired by a T2-weighted echo producing 37 slices 3.5 mm thick with no gap. The images were slightly tilted (30 degrees) to prevent artifacts due to nasal cavities. The functional scans were made in the axial plane [TR = 2 s; TE = 30 s; flip angle (α) = 70°; FOV = 224.0, 129.5, 224.0; in-plane resolution 64 × 62 pixels; isotropic voxels of 3.5 mm] and were scanned interleaved. The T1-weighted anatomical image (170 slices; isotropic voxels of 1 mm; TR = 9 ms; TE = 3.54 ms; α = 8°; FOV 256 mm) was acquired in the bicommissural plane, covering the whole brain.
Statistical analyses
Behavioral analyses were performed using SPSS 20 (SPSS Inc., Chicago, IL, USA). To calculate the main effect of condition on the negative affect rating scores from the emotion regulation task, a Friedman’s ANOVA was applied due to non-normality of the ratings scores. Post hoc analyses were performed with a Wilcoxon signed-rank test and corrected for multiple testing using a Bonferroni correction. To examine the effect of alexithymia on the rating scores, non-parametric Spearman’s correlations were performed. To examine whether the cognitive alexithymia dimension and the Fantasizing sub-factor were associated with demographic variables, ERQ scores and PANAS scores, two-sample t-tests and Pearson correlations were performed. The associations with the Emotionalizing sub-factor were calculated using non-parametric tests due to non-normality of this sub-factor. For all calculated associations with alexithymia, the significance level was set at P < .001 to control for multiple testing (Bonferroni correction for 39 tests). Furthermore, Spearman’s correlations were performed between the ERQ reappraisal and suppression scores and the negative affect rating scores of the emotion regulation task. For these analyses, the threshold was set to P < .006 (correcting for eight tests).
The fMRI data were analysed using Statistical Parametric Mapping (SPM 8) (www.fil.ion.ucl.ac.uk) using Matlab 7 (The MathWorks Inc., Natick, MA, USA). Before processing the data, all images were checked for artifacts. Slice timing was applied to the functional images and the functional data were spatially realigned, resliced and coregistered. The anatomical data were segmented. To enhance the accuracy of inter-subject alignment, the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) approach was used to create a grey matter template based on the grey matter segmented images of all subjects. This created template was used to normalize the functional images to and affine-transform them into Montreal Neurological Institute (MNI) stereotactic space. Data were smoothed with a full-width half-maximum Gaussian kernel of 6 mm. Subject head movement greater than 3 mm in more than one direction resulted in exclusion of the data.
Sixteen task-related regressors were modelled with a boxcar function convolving a haemodynamic response function. The regressors View and Relax were divided into View/Relax neutral and View/Relax negative. The other regressors—Regulation, Linger and Rating—were subdivided into a Reappraise, Suppress, Attend Negative and Attend Neutral part. Additionally, the realignment parameters and the first derivatives thereof were entered as covariates to correct for the effects related to head motion (Friston et al., 1996). Five contrasts were made for each participant: (i) View Neutral versus Baseline; (ii) View Negative versus View Neutral; (iii) Attend Negative versus Attend Neutral; (iv) Reappraise versus Attend Negative; (v) Suppress versus Attend Negative.
To examine task-related activation, one-sample t-tests were conducted. Sex, handedness and scanner site (Amsterdam vs Groningen) were entered as covariates of no interest. To examine the effect of the cognitive alexithymia dimension on task-related activity (positive and negative correlations), the demeaned scores of the cognitive dimension of the BVAQ were entered as a covariate of interest in a whole brain multiple regression analyses with sex, handedness, site and the sum of the Emotionalizing and Fantasizing sub-factors as nuisance variables. Additional regression models were created for the three cognitive subscales of the BVAQ separately as covariates of interest to explore whether the observed correlations with the cognitive dimension were driven by specific subscales. To examine brain activation related to the affective alexithymia dimension (positive and negative correlations), two separate multiple regression models were created for the Emotionalizing and Fantasizing sub-factors. In both models, the cognitive dimension, sex, handedness and site were included as nuisance variables.
To limit possible false positives due to multiple comparisons, effects had to meet P < .05 family-wise error (FWE) corrected at the cluster level to be considered statistically significant (initial height-threshold for all the analyses was set at P < .001). Because of specific hypotheses regarding the amygdala, a small volume correction (SVC) was applied if this region would not show in the whole-brain analyses.
RESULTS
Behavioral results
Alexithymia scores
Mean alexithymia scores, range and internal consistencies of the BVAQ are presented in Table 2. Alexithymia was not significantly related to any of the demographic variables, including sex, handedness, age and scanner site (see Table 1 for test statistics). The Fantasizing and Emotionalizing sub-factors were not significantly correlated to the cognitive dimension (r = −.13, P = .38; r = .08, P = .61, respectively). Of our 51 participants, 12 participants could be classified as alexithymic and 24 as non-alexithymic as indicated by the cut-off scores of Deborde et al. (2008) based on the recalculated scores of the BVAQ-B (Zech et al., 1999).
Table 2.
Mean, standard deviation, range and Cronbach’s alpha of the alexithymia scores
| Variable | Mean (SD) | Range | Cronbach’s alpha |
|---|---|---|---|
| Affective dimension | |||
| Emotionalizing | 20.0 (3.9) | 13–27 | .54 |
| Fantasizing | 21.6 (7.3) | 9–40 | .87 |
| Cognitive dimension | 48.0 (13.7) | 26–84 | .90 |
| Identifying | 13.8 (5.3) | 8–29 | .78 |
| Verbalizing | 18.9 (6.4) | 9–35 | .86 |
| Analysing | 15.3 (5.0) | 8–28 | .81 |
| Recalculated BVAQ-B | 44.5 (8.6) | 29–62 | .73 |
BVAQ, Bermond–Vorst alexithymia questionnaire; SD, standard deviation.
The cognitive dimension was positively correlated with the ERQ suppression scores, but not with ERQ reappraisal. The Fantasizing and Emotionalizing sub-factors did not correlate significantly with either reappraisal or suppression. Furthermore, no significant correlations were found between PANAS positive or negative factors and alexithymia, except from a small negative correlation between the Emotionalizing sub-factor and negative affect (not surviving multiple comparison correction) (see Table 1).
Rating scores of emotion regulation task
A significant main effect of condition (Attend Neutral, Attend Negative, Reappraise and Suppress) was observed on the ratings of the emotion regulation task [χ2(3) = 106.55, P < .001]. Post hoc tests revealed that negative stimuli were rated more negatively than neutral stimuli (Z = 6.15, P < .001). Furthermore, the participants were able to reduce their negative affect through reappraisal (Z = −5.24, P < .001) and suppression (Z = −3.02, P = .003) in comparison with attending negative stimuli. The cognitive dimension, Fantasizing and Emotionalizing, were not significantly associated with any of the rating scores, except for a small negative correlation between negative affect scores during attend and the Emotionalizing factor (not surviving multiple comparison correction) (see Table 1). The correlation between the ERQ reappraisal subscale and the rating scores of negative affect after reappraisal was marginally significant (ρ = −.356; P = .01, not reaching our threshold of P < .006). No other significant correlations were found between the ERQ and rating scores (P > .1).
NEUROIMAGING RESULTS
Main effects of emotion regulation task
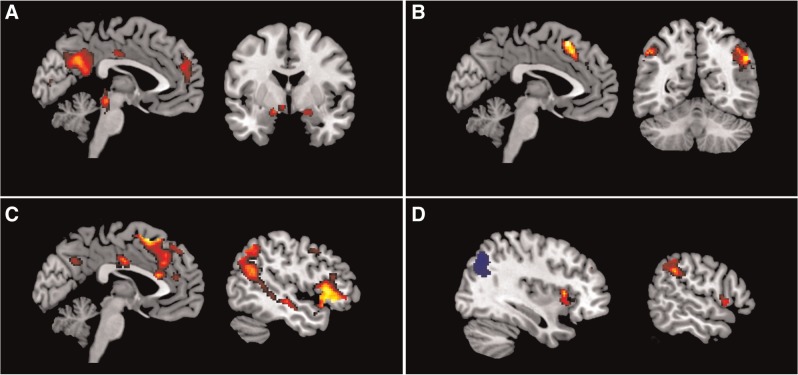
The first two seconds of viewing a negative picture compared with viewing a neutral picture revealed higher activation in various emotion processing areas, such as the right fusiform gyrus, left anterior insula, left middle cingulate gyrus, the bilateral inferior and superior parietal gyrus and the right amygdala (see Figure 1A; for more specific details and a full overview of the results, see Supplementary Table S1). The left amygdala reached significance after applying a SVC (k = 29; −16, −2, −14 [x, y, z]; Z = 4.33). The four seconds of attending to a negative picture (after two seconds of viewing) yielded significantly higher activation in the left medial frontal gyrus, left middle and inferior frontal gyrus and the bilateral angular and inferior parietal gyrus, in comparison with attending a neutral picture (see Figure 1B and Supplementary Table S1). Reappraising negative pictures in comparison with the attending condition yielded, amongst others, higher activation in the left inferior frontal gyrus, bilateral dorsal medial prefrontal cortex and dorsal ACC (see Figure 1C and see Supplementary Table S1 for full details on all the significant findings). No activation decreases were found for any of the abovementioned contrasts. Suppressing compared with attending negative pictures, led to higher activation in the bilateral insula, supramarginal gyrus, bilateral middle cingulate gyrus, right middle frontal gyrus and the right superior frontal gyrus. Furthermore, activation was lower in the bilateral occipital cortex and the left postcentral gyrus (see Figure 1D and Supplementary Table S1).
Fig. 1.
Main effects of emotion processing and emotion regulation. (A) Increased activation during negative picture viewing compared with neutral picture viewing. (B) Increased activation during the 3rd till 6th second of attending a negative picture compared with a neutral picture. (C) Increased activation during the reappraisal of a negative picture compared with attending. (D) Increased (red) and decreased (blue) activation during the suppression of a negative picture compared with attending. Results are displayed at P < .001, with a P < .05 FWE correction at cluster level and overlaid on a MNI template brain.
Alexithymia-related brain activation
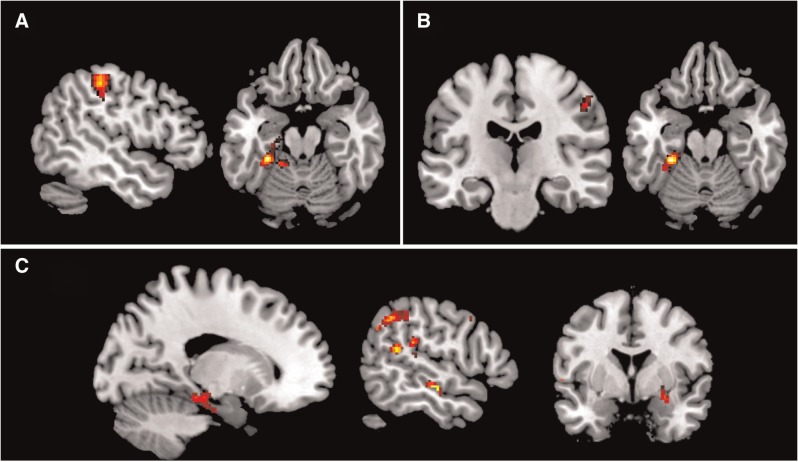
During the contrast ‘view negative > view neutral’ the cognitive dimension of alexithymia was associated with lower activation in the left inferior parietal cortex, the left fusiform gyrus and the bilateral precuneus (see Figure 2A and Table 3). This decrease in activation was driven by the sub-factor Identifying and the sub-factor Verbalizing. Verbalizing was associated with lower activation in the right precuneus, left fusiform gyrus and the right postcentral gyrus (see Figure 2B and Table 3). The Identifying sub-factor correlated negatively with various emotion processing areas, such as the bilateral inferior frontal gyrus, left parietal cortex, the right precuneus, the right supramarginal gyrus and left superior temporal cortex (see Figure 2C and Table 3). The amygdala was not found to be significantly correlated to alexithymia in the whole brain analyses. However, significant lower activation in the right amygdala in association with the Identifying subscale was reached after applying a SVC (k = 25; 28, 0, −12 [x, y, z]; Z = 4.12). No positive correlations were found with the cognitive dimension. Furthermore, the Fantasizing and Emotionalizing sub-factors did not correlate (positive nor negative) with brain activation during negative emotion picture viewing. Scatterplots of all alexithymia-related brain activation differences are depicted in Supplementary Figures S1–S3. The Identifying sub-factor contained one outlier (>3 s.d.). To examine the influence of this outlier, the Cook’s distance was calculated and the scatterplots were visually inspected. This procedure resulted in the exclusion of two findings (depicted in red, in Supplementary Figure S2). No significant alexithymia-related activation (positive nor negative) was found during the Attend, Reappraise or Suppress condition with either the cognitive dimension nor the Fantasizing and Emotionalizing sub-factors.
Fig. 2.
Alexithymia-related brain activation differences during emotion processing and expressive suppression. (A) Decreased activation during negative picture viewing associated with the cognitive alexithymia dimension. (B) Decreased activation during negative picture viewing associated with the Verbalizing subscale. (C) Decreased activation during negative picture viewing associated with the Identifying subscale. Results are overlaid on an MNI template brain and displayed at P < .001, with a P < .05 FWE correction at cluster level, except for the amygdala (right picture under C), which displays the result of the SVC.
Table 3.
Summary of significant findings of neural correlates associated with alexithymia during negative emotion processing
| Brain region | Hemisphere | k voxels | MNI coordinates |
Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| View Negative > View Neutral | ||||||
| Negative correlation with cognitive dimension | ||||||
| Inferior parietal gyrus | L | 146 | −52 | −30 | 46 | 4.67 |
| Precuneus | R | 160 | 8 | −72 | 52 | 4.67 |
| 10 | −66 | 58 | 3.98 | |||
| 8 | −62 | 36 | 3.74 | |||
| Fusiform gyrus | L | 197 | −30 | −36 | −20 | 4.60 |
| −16 | −20 | −12 | 3.84 | |||
| −20 | −40 | −20 | 3.83 | |||
| Precuneus | L | 144 | −12 | −46 | 64 | 3.94 |
| 4 | −50 | 64 | 3.66 | |||
| −28 | −38 | 66 | 3.61 | |||
| Negative correlation with identifying | ||||||
| Superior temporal gyrus | L | 94 | −56 | −6 | 0 | 5.30 |
| Inferior parietal gyrus | L | 338 | −52 | −28 | 48 | 4.72 |
| −50 | −28 | 38 | 4.07 | |||
| −40 | −48 | 56 | 3.71 | |||
| Middle and inferior frontal gyrus | L | 152 | −34 | 36 | 18 | 4.70 |
| −44 | 34 | 22 | 3.77 | |||
| −44 | 32 | 30 | 3.70 | |||
| Inferior frontal gyrus and precentral gyrus | R | 140 | 42 | 10 | 34 | 4.45 |
| 38 | 16 | 30 | 3.46 | |||
| 56 | 10 | 40 | 3.24 | |||
| Supramarginal gyrus and inferior parietal gyrus | R | 231 | 52 | −44 | 46 | 4.43 |
| 56 | −48 | 40 | 3.92 | |||
| 54 | −36 | 40 | 3.76 | |||
| Parahippocampal gyrus and Cerebellum | L | 261 | −10 | −24 | −24 | 4.27 |
| −8 | −34 | −26 | 4.12 | |||
| −22 | −20 | −22 | 3.84 | |||
| Middle temporal gyrus | R | 97 | 56 | −16 | −10 | 4.20 |
| 46 | −10 | −10 | 3.44 | |||
| 60 | −8 | −4 | 3.17 | |||
| Precuneus | R | 149 | 2 | −66 | 42 | 3.86 |
| 4 | −60 | 36 | 3.85 | |||
| 10 | −66 | 58 | 3.73 | |||
| Negative correlation with verbalizing | ||||||
| Precuneus and superior parietal gyrus | R | 162 | 8 | −72 | 52 | 4.77 |
| 20 | −68 | 56 | 3.74 | |||
| 20 | −60 | 62 | 3.64 | |||
| Fusiform gyrus | L | 135 | −28 | −36 | −20 | 4.72 |
| −34 | −42 | −22 | 3.97 | |||
| Postcentral gyrus | R | 155 | 52 | −20 | 44 | 3.77 |
| 50 | −28 | 52 | 3.64 | |||
| 40 | −26 | 48 | 3.49 | |||
L, left; R, right.
DISCUSSION
The aim of the current study was to examine neural correlates as a function of alexithymia during emotion perception and regulation. Furthermore, we wanted to investigate whether these neural correlates differed for the cognitive dimension and the sub-factors of the affective alexithymia dimension. Our results indicate that the cognitive alexithymia dimension is associated with lower activation in a widespread emotion attention and recognition network during the initial phase of emotion perception. No alexithymia-related brain activation differences were found in a later phase of emotion regulation.
Basic emotion processing
Our emotion perception task reliably activated key regions that have been shown to be involved in emotion processing, such as the amygdala, insula and medial prefrontal cortex (Phan et al., 2004; Fusar-Poli et al., 2009). In association with cognitive alexithymia (Identifying sub-factor), we found lower activation in a part of the ventral system as defined by Phillips and colleagues (2003), namely the right amygdala, during emotion perception. This is in concordance with previous studies that also reported negative correlations between right amygdala activation and the Identifying sub-factor (Kugel et al., 2008; Pouga et al., 2010; Reker et al., 2010). The amygdala is an important area in directing bottom–up attention towards emotional stimuli (Vuilleumier, 2005). When presented with a relevant stimulus, the amygdala becomes activated and subsequently activates occipital areas directing visual attention towards the stimulus (Vuilleumier, 2005; Jacobs et al., 2012). Therefore, lower activation in this area might indicate that attention is less automatically directed towards emotional stimuli in alexithymia and might explain the emotional attention deficits in alexithymia as reported by various behavioral studies (Suslow et al., 2003; Mueller et al., 2006).
Furthermore, we found lower activation in association with cognitive alexithymia in several other emotion processing areas, such as the inferior parietal lobe, the superior temporal cortex, the somatosensory cortex and the parahippocampal gyrus, which is in accordance with previous findings (Kano et al., 2003; Duan et al., 2010; Reker et al., 2010). According to Adolphs (2002a,b), all of these regions are involved in emotion recognition. After the fast direction of attention involving the amygdala and occipital cortex, the superior temporal cortex and hippocampal formation become activated to retrieve conceptual knowledge about the emotion and to generate an emotional reaction (Adolphs, 2002b). Subsequently, connections to, amongst others, the motor and somatosensory areas lead to emotional awareness and recognition (Adolphs, 2002a). These results might explain the worse performance of alexithymic individuals on emotional perception and recognition tasks (Lane et al., 1996; Swart et al., 2009) and might account for the problems in recognizing feelings in themselves and others.
Furthermore, the DLPFC, part of the dorsal system, was less activated during emotion perception. Together with the lower activation found in the inferior parietal lobe this might suggest less functioning of the frontoparietal attention network (Corbetta, 1998; Corbetta et al., 2008). When attention is not automatically directed towards emotional stimuli, visual attention can be enhanced by this top–down attention network (Ochsner et al., 2009). Lower activation in this network could suggest that individuals with higher scores on alexithymia are overall less inclined to direct their visual attention towards emotional stimuli.
In contrast to our hypotheses, we did not find any significant associations between the Fantasizing or Emotionalizing sub-factors of the affective dimension and brain activation during emotion perception, while previous studies did suggest specific neural correlates to underlie this dimension (Pouga et al., 2010; Goerlich et al., 2012). These findings are in accordance with the EEG study of Goerlich and colleagues (2012) in which the cognitive dimension was associated with larger early N1 potentials, while the affective dimension did not influence these early brain potentials. It is possible that the lower activation in the early emotional attention regions is specifically underlying the problems in the cognitive processing of emotion, such as identifying, and that it is less important for the guidance of physiological responses to emotions (e.g. emotion arousal). However, Goerlich and colleagues (2012) showed that the affective dimension was specifically related to a reduction of brain potentials in a later phase of emotion processing (e.g. P3), while we were unable to show activation differences related to either the cognitive or affective (Emotionalizing and Fantasizing) dimension in a later phase of emotion processing (the Attend condition). It could be that these effects were too subtle to detect in the current task. Future research combining fMRI and physiological data could give more insight into these processes. Furthermore, research applying tasks specifically designed to generate affective states or imagination (for example Aust et al., 2014; Mantani et al., 2005; Karlsson et al., 2008) might be useful to gain more insight into the neural correlates of the Emotionalizing and Fantasizing sub-factors. Distinguishing neural correlates related to either the cognitive or affective alexithymia dimension is important to further disentangle alexithymia-related brain activation patterns.
Emotion regulation
To our knowledge, this is the first fMRI study on emotion regulation and alexithymia. During reappraisal, participants activated a large part of the dorsal system, such as the left inferior frontal gyrus and the dorsal ACC, which is in accordance with previous fMRI studies on reappraisal (Diekhof et al., 2011). We hypothesized that during reappraisal, activation in this dorsal system would be lower in individuals with high alexithymia scores. However, we were unable to find any alexithymia-related activation differences. This result is in accordance with a recent EEG study that also did not find any differences in brain potentials during reappraisal associated with alexithymia (Walker et al., 2011). However, another study reported that low alexithymia was related to reduced P3 and slow wave potentials (Pollatos and Gramann, 2012). These divergent results might be explained by the fact that part of the regions in which decreased brain potentials were found (fusiform gyrus and inferior temporal gyrus) are not generally activated during reappraisal (Diekhof et al., 2011). Furthermore, these regions were not activated in the main effect of the current reappraisal paradigm, which might explain the lack of an association between alexithymia and these areas in the current study. The lack of alexithymia-related activation differences during reappraisal together with the fact that participants with high alexithymia scores were able to down-regulate their negative affect through reappraisal as indicated by the rating scores, suggests that individuals with alexithymia are able to use cognitive reappraisal, at least when they are explicitly trained and cued to do so.
The fMRI results of the main effect of suppression were in accordance with previous literature (Goldin et al., 2008; Vanderhasselt et al., 2012). In relation to alexithymia, no significant activation differences were found. This finding is in contrast with an EEG study in which lower P2, N2 and late positive potentials were found in association with cognitive alexithymia during suppression (Walker et al., 2011). The discrepancy between the findings of the current study and the study of Walker and colleagues might be explained by the use of a different questionnaire (TAS-20). However, it is unlikely that this difference can fully explain the different findings, as the TAS-20 total score and BVAQ cognitive dimension are highly correlated (Vorst and Bermond, 2001). In line with the current fMRI results, no differences in rating scores after suppression were found. This indicates that individuals with higher alexithymia scores were capable of decreasing negative affect through suppression which was also found in the study of Walker et al. (2011). Therefore, these results indicate that individuals with alexithymia do not seem to be impaired in the use of suppression. Future research is necessary to see if this finding can be replicated.
Limitations and future implications
Some limitations of the current study should be addressed. First, we did not include depression or anxiety measures, despite the fact that correlations between these and alexithymia have been found (Berthoz et al., 1999). We should note, however, that none of our subjects had a clinical psychiatric diagnosis of mood disorder or otherwise. Moreover, alexithymia was not related to positive or negative affect prior to scanning, as measured with the PANAS. Second, our results indicate neural correlates to differ already early in time during emotion processing in alexithymia. However, due to the low temporal resolution of fMRI, it is not possible to indicate that these differences already occurred very early. Therefore, we suggest that future studies combine EEG and fMRI to gain both good temporal and spatial resolution. Third, the regulation blocks in the current task were too short to calculate reliable connectivity patterns. We suggest that future research should apply tasks with longer regulation periods to examine functional connectivity patterns associated with alexithymia during emotion regulation. Furthermore, the rating scale of negative affect during the emotion regulation task might not have been sensitive enough to detect associations with alexithymia. Use of a larger scale may overcome this. Finally, the neural correlates were examined through a dimensional approach. Although some of the participants exceeded the cut-off score for clinical alexithymia [N = 12; ≥53 (Deborde et al., 2008)], participants were not specifically selected on this criterion and no high vs low alexithymia comparison could be made (due to low power). Therefore, future research is necessary to examine whether the intact functioning of emotion regulation areas also applies to clinical alexithymia.
CONCLUSIONS
The results of this study showed that the cognitive dimension, but not the sub-factors of the affective dimension of alexithymia, seems to affect emotion processing during the perception but not the regulation phase. Activation was lower in a widespread emotional attention and emotional recognition brain network, probably underlying emotional attention and identification deficits in alexithymia. Activation of neural networks during the later phase of emotion regulation did not differ, which is at odds with views of alexithymia as an emotion regulation deficit. In sum, these results suggest that alexithymia may result from an early emotion processing deficit instead of deviant activation in networks subserving emotion regulation. Further research is necessary to investigate whether individuals with high alexithymia scores are also capable of regulating their emotions in daily life.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgements
The GROUP study is supported by a grant from ZonMw, within the Mental Health programma (project number: 10.000.1002). A.A. is supported by a VICI grant from N.W.O., grant number 435-11-004. L.K. is supported in part by a VICI grant from N.W.O., grant number: 435-11-005. We would like to thank the families who invested time and effort to make the GROUP project possible. Furthermore, we would like to acknowledge Anita Sibeijn-Kuiper, Judith Streurman, Edith Liemburg and Michelle Servaas for their assistance with fMRI scanning and Dr Remco Renken for his advice regarding fMRI statistics.
References
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002a;12(2):169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews. 2002b;1(1):21–62. doi: 10.1177/1534582302001001003. [DOI] [PubMed] [Google Scholar]
- Aleman A. Feelings you can't imagine: towards a cognitive neuroscience of alexithymia. Trends in Cognitive Sciences. 2005;9(12):553–5. doi: 10.1016/j.tics.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Archives of General Psychiatry. 1992;49(8):615–23. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Aust S, Alkan Härtwig E, Koelsch S, Heekeren H, Heuser I, Bajbouj M. How emotional abilities modulate the influence of early life stress on hippocampal functioning. Social Cognitive and Affective Neuroscience. 2014;9(7):1038–45. doi: 10.1093/scan/nst078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby RM, Quilty LC, Taylor GJ, et al. Are there subtypes of alexithymia? Personality and Individual Differences. 2009;47:413–18. [Google Scholar]
- Bailey PE, Henry JD. Alexithymia, somatization and negative affect in a community sample. Psychiatry Research. 2007;150(1):13–20. doi: 10.1016/j.psychres.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Bekker MJ, Bachrach N, Croon MA. The relationship of antisocial behavior with attachment styles, autonomy-connectedness, and alexithymia. Journal of Clinical psychology. 2007;63(6):507–27. doi: 10.1002/jclp.20363. [DOI] [PubMed] [Google Scholar]
- Bermond B, Clayton K, Liberova A, et al. A cognitive and an affective dimension of alexithymia in six languages and seven populations. Cognition and Emotion. 2007;21(5):1125–36. [Google Scholar]
- Bermond B, Bierman DJ, Cladder MA, Moormann PP, Vorst HM. The cognitive and affective alexithymia dimensions in the regulation of sympathetic responses. International Journal of Psychophysiology. 2010;75(3):227–33. doi: 10.1016/j.ijpsycho.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Bermond B, Vorst HCM, Moormann P. Cognitive neuropsychology of alexithymia: implications for personality typology. Cognitive Neuropsychiatry. 2006;11(3):332–60. doi: 10.1080/13546800500368607. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Consoli S, Perez Diaz F, Jouvent R. Alexithymia and anxiety: compounded relationships? A psychometric study. European Psychiatry. 1999;14(7):372–8. doi: 10.1016/s0924-9338(99)00233-3. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Ouhayuon B, Perez-Diaz F, Consoli SM, Jouvent R. Comparison of the psychometric properties of two self-report questionnaires measuring alexithymia: Confirmatory factor analysis of the 20-item Toronto Alexithymia Scale and the Bermond-Vorst Alexithymia Questionnaire. European Review of Applied Psychology. 2000;50(4):359–68. [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):831–8. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman G. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J, Henry J. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43(3):245–65. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Deborde A, Berthoz S, Wallier JM, et al. The Bermond-Vorst Alexithymia Questionnaire cutoff scores: a study in eating-disordered and control subjects. Psychopathology. 2008;41(1):43–9. doi: 10.1159/000109955. [DOI] [PubMed] [Google Scholar]
- Diekhof E, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58(1):275–85. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Duan X, Dai Q, Gong Q, Chen H. Neural mechanism of unconscious perception of surprised facial expression. Neuroimage. 2010;52(1):401–7. doi: 10.1016/j.neuroimage.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35(3):346–55. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience: JPN. 2009;34(6):418–32. [PMC free article] [PubMed] [Google Scholar]
- Geenen R, van Ooijen-van der Linden L, Lumley M, Bijlsma JWJ, van Middendorp H. The match-mismatch model of emotion processing styles and emotion regulation strategies in fibromyalgia. Journal of Psychosomatic Research. 2012;72(1):45–50. doi: 10.1016/j.jpsychores.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Goerlich-Dobre KS, Bruce L, Martens S, Aleman A, Hooker CI. Distinct associations of insula and cingulate volume with the cognitive and affective dimensions of alexithymia. Neuropsychologia. 2014;53:284–92. doi: 10.1016/j.neuropsychologia.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Goerlich-Dobre K, Witteman J, Schiller N, van Heuven VJP, Aleman A, Martens S. Blunted feelings: Alexithymia is associated with a diminished neural response to speech prosody. Social Cognitive and Affective Neuroscience. 2014;9(8):1108–17. doi: 10.1093/scan/nst075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerlich K, Aleman A, Martens S. The sound of feelings: electrophysiological responses to emotional speech in alexithymia. PLoS One. 2012;7(5):e36951. doi: 10.1371/journal.pone.0036951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74(1):224–37. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Grynberg D, Chang B, Corneille O, et al. Alexithymia and the Processing of Emotional Facial Expressions (EFEs): Systematic review, unanswered questions and further perspectives. PLoS One. 2012;7(8):e42429. doi: 10.1371/journal.pone.0042429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RH, Renken R, Aleman A, Cornelissen F. The amygdala, top-down effects, and selective attention to features. Neuroscience Biobehavioral Reviews. 2012;36(9):2069–84. doi: 10.1016/j.neubiorev.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Kano M, Fukudo S, Gyoba J, et al. Specific brain processing of facial expressions in people with alexithymia: An H2 15O-PET study. Brain. 2003;126(6):1474–84. doi: 10.1093/brain/awg131. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Näätänen P, Stenman H. Cortical activation in alexithymia as a response to emotional stimuli. British journal of Psychiatry. 2008;192(1):32–8. doi: 10.1192/bjp.bp.106.034728. [DOI] [PubMed] [Google Scholar]
- Kessler H, Kammerer M, Hoffmann H, Traue H. Regulation of emotions and alexithymia: a correlative study [in German] Psychotherapie Psychosomatik medizinische Psychologie. 2010;60(5):169–74. doi: 10.1055/s-0029-1234046. [DOI] [PubMed] [Google Scholar]
- Korver N, Quee P, Boos HBM, Simons CJP, de Haan L. Genetic Risk and Outcome of Psychosis (GROUP), a multi-site longitudinal cohort study focused on gene-environment interaction: objectives, sample characteristics, recruitment and assessment methods. International Journal of Methods in Psychiatric Research. 2012;21(3):205–21. doi: 10.1002/mpr.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugel H, Eichmann M, Dannlowski U, et al. Alexithymic features and automatic amygdala reactivity to facial emotion. Neuroscience Letters. 2008;435(1):40–4. doi: 10.1016/j.neulet.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Lane RD, Sechrest L, Reidel R, Weldon V, Kaszniak A, Schwartz GE. Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosomatic Medicine. 1996;58(3):203–10. doi: 10.1097/00006842-199605000-00002. [DOI] [PubMed] [Google Scholar]
- Liemburg EJ, Vercammen A, Ter Horst GJ, Curcic-Blake B, Knegtering H, Aleman A. Abnormal connectivity between attentional, language and auditory networks in schizophrenia. Schizophrenia Research. 2012;135(1–3):15–22. doi: 10.1016/j.schres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Mantani T, Okamoto Y, Shirao N, Okada G, Yamawaki S. Reduced activation of posterior cingulate cortex during imagery in subjects with high degrees of alexithymia: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57(9):982–90. doi: 10.1016/j.biopsych.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Moormann PP, Bermond B, Vorst HCM, Bloemendaal AFT, Teijn SM, Rood L. New avenues in alexithymia research: The creation of alexithymia types. In: Denollet J, Vingerhoets AJM, Nyklicek T, editors. Emotion Regulation: Conceptual and Clinical Issues. New York, New York: Springer; 2008. pp. 27–42. [Google Scholar]
- Mueller J, Alpers G, Reim N. Dissociation of rated emotional valence and Stroop interference in observer-rated alexithymia. Journal of Psychosomatic Research. 2006;61(2):261–9. doi: 10.1016/j.jpsychores.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Nemiah JC, Sifneos PE. Psychosomatic illness: a problem in communication. Psychotherapy and Psychosomatics. 1970;18(1):154–60. doi: 10.1159/000286074. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Ray R, Hughes B, et al. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychological Science. 2009;20(11):1322–1. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS spectrums. 2004;9(4):258–66. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Phillips M, Drevets W, Rauch S, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003;54(5):504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K. Attenuated modulation of brain activity accompanies emotion regulation deficits in alexithymia. Psychophysiology. 2012;49(5):651–8. doi: 10.1111/j.1469-8986.2011.01348.x. [DOI] [PubMed] [Google Scholar]
- Pouga L, Berthoz S, de Gelder B, Grzes J. Individual differences in socioaffective skills influence the neural bases of fear processing: the case of alexithymia. Human Brain Mapping. 2010;31(10):1469–81. doi: 10.1002/hbm.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reker M, Ohrmann P, Rauch A, et al. Individual differences in alexithymia and brain response to masked emotion faces. Cortex. 2010;46(5):658–67. doi: 10.1016/j.cortex.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Roedema T, Simons RF. Emotion-processing deficit in alexithymia. Psychophysiology. 1999;36(3):379–87. doi: 10.1017/s0048577299980290. [DOI] [PubMed] [Google Scholar]
- Sifneos PE. The prevalence of ‘alexithymic' characteristics in psychosomatic patients. Psychotherapy and Psychosomatics. 1973;22(2):255–62. doi: 10.1159/000286529. [DOI] [PubMed] [Google Scholar]
- Stasiewicz P, Bradizza C, Gudleski G, et al. The relationship of alexithymia to emotional dysregulation within an alcohol dependent treatment sample. Addictive Behaviors. 2012;37(4):469–76. doi: 10.1016/j.addbeh.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Kersting A, Arolt V. Alexithymia and incidental learning of emotional words. Psychological Reports. 2003;93(3):1003–12. doi: 10.2466/pr0.2003.93.3f.1003. [DOI] [PubMed] [Google Scholar]
- Swart M, Bruggeman R, Laroi F, et al. COMT Val158Met polymorphism, verbalizing of emotion and activation of affective brain systems. Neuroimage. 2011;55(1):338–44. doi: 10.1016/j.neuroimage.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Swart M, Kortekaas R, Aleman A. Dealing with feelings: characterization of trait alexithymia on emotion regulation strategies and cognitive-emotional processing. PLoS One. 2009;4(6):e5751. doi: 10.1371/journal.pone.0005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Parker JD. Disorders of Affect Regulation: Alexithymia in Medical and Psychiatric Illness. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Taylor G, Bagby RM. New trends in alexithymia research. Psychotherapy and Psychosomatics. 2004;73(2):68–77. doi: 10.1159/000075537. [DOI] [PubMed] [Google Scholar]
- Van der Meer L, Van't Wout M, Aleman A. Emotion regulation strategies in patients with schizophrenia. Psychiatry Research. 2009;170(2–3):108–13. doi: 10.1016/j.psychres.2009.07.010. [DOI] [PubMed] [Google Scholar]
- van der Velde J, Servaas M, Goerlich K, et al. Neural correlates of alexithymia: A meta-analysis of emotion processing studies. Neuroscience Biobehavioral Reviews. 2013;37(8):1774–85. doi: 10.1016/j.neubiorev.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt M, Kühn S, De Raedt R. ‘Put on your poker face': neural systems supporting the anticipation for expressive suppression and cognitive reappraisal. Social Cognitive and Affective Neuroscience. 2012;8(8):903–10. doi: 10.1093/scan/nss090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorst HC, Bermond B. Validity and reliability of the Bermond-Vorst Alexithymia Questionnaire. Personality and Individual Differences. 2001;30:413–34. [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9(12):585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Walker S, O'Connor D, Schaefer A. Brain potentials to emotional pictures are modulated by alexithymia during emotion regulation. Cognitive, Affective Behavioral Neuroscience. 2011;11(4):463–75. doi: 10.3758/s13415-011-0042-1. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weiss E, Schulter G, Freudenthaler HH, Hofer E, Pichler N, Papousek I. Potential markers of aggressive behavior: The fear of other persons' laughter and its overlaps with mental disorders. PLoS One. 2012;7(5):e38088. doi: 10.1371/journal.pone.0038088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Archives of General Psychiatry. 1990;47(6):589–93. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- Wingbermühle E, Theunissen H, Verhoeven WMA, Kessels RPC, Egger JIM. The neurocognition of alexithymia: evidence from neuropsychological and neuroimaging studies. Acta Neuropsychiatrica. 2012;24(2):67–80. doi: 10.1111/j.1601-5215.2011.00613.x. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Riedesel K, Petrovic Z, et al. Impact of childhood trauma, alexithymia, dissociation, and emotion suppression on emotional Stroop task. Journal of Psychosomatic Research. 2011;70(1):53–8. doi: 10.1016/j.jpsychores.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Zech E, Luminet O, Rimé B, Wagner H. Alexithymia and its measurement: confirmatory factor analyses of the 20-item Toronto Alextihymia Scale and the Bermond-Vorst Alexithymia Questionnaire. European Journal of Personality. 1999;13(6):511–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.