Abstract
Humans often judge others egocentrically, assuming that they feel or think similarly to themselves. Emotional egocentricity bias (EEB) occurs in situations when others feel differently to oneself. Using a novel paradigm, we investigated the neurocognitive mechanisms underlying the developmental capacity to overcome such EEB in children compared with adults. We showed that children display a stronger EEB than adults and that this correlates with reduced activation in right supramarginal gyrus (rSMG) as well as reduced coupling between rSMG and left dorsolateral prefrontal cortex (lDLPFC) in children compared with adults. Crucially, functional recruitment of rSMG was associated with age-related differences in cortical thickness of this region. Although in adults the mere presence of emotional conflict occurs between self and other recruited rSMG, rSMG-lDLPFC coupling was only observed when implementing empathic judgements. Finally, resting state analyses comparing connectivity patterns of rSMG with that of right temporoparietal junction suggested a unique role of rSMG for self-other distinction in the emotional domain for adults as well as for children. Thus, children’s difficulties in overcoming EEB may be due to late maturation of regions distinguishing between conflicting socio-affective information and relaying this information to regions necessary for implementing accurate judgments.
Keywords: functional and structural brain development, socio-affective development, emotional egocentricity bias, self–other distinction, supramarginal gyrus, dorsolateral prefrontal cortex
INTRODUCTION
Humans tend to rely on their own experiences and mental states to infer those of other people (Gallese and Goldman, 1998; Singer et al., 2004; Keysers and Gazzola, 2007). This works efficiently when mental states between oneself and others align, but such a mechanism will betray a bias to judging others egocentrically when this is not the case, for example, judging others as happier when they are evidently upset merely because oneself is happy. Children are particularly prone to various kinds of blatant egocentricity (Piaget and Gabain, 1932; Kohlberg, 1984). Although the majority of the evidence for such egocentricity comes from studying the development of cognitive perspective-taking and theory of mind (Elkind, 1967; Flavell, 1999; Royzman et al., 2003), very little is known in contrast about how emotional egocentricity manifests itself in development and what the underlying neural and associated cognitive processes are that give rise to such emotional egocentricity in childhood. Emotional egocentricity lies at the heart of much of human interpersonal (Thompson and Loewenstein, 1992) and group conflict (Chambers et al., 2006). Peer conflicts are rife in childhood; lacking the ability to resolve them can lead to maladjustment and social rejection (Newcomb et al., 1993), causing problems in life-span development and health (Murphy et al., 2013). Unraveling the mechanisms that give rise to emotional egocentricity in development can, thus, help to identify the causes of this socially detrimental behavior early in life, offering the potential for targeted intervention.
To address our first major aim, we wanted to compare the emotional egocentricity bias (EEB) between children and adult. To this end, we developed a novel paradigm, a performance-based monetary reward and punishment task (the REAP task). Based on previous work on emotional egocentricity in adults (Silani et al., 2013), we refer to EEB when one’s empathic judgments of another persons’ feeling state is biased toward one’s own simultaneously experienced, albeit emotionally incongruent, state. In other words, EEB expresses the inability to disregard one’s own feeling states to accurately judge the state of another, who is feeling something different to oneself. Previous studies have shown that attributing desires to others incongruent with one’s own is something already young children around 18 months successfully do (Repacholi and Gopnik, 1997). However, behavioral evidence suggests that the ability to attribute beliefs and desires to others incongruent to one’s own is protracted and continues into adulthood; in turn, difficulties in such cognitive perspective taking are generally found to be much larger in children (Keysar et al., 2003; Apperly et al., 2011). In spite of this wealth of behavioral studies on egocentricity in the cognitive domain, the developmental trajectory of emotional egocentricity remains unknown. Thus, in addition to investigating the developmental course of EEB from childhood into adulthood using a novel paradigm, we also sought to identify cognitive mechanisms accounting for potential age-differences in EEB. These include attentional reorienting, as well as response inhibition, fluid intelligence, and cognitive perspective-taking (see Supplementary Materials).
A second goal of the study was to understand the neuronal mechanisms underlying age-related differences in overcoming such EEB from childhood to adulthood. Adults have been shown to be prone to egocentric judgments both in the cognitive domain (Royzman et al., 2003; Pronin, 2008) as well as when attributing visceral (Van Boven and Loewenstein, 2003; O'Brien and Ellsworth, 2012) and affective states (Silani et al., 2013). A recent study from our group combining transcranial magnetic stimulation (TMS) and functional magnetic resonance imaging (fMRI) was able to demonstrate that, in adults, right supramarginal gyrus (rSMG) is critically involved in overcoming emotional egocentricity when making judgments on the emotional states of others as disrupting rSMG with TMS lead to a drastic increased in the EEB. SMG is known to mature late in ontogeny (Gogtay et al., 2004; Shaw et al., 2008) and a further goal was to see if its functional development can account for a heightened EEB in children compared to adults. In this regard, it was also considered important to what extent age-related differences in functional recruitment of brain regions relevant to overcoming the EEB can be explained by structural development of the neocortex.
A third goal of the present study was to explore the exact processes that may be required by rSMG in overcoming EEB. The observed involvement of rSMG in overcoming EEB in adults (Silani et al., 2013) could reflect early processes of distinguishing between one’s own and another’s affective experience or the later implementation of such empathic judgments. Thus, the current paradigm was extended to allow for a distinction between whether rSMG is recruited only when explicit empathic judgments are required, or whether rSMG is already involved when no such judgments have to be made but participants are simply exposed to a situation involving incongruent emotional states between self and others. If rSMG implements overcoming EEB, we would expect greater activation during runs when empathic judgments are required compared to when this is not required. Conversely, if rSMG plays a role in detecting socio-affective incongruency and self–other distinction, activation in this area should be observed independently of the demands of making a judgment. In that case we would, however, have to expect the involvement of additional brain regions known to implement social judgments and decisions, such as dorsolateral prefrontal cortex (DLPFC; Greene et al., 2004; Knoch et al., 2006; Spitzer et al., 2007; Guroglu et al., 2011; Steinbeis et al., 2012; Tassy et al., 2012). Activation in DLPFC should, however, then only be involved in the explicit judgement condition.
Finally, the findings by (Silani et al., 2013) suggested that rSMG and not temporoparietal junction (TPJ) is particularly involved in overcoming emotional egocentricity. These findings were buttressed by recent evidence of differential intrinsic functional connectivity of tractography-based parcellated subregions of the temporoparietal cortex (Mars et al., 2012). This study showed that although posterior portions comprising TPJ have greater intrinsic functional connectivity with medial prefrontal (PFC) and posterior cingulate cortex, regions known to be involved in cognitive perspective taking, anterior regions, including SMG have greater connectivity with the anterior insula, a region known to be involved in empathic judgments when in a congruent emotional or neutral state (Bernhardt and Singer, 2012). This implies that rSMG may possess the connectivity profile to overcome specifically emotional egocentricity. Given recent debates on the precise function of temporoparietal areas in social cognition (Mitchell, 2008; Scholz et al., 2009; Carter and Huettel, 2013), such analyses are crucial in obtaining a clearer picture of the unique suitability of specific brain regions to perform specific socio-cognitive functions. Thus, our fourth and final goal was to see if these findings could be replicated with seeds derived from the functional activations elicited by a task designed to induce an EEB. Further, we were also interested in expanding the findings by Mars and colleagues to development in testing whether these differential networks of intrinsic functional connectivity are already present during childhood.
MATERIALS AND METHODS
Participants
Study 1 (Behavioral)
Participants were 168 children (70 boys; mean ± s.d. age = 10.14 ± 1.79 years; age range = 7–13 years). The majority (163) of them were Swiss, two were German, one was Thai, one was Swedish and one was English. Parents of all children gave informed consent, and the study was approved by the ethics committees of the University of Zurich and of the Canton of Zurich (E68/2008). Children were recruited from primary schools in the town of Rapperswil, in the canton St Gallen, Switzerland. All children were normal developing and socioeconomic status was mixed.
Study 2 (Behavioral and MRI)
We studied 30 children and 20 adults in the MRI experiment. Three children had to be excluded owing to excessive motion (i.e. interscan motion over 3 mm), leaving 27 children for task-based fMRI analysis (14 boys; N = 27; mean age ± s.d. = 10.22 ± 2.11 years, age range = 6–13 years). We also studied 20 adults who participated in the MRI experiment (10 men; mean ± s.d. age = 26.01 ± 2.34 years; age range = 22–31 years). All participants were German, with a mixed socioeconomic status. Enrolled children were normally developing, no participant had a history of neurological or psychiatric disorder and routine radiological assessment of all subjects did not indicate any mass lesion. Adult participants and children’s parents gave informed consent, and the study was approved by the ethics committee of Leipzig University (E029-11-24012011).
The REAP task
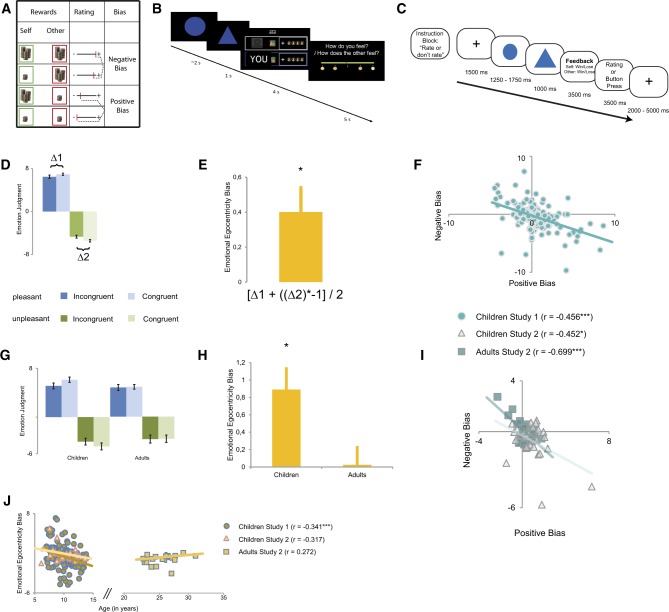
In our monetary reward and punishment paradigm, participants played a competitive game with another interacting partner. Using a speeded reaction time task with monetary incentives, participants were asked to react as fast as possible to a change in stimulus. They were told that fast reaction times below a threshold set by an experimenter would result in a reward of four monetary units (henceforth MUs). Conversely, slow reaction times would result in a punishment of having four MUs subtracted (Figure 1A). In the experiment, wins were indicated by a plus symbol in front of four coins together with a bell sound; losses were indicated by a minus symbol in front of four coins together with a buzz sound (Figure 1B).
Fig. 1.
Experimental design and behavioral results. (A) Operationalization of the EEB. (B) In a speeded reaction time task, participants first reacted as fast as they could, then they saw whether both they and the other had won or lost and finally they indicated how they felt. (C) In the fMRI study, participants underwent blocks in which they had to either provide a judgment or not. (D) In Study 1, children showed a significant effect of congruency between their own affective state and that of the other person, whereby they rated the other as feeling more negative when they themselves had lost and more positive when they themselves had won, which (E) resulted in a significant EEB. (F) The EEB in the positive and negative domain were significantly correlated. (G) In Study 2, children showed a significant effect of congruency, whereas adults did not, which (H) led to a significant EEB only in children and not in adults. (I) The EEB during wins and losses was significantly correlated for both children and adults. (J) In Study 1, there was also an age-related decrease in the EEB in the sample of children, and a trend in the sample of children in Study 2.
They played four blocks, but for the present purpose only the fourth block is relevant (see Supplementary Materials for further details). In this block, both participants performed the task simultaneously and saw feedback of their own performance and that of the other participant on the screen. Subsequent to the display of win or loss, participants were asked to indicate how they thought the other felt (henceforth, Simultaneous Other condition). Trial design in this condition ensured an equal number of wins and losses and an equal number of trials where the valence between the participants was either an outcome that was congruent (i.e. both participants win or lose) or incongruent (i.e. the participant won and simultaneously saw the other participant losing, or vice versa). In the behavioral study, the experimental setup to calculate emotional egocentricity, therefore, led to a 2 × 2 factorial design with the factors valence (wins, losses) and congruency (congruent, incongruent wins and losses for participant and the other; Figure 1A).
In the fMRI study, the experimental block was further subdivided into two subblocks. In one subblock, participants had to give a judgment after seeing their own and the other’s wins and losses. In the other subblock, participants had to press two buttons on the response device once each (Figure 1C). The fMRI experiment was, hence, organized according to a 2 × 2 × 2 factorial design with the factors valence, congruency and judgment (judgment, no judgment).
In terms of our paradigm, emotional egocentricity would be indicated by an influence of one’s own emotional state in the moment of making a judgment of the state of the other. For instance, after seeing the other loose, egocentricity would relate to judging the other as feeling better when the participant had just won (i.e. incongruent trial) compared with when they had also lost (i.e. congruent trial). Similarly, when judging the other after seeing them win egocentricity would be apparent when judging the other as feeling worse when the participant had just lost compared with when they had also won (Figure 1A). We calculated the difference in judgment between incongruent and congruent trials during the Simultaneous Other condition to quantify emotional egocentricity. Specifically, the EEB was calculated as follows: unpleasant incongruent—unpleasant congruent in the negative—and pleasant incongruent—pleasant congruent in the positive domain.
Several behavioral tests were carried out with the subjects following the scanning sessions, in a counterbalanced order across subjects. Tests included Coloured Progressive Matrices (Raven et al., 2003), a test of reorienting attention (Posner, 1980), belief attribution (Apperly et al., 2011) and the Stop-Signal Reaction Time Task (Logan et al., 1997). For more details also of a description of the experimental procedure, data acquisition and data analysis procedure please see Supplementary Material.
RESULTS
EEB—behavioral results
In Study 1, to assess our first goal we tested whether we could detect an EEB in a sample of children (7–13 years old) with the REAP task. Emotional egocentricity was assessed through repeated-measures analysis of variance (ANOVA) of the emotional ratings when judging the other, with the factors valence and congruency. We observed a main effect of valence [F(1,166) = 36.73; P < 0.001, 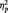 = 0.18], whereby wins were rated more strongly (i.e. more strongly significant from zero) than losses, and a main effect of congruency [F(1,166) = 7.01; P < 0.01,
= 0.18], whereby wins were rated more strongly (i.e. more strongly significant from zero) than losses, and a main effect of congruency [F(1,166) = 7.01; P < 0.01, 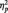 = 0.04], but no interaction between both [F(1,166) < 1; see Figure 1D and E]. Furthermore, there was a negative relationship between the size of the EEB when the other participant had won and when the other participant had lost (r = −0.47; P < 0.001; Figure 1F).
= 0.04], but no interaction between both [F(1,166) < 1; see Figure 1D and E]. Furthermore, there was a negative relationship between the size of the EEB when the other participant had won and when the other participant had lost (r = −0.47; P < 0.001; Figure 1F).
In Study 2, the fMRI study, a repeated-measures ANOVA of the ratings with the factors valence and congruency revealed that children showed a main effect of valence [F(1,26) = 10.10; P < 0.01; 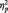 = 0.28], a main effect of congruency [F(1,26) = 13.67; P < 0.001;
= 0.28], a main effect of congruency [F(1,26) = 13.67; P < 0.001; 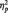 = 0.345] and no interaction between valence and congruency. Adults, on the other hand, only showed a main effect of valence [F(1,19) = 9.50; P < 0.01;
= 0.345] and no interaction between valence and congruency. Adults, on the other hand, only showed a main effect of valence [F(1,19) = 9.50; P < 0.01; 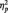 = 0.33], but no main effect of congruency [F(1,19) < 0.2] or a valence by congruency interaction. When including the factor age in the analyses, the interaction between factors congruency and age was significant [F(1,45) = 6.92; P < 0.05;
= 0.33], but no main effect of congruency [F(1,19) < 0.2] or a valence by congruency interaction. When including the factor age in the analyses, the interaction between factors congruency and age was significant [F(1,45) = 6.92; P < 0.05; 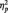 = 0.13; Figure 1G and H]. This age difference persisted when including reaction times, emotional engagement during the task as well as developmental changes in our additional measures of lower and higher cognitive tasks (Supplementary Figures S1–S4). Further, none of these variables correlated with individual differences in the EEB, neither in children (r < 0.3; P > 0.15) nor in adults (r < 0.33; P > 0.16). Similar to the results in Study 1 in children, data from Study 2 in children as well as adults revealed a negative correlation between the EEB when seeing the other winning and loosing (adults: r = −0.70; P < 0.001; children: r = −0.45; P < 0.05; Figure 1I).
= 0.13; Figure 1G and H]. This age difference persisted when including reaction times, emotional engagement during the task as well as developmental changes in our additional measures of lower and higher cognitive tasks (Supplementary Figures S1–S4). Further, none of these variables correlated with individual differences in the EEB, neither in children (r < 0.3; P > 0.15) nor in adults (r < 0.33; P > 0.16). Similar to the results in Study 1 in children, data from Study 2 in children as well as adults revealed a negative correlation between the EEB when seeing the other winning and loosing (adults: r = −0.70; P < 0.001; children: r = −0.45; P < 0.05; Figure 1I).
We also computed correlations between size of EEB and age within our two samples of children. In the large sample of children from Study 1, there was an age-related decrease in emotional egocentricity (r = −0.34; P < 0.001; Figure 1J). While effect size was similar, this age-related decrease in emotional egocentricity only reached marginal significance in our smaller sample of children from Study 2 (r = −0.32; P = 0.11). Analyzing data from the adults of Study 2 also did not reveal any age-related changes within the sample of adults (r = 0.27; P = 0.25).
Task-related functional MRI data
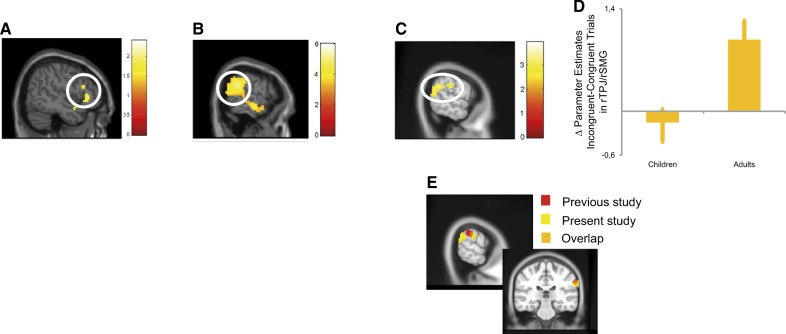
We analyzed the fMRI data using a flexible factorial design, with the factors valence, congruency, judgment as within-subject factors and age as a between-subject factor. To address our second goal, we assessed brain processes involved in the EEB; for each of the two age groups, we looked at the main effect of the factor congruency (Simultaneous Other Incongruent Trials > Simultaneous Other Congruent Trials). Seeing that in the behavioral ratings no interaction between valence and congruence was found, we pooled these data to compute the contrast of congruency. Similar to the analysis of the behavioural ratings, analyses for each group were run separately before carrying out a direct comparison. In a first step, we tested whether we could replicate previous findings from our lab observed in adults in the domain of affective touch (Silani et al., 2013), showing a crucial role of rSMG in overcoming EEB. Indeed, adults showed (Figure 2A; all at pfwe < 0.05; see Supplementary Materials, Supplementary Table S1) marked activation differences in right (x = 63, y = −46, z = 25) and left TPJ (x = −60, y = −43, y = 25), each also comprising rSMG, as well as right anterior insula (x = 36, y = 20, z = −11), left anterior insula (x = −30, y = 17, z = −14) and anterior cingulate cortex (x = −6, y = 38, z = 25). In children, activation differences between congruent and incongruent trials were subtle and did not survive correction for multiple comparisons (here for illustrative purposes thresholded at P < 0.05 uncorrected; Figure 2B).
Fig. 2.
fMRI results: (A) When comparing congruent with incongruent trials, adults showed strong activitation of rTPJ and rSMG. (pfwe < 0.05), whereas (B) children showed activity in right inferior frotal gyrus (P < 0.05 uncorrected). (C and D) The direct comparison between adults and children showed that recruitment of rTPJ and rSMG was stronger in adults than in children (psvc < 0.005). (E) The group difference between adults and children in rSMG (thresholded at psvc < 0.005) overlapped with regions previously identified to be crucial for overcoming emotional egocentricity in adults (Silani et al., 2013).
Age-related changes in activitation of rSMG
The main focus of our second goal was to investigate age-related differences in the rSMG when overcoming emotional egocentricity. When comparing fMRI activation during incongruent compared with congruent trials between the two groups, the only regions revealed by this contrast were rTPJ (x = 66, y = −49, z = 31) and rSMG (Figure 2C and D; x = 72, y = −25, z = 34; psvc < 0.01; Supplementary Figure S5). Importantly, rSMG activation overlapped by 80 mm3 with the rSMG activation reported in previous studies (Figure 2E). Consistent with these brain data, the above behavioral analyses showed an increased EEB in the children sample compared with adults, suggesting a role of rSMG in overcoming EEB.
Age-related functional connectivity from rSMG
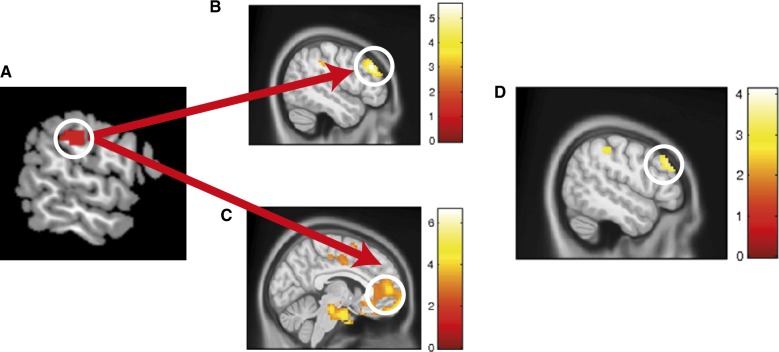
No brain–behavior correlations between individual differences in the EEB and brain activity was found, neither in children nor in adults, similar to previous studies (Silani et al., 2013). In a next step and to further address our second goal, we assessed whether such brain–behavior relationships would become evident in the functional connectivity of rSMG and distal brain regions using psychophysiological interaction (PPI) analysis (Friston et al., 1997; McLaren et al., 2012). As a seed region, we used the cluster of rSMG activation from the contrast AdultsIncongruent > Congruent > ChildrenIncongruent > Congruent. Our findings revealed that for children, a smaller EEB related to stronger functional connectivity between rSMG and medial PFC (Figure 3C; x = 6, y = 56, z = 7; pfwe < 0.05; Supplementary Table S2). For adults, on the other hand, a smaller EEB was associated with greater connectivity between rSMG and left DPLFC (Figure 3B; x = −48, y = 32, z = 34; psvc < 0.05; Supplementary Table S3 and Supplementary Figure S6). Although we also found increased connectivity of rSMG with right DLPFC when the EEB was smaller, this effect did not survive our threshold set to correct for multiple comparisons. Comparing both age groups indicated that the association in lDLPFC was more marked in adults than in children (Figure 3D; x = −51, y = 32, z = 34; psvc < 0.05; Supplementary Table S4 and Supplementary Figure S6). Looking at the reverse of this comparison, no significant differences were found between the two groups. These findings suggest that individual differences in the EEB manifest themselves rather in the functional coupling of rSMG with lateral and medial prefrontal regions, and not in activity of rSMG per se. When seeding from the cluster of rTPJ activation from the contrast AdultsIncongruent > Congruent > ChildrenIncongruent > Congruent, no significant association between the rTPJ connectivity with frontal brain regions and individual differences in the EEB were found.
Fig. 3.
PPI: (A) When seeding from rSMG, (B) adults with smaller EEB show an increased functional coupling of rSMG and lDLPFC (psvc < 0.005). (C) In children, on the other hand, those with smaller EEB showed increased functional coupling of rSMG and medial PFC (pfwe < 0.05). (D) When comparing the association between individual differences in EEB and functional coupling of rSMG, adults showed significantly stronger connectivity with lDLPFC than children (psvc < 0.005).
Comparison of rSMG activity in the judgement and no-judgement condition
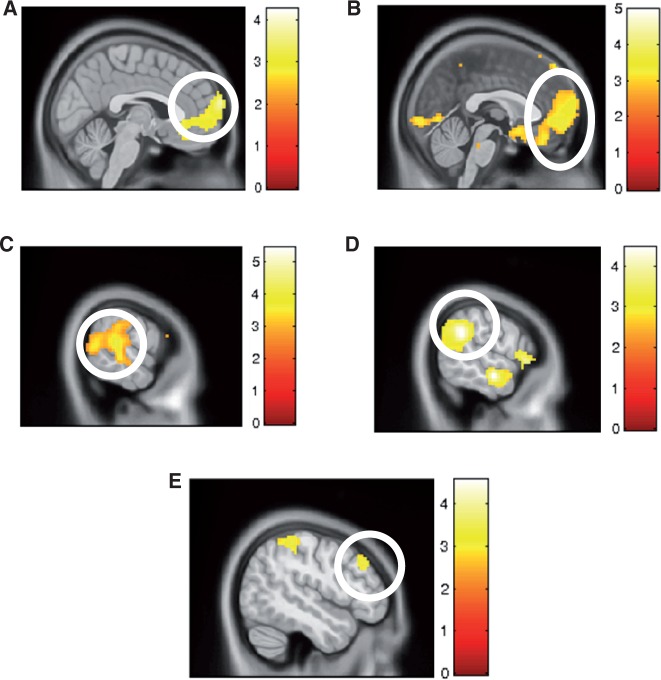
To address our third goal, namely to explore the functional role of rSMG in overcoming affective egocentricity, we looked at the judgment and no-judgment runs separately. In children, analysis of the judgement condition revealed that only dorsomedial PFC was activated as a function of incongruency (Figure4A; x = 12, y = 71, z = 19; pfwe < 0.05; Supplementary Table S5 and Supplementary Figure S7). During the no-judgment condition, no significant activations were observed. Directly comparing judgement vs no-judgement conditions as a function of congruency, we observed a large cluster in dorsomedial PFC (Figure 4B; x = 9, y = 71, z = 19; pfwe < 0.05; Supplementary Table S6) and supplementary motor area (x = 42, y = −22, z = 70; pfwe < 0.05).
Fig. 4.
Comparing judgment and no-judgment trials. (A) When children had to provide a judgment, medial PFC was significantly activated (pfwe < 0.05). (B) For children, medial PFC was activated during runs when a judgment had to be made, compared with when it did not have to be made (pfwe < 0.05). For adults, rSMG activity was recruited both when (C) a judgment had to be made (pfwe < 0.05) and (D) also when no judgment had to be made (pfwe < 0.05). (E) When comparing the functional connectivity with rSMG during runs when a judgment had to be made compared with when it did not, there was greater functional coupling with lDLPFC for adults (psvc < 0.005).
In adults, in contrast, analysis of incongruent vs congruent trials in the judgment blocks revealed activation in several brain regions (Figure 4C, all at pfwe < 0.05; see Supplementary Materials; Supplementary Table S7) including right insula (x = 33, y = 14, z = −11), left anterior insula (x = −39, y = 14, z = −8), anterior cingulate (x = 3, y = 44, z = 7), as well as left TPJ/SMG (x = −56, y = −67, z = 7), and right TPJ/SMG (x = 66, y = −34, z = 16; see Figure 4C and Supplementary Figure S8). During the no-judgment blocks, a similar set of brain regions was activated (Figure 4D; all at pfwe < 0.05; Supplementary Table S8), comprising left TPJ/SMG (x = −63, y = −46, z = 31), right TPJ/SMG (x = 60, y = −46, z = 28; Figure 4D and Supplementary Figure S8), right IFG extending into the right anterior insula (x = 51, y = 20, z = −2), as well as dorsal anterior cingulate cortex (x = −6, y = 23, z = 34). When comparing activity as a function of congruency between judgment and no-judgment blocks, there were no significant activations.
Comparison of functional connectivity from rSMG in the judgement and no-judgement condition
To further address our third goal, we also sought to specify if the increased connectivity between rSMG and left DLPFC as a function of a decrease in EEB in adults also occurs during those trials in which no empathic judgement is required. For adults, there was no association between connectivity of rSMG during the no-judgment block and a decrease in the EEB with any other brain region (P > 0.05 uncorrected). Further, comparing the increase in connectivity with rSMG as a function of a decrease in the EEB between judgment and no-judgment blocks revealed stronger connectivity to left DLPFC during judgment compared with no-judgment blocks (Figure 4E; x = −48, y = 32, z = 34; psvc < 0.05; Supplementary Table S9 and Supplementary Figure S9).
Structural and task-free functional MRI data
We complemented the task-based functional MRI analysis from the previous section with a comprehensive assessment of structural and intrinsic functional brain markers to further explore the specific roles of rSMG as compared with rTPJ in overcoming EEB. Analyzing cortical thickness as a marker of brain maturation (to address our second goal, i.e. to better understand brain mechanisms underlying age-related differences), we found a significant age-related decrease in cortical thickness for both rTPJ and rSMG regions identified previously in the functional run [rTPJ: F(1,46) = 76.509; P < 0.001; rSMG: F(1,46) = 76.336; P < 0.001; Supplementary Figure S10]. Using an independent region of interest of rSMG from a previous study (Silani et al., 2013), we correlated cortical thickness and functional activation during incongruency of this region, showing that they were negatively related (r = −0.370; P < 0.05; Supplementary Figure S11). To see if the shared variance of age and cortical thickness of rSMG could account for the individual differences in functional recruitment of this region over and above each predictor (age and thickness) alone, we computed a commonality analysis (Supplementary Materials). We found that the degree to which this region was preferentially activated during incongruent compared with congruent trials was best accounted for by the shared variance component of age and cortical thickness of rSMG (13.2% of the explained variance). These findings indicate that the extent of activation of rSMG can, in part, be explained by the age-related maturation of this brain region (Table 1 and Supplementary Figure S12). Note that the above age-related effects were also consistent when additionally correcting for sex effects in all the above analyses. Further, to test for a certain specificity of this effect, we show that when taking cortical thickness of the closely adjacent precentral gyrus (Supplementary Figure S13) there is no correlation with activation of rSMG (r = −0.151; P > 0.2) and that the correlation between thickness and activation of rSMG persisted when controlling for individual differences in thickness of right precentral gyrus (r = −0.299; P < 0.05).
Table 1.
Results of commonality analysis
| Unique and common variance contributors | Functional activation of rSMG |
|---|---|
| U1 (Age) | 0.032 |
| U2 (Cortical Thickness) | 0.0017 |
| C12 (Age + Cortical Thickness) | 0.132 |
Numbers refer to unique (U) and common (C) contributions in explaining variance in functional activation of rSMG. Predictors were (i) age and (ii) cortical thickness of rSMG. Significant contributions are shown in bold.
To address our fourth and final goal, we also assessed whether our task-based peaks of rTPJ and rSMG would be embedded into different intrinsic functional networks, which in turn might explain the unique suitability of rSMG involvement in overcoming EEB. We used the same peaks of rSMG and rTPJ as outlined previously, taken from the contrast AdultsIncongruent > Congruent > ChildrenIncongruent > Congruent and assessed intrinsic functional networks in children and adults by performing an analysis of task-free fMRI data. To this end, we directly compared the voxel-wise connectivity strength of both regions within subjects, both within the sample of adults and children. In both children and adults, rSMG showed more marked connectivity patterns relative to rTPJ to other SMG subregions as well as the bilateral insula (Figure 5A and B). Conversely, rTPJ showed a stronger functional embedding than rSMG with medial PFC, ventrolateral PFC, as well as lateral and medial parietal regions (Figure 5A and B).
Fig. 5.
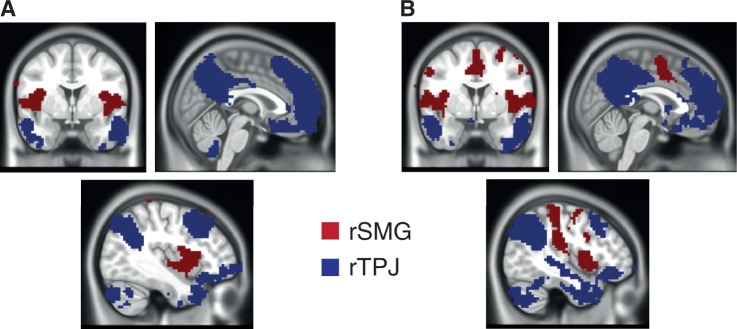
Functional connectivity differences between rSMG and rTPJ in children and adults (pfwe < 0.05). (A) In adults, rSMG showed more marked connectivity relative to rTPJ to bilateral insula and rSMG; conversely, rTPJ was more strongly connected than rSMG to large portions of medial PFC, precuneus, TPJ, the temporal poles and superior frontal gyrus. (B) For children, these patterns were virtually identical, with the exception of increased connectivity between rSMG and medial cingulate cortex.
DISCUSSION
Using a novel monetary reward and punishment task capable of inducing strong emotional states in children and adults in response to winning and losing money during a speeded reaction time task, we could replicate the crucial involvement of rSMG in overcoming the EEB in adults, despite considerable differences between the two paradigms. This evidence converges to show that over different paradigms rSMG had a crucial role in overcoming EEB, suggesting that the process implemented in rSMG is largely independent of stimulus type and sensory modality.
Even though this study was primarily designed to explore the neurocognitive mechanisms underlying age-related changes in overcoming the EEB, we begin the discussion of the findings with reference to our third experimental goal on the precise function of rSMG, seeing that this provides the backbone of interpreting the developmental findings. Using functional connectivity analysis, we were able to show that rSMG connectivity to lDLPFC was modulated by individual differences in EEB, whereby stronger connectivity led to a smaller EEB. Interestingly, comparing the judgment with the no-judgment condition, rSMG activation in adults was observed even during those runs when no affective judgment had to be made. Moreover, it was the functional coupling between rSMG and DLPFC and only when affective judgments had to be made that correlated with individual differences in the EEB. Such findings are in line with a role of DLPFC in the implementation of social judgement and decision making. Thus, previous fMRI studies have provided considerable evidence that DLPFC is required for the implementation of basic perceptual (Heekeren et al., 2004, 2006) as well as complex social decisions and judgments (Knoch et al., 2006, 2009; Spitzer et al., 2007; Steinbeis et al., 2012; Tassy et al., 2012). Regions in the TPJ, such as rTPJ and rSMG, on the other hand, have been associated among other things with processes related to self-other distinction in the social domain (Decety and Lamm, 2007; Brass et al., 2009; Santiesteban et al., 2012). Specifically, a recent functional role ascribed to rSMG in overcoming EEB is to disambiguate the perceptual information pertaining to oneself from that relevant to others during incongruency between self- and other-related affective states (Silani et al., 2013). Further, recent electrophysiological data suggest activity of the posterior parietal cortex to relate the calculation and representation of the perspective of others, whereas frontal regions resolve the conflict between self and other perspective when having to respond (McCleery et al., 2011). Our findings suggest that rSMG may be involved in early processes required to engage in self–other distinction and more specifically to accurately calculate and weight the information related to ones own affective state and differentiate this from the information related to the other. On the other hand, DLPFC may then use this information from rSMG to subsequently implement a concrete decision in form of a social judgment. Future studies using TMS to differentially disrupt either rSMG or DLPFC functions while subjects engaging in empathic judgments may help validating such an interpretation.
With reference to our forth goal, the present study set out to better differentiate between the respective roles of rTPJ and rSMG in emotional egocentricity. Thus, although rSMG was found now twice to be engaged in overcoming incongruent affective judgements, rTPJ has been usually discussed to be crucial in the context of self–other distinction and cognitive perspective-taking and other less affective aspects of social cognition (Saxe and Kanwisher, 2003; Decety and Lamm, 2007; Carter and Huettel, 2013). The task-based connectivity analysis indicates that individual differences in the EEB are best explained by connectivity from rSMG and not from rTPJ to lateral PFC. In combination, with the task-free connectivity analyses using seeds that emerged from the task-based fMRI analysis, this suggests that rSMG is uniquely suited to perform such an operation, by resolving the kind of demands posed by the experience of affective incongruency in social cognition as a function of its specific connectivity with emotion-relevant brain regions (see Supplementary Materials for further discussion). More importantly, this suitability appears to be present already during childhood. These findings highlight the necessity for a more differentiated account of the functional role of subregions of temporoparietal regions in social cognition and decision making and stress the suitability of rSMG in specifically resolving socio-affective incongruency and relaying this resolution to regions implementing appropriate empathic judgments.
Despite the investigation of neurocognitive mechanisms underlying overcoming EEB in adults, the first and main goal of the present study was to study the developmental time course of our ability to overcome emotional egocentricity. We could confirm here our hypothesis that children are considerably more egocentric than adults when having to make empathic judgments about others’ affective states in a situation where those are incongruent to their own. Moreover, by using a comprehensive battery of additional tasks that probed low- and high-level cognitive function, such as fluid intelligence, attentional reorienting and motor inhibition, we were able to dispel that this age-related difference is due to more general cognitively demanding aspects of the task. Crucially, we could show that individual differences in cognitive perspective taking neither correlated with individual differences in the EEB nor could they account for age-related differences in overcoming the EEB. These findings thus suggest a unique developmental trajectory of the social skill to overcome one’s own egocentric stance when judging the emotional states of others.
Our second goal we addressed using complimentary functional MRI analysis. Here, we were able to show that, unlike adults, children show smaller activation in rSMG when having to make incongruent empathic judgments. This observation was in line with their increased EEB, suggesting difficulties in overcoming such egocentric tendencies to be linked to reduced recruitment of brain regions critical for overcoming emotional egocentricity (Silani et al., 2013). Further, we were able to show that age-related changes in cortical thickness of rSMG could account for the extent to which these regions are functionally recruited when having to overcome emotional egocentricity. Moreover, the task-related functional connectivity of this region with lDLPFC was associated with individual differences in the EEB and in turn weaker for children than for adults. Children, on the other hand, recruited medial PFC when giving affectively incongruent judgments. Thus, the developmental decrease in the EEB observed in the present sample can be potentially attributed to two mechanisms. First, the reduced activity in rSMG may reflect a calculation biased in favor of one’s own affective state. Second, the transmission of calculated signals to regions that may ultimately implement such empathic judgments and decisions, such as lDLPFC, also appears to be diminished early in development. DLPFC has been shown to play a crucial role in the context of implementing social decisions and previous evidence that this function still undergoes considerable development (Guroglu et al., 2011; Steinbeis et al., 2012; Crone, 2013). These findings potentially suggest age-related improvements in implementing accurate affectively incongruent judgments of other’s emotions. A previous study used eye-tracking to show that age-related differences in egocentric visual perspective taking occurs as a function of developmental changes in controlled processes of correcting and implementing judgments (Epley et al., 2004). Our data seem to suggest, that on top of this, the root of heightened emotional egocentrism in childhood may already occur at the level of inadequately calculating another’s emotional state incongruent to one’s own. Although the present study explored the mechanisms of EEB during childhood and in comparison with adulthood, future studies may also wish to focus on its development during adolescence. It is also important to note that our structure–function association in the rSMG is presumably a result of general cortical development also comprising the rSMG. This suggests that our findings of an age-related increase in functional recruitment of rSMG during our task occur as a function of cortical development per se and not just because of the development of rSMG. However, these findings are still specific enough to not pertain to the entire cortical mantle as indicated by a lack of significant associations between function of rSMG and thickness of precentral gyrus.
The present task was designed in such a way that participants experienced emotions in response to wins and losses throughout each run. Although it is possible that participants may not actually experience the emotion but rather reactivate a remembered experience of the emotion in the Simultaneous Other condition, there are several lines of evidence that suggest this was not the case. First, in our experiment, emotional intensity in response to one’s own wins and losses did not decrease throughout the experiment, which suggests that the Simultaneous Other condition induced strong emotions to one’s own wins and losses. Second, a previous assessment on emotional egocentricity based on visuo-tactile stimulation (Silani et al., 2013) has shown increased rSMG connectivity to visual and somatosensory cortical areas during incongruent compared with congruent trials, suggestive that the immediate perceptual input (and not its memory) is processed and overcome. In terms of the role of rSMG in terms of overcoming the EEB, while there is evidence that rSMG subserves this function in a visuo-tactile version of the present paradigm (Silani et al., 2013), there is no direct evidence with the present paradigm for rSMG serving the same function. However, in our view, it is possible to see both rTMS and the developing brain as two sides of the same coin in terms of a lesion model of rSMG function, which when functionally impaired/immature in both cases leads to a greater EEB. To really make this point, however, we propose two solutions: (i) an inclusion of an rTMS study with the present paradigm; (ii) running both previous paradigms and the present one in a single sample and testing for significant relationships between the two observed biases.
In sum, the present findings represent an important advance in the understanding of the neurocognitive mechanisms underlying social judgment and decision making, by showing an important role of rSMG in contrast to rTPJ in overcoming emotional egocentricity in the context of social interaction. Specifically, our data reveal that although rSMG appears to distinguish between one’s own and another’s emotional states when these are incongruent, conflict resolution is subsequently implemented by lDLPFC when having to provide an accurate empathic judgment. Importantly, neither individual differences in cognitive perspective taking and EEB correlated, nor could connectivity from rTPJ account for any individual- or age-related differences in the EEB. Together, with the strikingly different task-free resting state connectivity between rSMG and rTPJ for both children and adults, this strongly suggest that rSMG possesses the necessary features to resolve the incongruence between one’s own and another’s emotional but not cognitive state; the latter being required for mentalizing and theory-of-mind tasks. These findings call for more comprehensive models of social judgment and decision making. Most importantly, our findings shed light on the development of emotional egocentricity in showing that children in comparison with adults show a considerable increase in EEB, which is reduced by age. Brain imaging data reveal that children still lack the full set of mechanisms that help to make accurate judgments of other’s emotions and presumably also predictions of their behavior in moments when their emotions conflict with those of others. The ability to fully overcome this with adulthood presumably occurs as a function of the maturation of late developing cortical regions, such as rSMG and lDLPFC.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This research was funded by the Swiss National Science Foundation (“Neuronal and developmental basis of empathy and emotion control: fMRI studies of adults and children aged 6 to 12 years”; to T.S.), and the University Research Priority Programs (URPP) of the University of Zurich.
REFERENCES
- Apperly IA, Warren F, Andrews BJ, Grant J, Todd S. Developmental continuity in theory of mind: speed and accuracy of belief-desire reasoning in children and adults. Child Development. 2011;82(5):1691–703. doi: 10.1111/j.1467-8624.2011.01635.x. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Singer T. The neural basis of empathy. Annual Review of Neuroscience. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Brass M, Ruby P, Spengler S. Inhibition of imitative behaviour and social cognition. Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364(1528):2359–67. doi: 10.1098/rstb.2009.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Huettel SA. A nexus model of the temporal-parietal junction. Trends in Cognitive Sciences. 2013;17(7):328–36. doi: 10.1016/j.tics.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JR, Baron RS, Inman ML. Misperceptions in intergroup conflict - Disagreeing about what we disagree about. Psychological Science. 2006;17(1):38–45. doi: 10.1111/j.1467-9280.2005.01662.x. [DOI] [PubMed] [Google Scholar]
- Crone EA. Considerations of fairness in the adolescent brain. Child Development Perspectives. 2013;7(2):97–103. [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13(6):580–93. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Elkind D. Egocentrism in adolescence. Child Development. 1967;38(4):1025–41. [PubMed] [Google Scholar]
- Epley N, Morewedge CK, Keysar B. Perspective taking in children and adults: equivalent egocentrism but differential correction. Journal of Experimental Social Psychology. 2004;40(6):760–8. [Google Scholar]
- Flavell JH. Cognitive development: children's knowledge about the mind. Annual Review of Psychology. 1999;50:21–45. doi: 10.1146/annurev.psych.50.1.21. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences. 1998;2(12):493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44(2):389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Guroglu B, van den Bos W, van Dijk E, Rombouts SA, Crone EA. Dissociable brain networks involved in development of fairness considerations: understanding intentionality behind unfairness. Neuroimage. 2011;57(2):634–41. doi: 10.1016/j.neuroimage.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431(7010):859–62. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ruff DA, Bandettini PA, Ungerleider LG. Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(26):10023–8. doi: 10.1073/pnas.0603949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysar B, Lin SH, Barr DJ. Limits on theory of mind use in adults. Cognition. 2003;89(1):25–41. doi: 10.1016/s0010-0277(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends in Cognitive Sciences. 2007;11(5):194–6. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314(5800):829–32. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Knoch D, Schneider F, Schunk D, Hohmann M, Fehr E. Disrupting the prefrontal cortex diminishes the human ability to build a good reputation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(49):20895–99. doi: 10.1073/pnas.0911619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlberg L. The Psychology of Moral Development: The Nature and Validity of Moral Stages. San Francisco: Harper & Row; 1984. [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8(1):60–4. [Google Scholar]
- Mars RB, Sallet J, Schuffelgen U, Jbabdi S, Toni I, Rushworth MF. Connectivity-based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cerebral Cortex. 2012;22(8):1894–903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- McCleery JP, Surtees ADR, Graham KA, Richards JE, Apperly IA. The neural and cognitive time course of theory of mind. Journal of Neuroscience. 2011;31(36):12849–54. doi: 10.1523/JNEUROSCI.1392-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu GF, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex. 2008;18(2):262–71. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Murphy ML, Slavich GM, Rohleder N, Miller GE. Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status. Clinical Psychological Science. 2013;1(1):30–40. doi: 10.1177/2167702612455743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb AF, Bukowski WM, Pattee L. Childrens peer relations—a metaanalytic review of popular, rejected, neglected, controversial, and average sociometric status. Psychological Bulletin. 1993;113(1):99–128. doi: 10.1037/0033-2909.113.1.99. [DOI] [PubMed] [Google Scholar]
- O'Brien E, Ellsworth PC. More than skin deep: visceral states are not projected onto dissimilar others. Psychological Science. 2012;23(4):391–6. doi: 10.1177/0956797611432179. [DOI] [PubMed] [Google Scholar]
- Piaget J, Gabain M. The Moral Judgment of the Child. London: K. Paul, Trench, Trubner & Co. ltd; 1932. [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Pronin E. How we see ourselves and how we see others. Science. 2008;320(5880):1177–80. doi: 10.1126/science.1154199. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven's Progressive Matrices and Vocabulary Scales. San Antonio, TX: Pearson Assessment; 2003. [Google Scholar]
- Repacholi BM, Gopnik A. Early reasoning about desires: evidence from 14- and 18-month-olds. Developmental Psychology. 1997;33(1):12–21. doi: 10.1037//0012-1649.33.1.12. [DOI] [PubMed] [Google Scholar]
- Royzman EB, Cassidy KW, Baron J. “I know, you know”: epistemic egocentrism in children and adults. Review of General Psychology. 2003;7(1):38–65. [Google Scholar]
- Santiesteban I, Banissy MJ, Catmur C, Bird G. Enhancing social ability by stimulating right temporoparietal junction. Current Biology. 2012;22(23):2274–7. doi: 10.1016/j.cub.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people—the role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19(4):1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One. 2009;4(3) doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28(14):3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G, Lamm C, Ruff C, Singer T. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. Journal of Neuroscience. 2013;33(39):15466–76. doi: 10.1523/JNEUROSCI.1488-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Fischbacher U, Herrnberger B, Gron G, Fehr E. The neural signature of social norm compliance. Neuron. 2007;56(1):185–96. doi: 10.1016/j.neuron.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Steinbeis N, Bernhardt BC, Singer T. Impulse control and underlying functions of the left DLPFC mediate age-related and age-independent individual differences in strategic social behavior. Neuron. 2012;73(5):1040–51. doi: 10.1016/j.neuron.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Tassy S, Oullier O, Duclos Y, et al. Disrupting the right prefrontal cortex alters moral judgement. Social Cognitive and Affective Neuroscience. 2012;7(3):282–8. doi: 10.1093/scan/nsr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L, Loewenstein G. Egocentric Interpretations of Fairness and Interpersonal Conflict. Organizational Behavior and Human Decision Processes. 1992;51(2):176–97. [Google Scholar]
- Van Boven L, Loewenstein G. Social projection of transient drive states. Personality and Social Psychology Bulletin. 2003;29(9):1159–68. doi: 10.1177/0146167203254597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.