Abstract
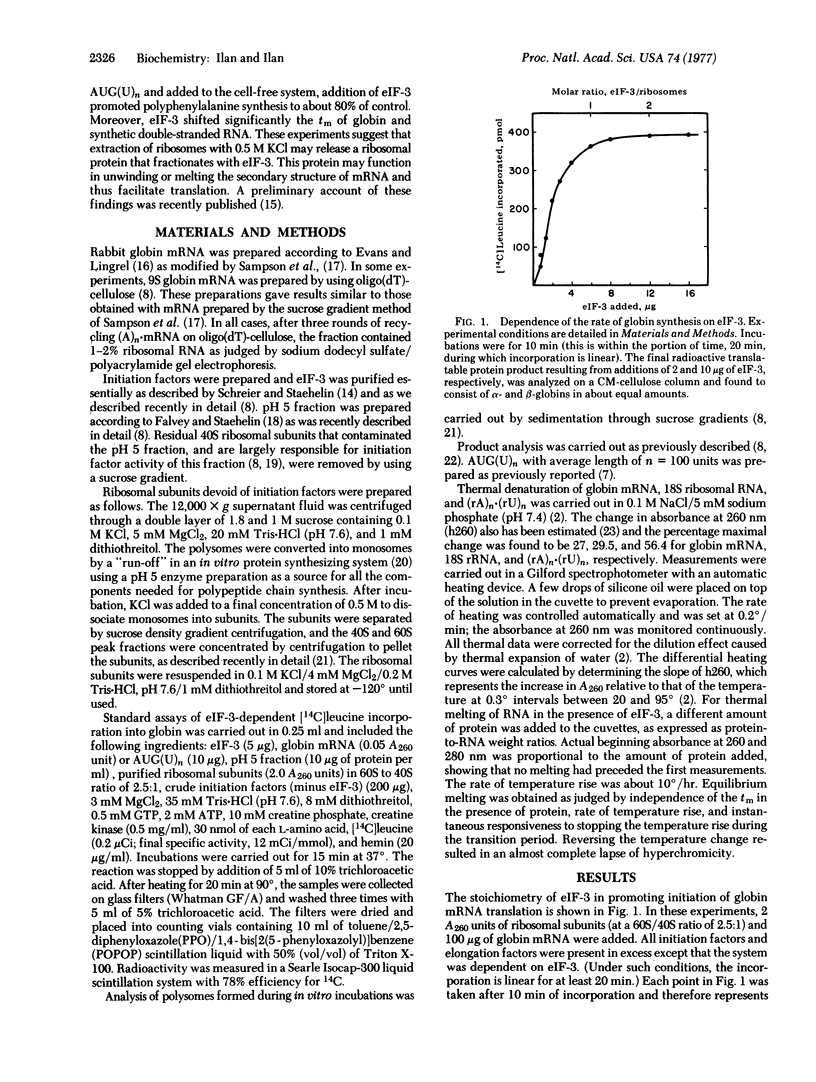
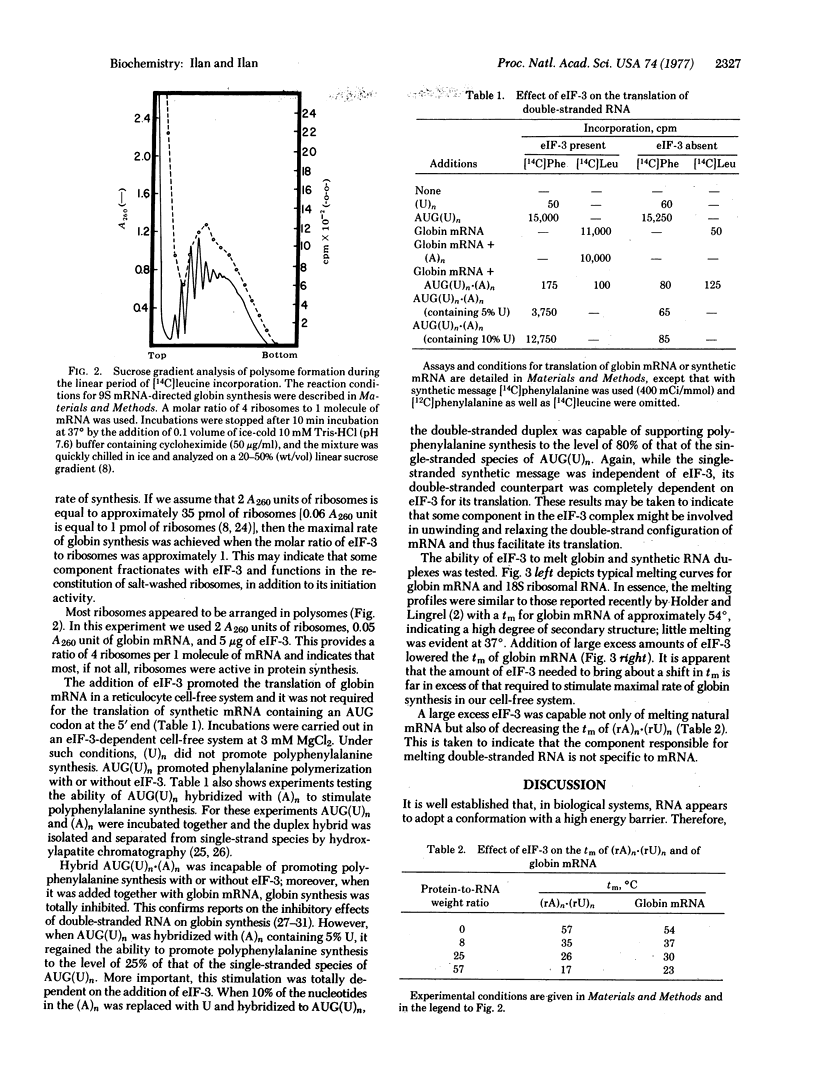
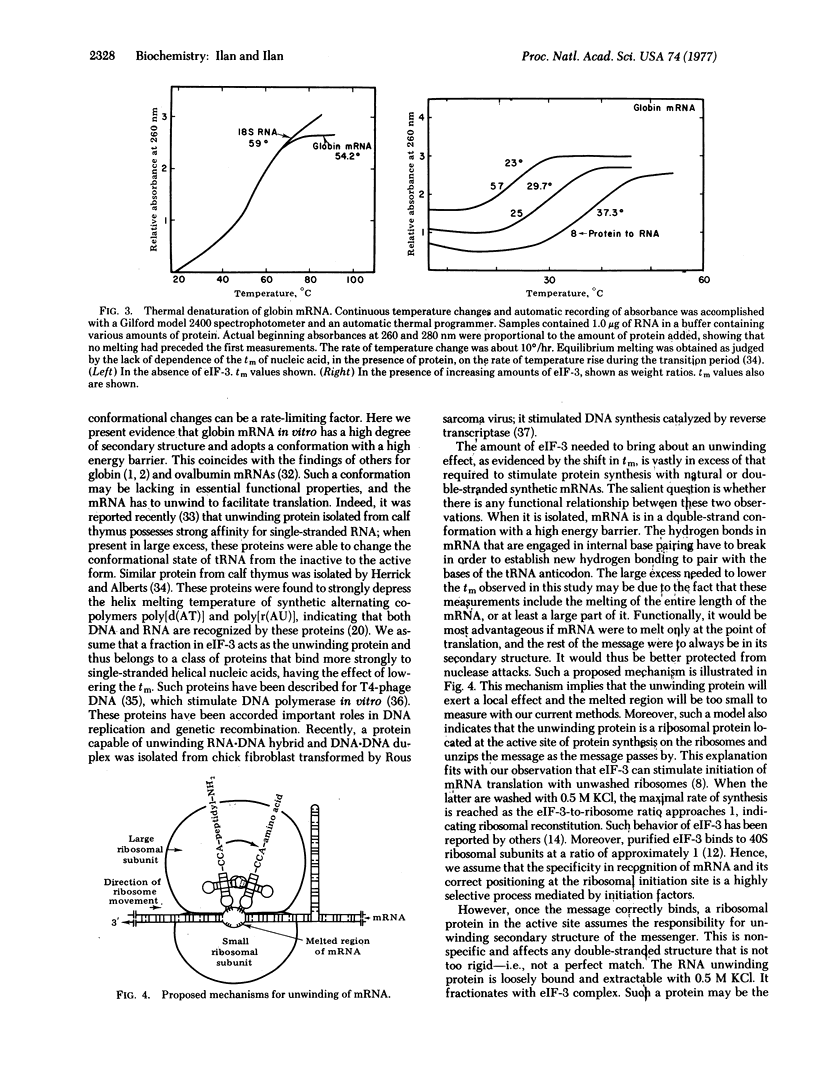
Experiments with a rabbit reticulocyte cell-free system dependent on the addition of initiation factor 3 (eIF-3) and mRNA were carried out. In this system, using ribosomal subunits, AUG(U)n can direct polyphenylalanine synthesis in the absence of eIF-3 at 3 mM MgCl2. Globin mRNA was not translated under similar conditions; its translation requires the addition of eIF-3. Moreover, the maximal rate of globin synthesis was achieved when the molar ratio of eIF-3 to ribosomes was approximately 1. This was taken to indicate that some ribosomal proteins were fractionated with eIF-3 and functioned in reconstitution of salt-washed ribosomes. In our system, almost all ribosomes were active, as evident from the fact that all were found in polysomes when analyzed at the time of linear incorporation, and the molar ratio of ribosomes to mRNA was maintained at 4:1. When AUG(U)n was hybridized with poly(A), it could not direct polyphenylalanine synthesis with or without eIF-3 and was a potent inhibitor of the translation of globin mRNA in the presence of eIF-3. When poly(A) containing 10% U was hybridized with AUG(U)n and added to the cell-free system, addition of eIF-3 promoted polyphenylalanine synthesis to about 80% of control. Moreover, eIF-3 was seen to shift significantly the melting temperature of globin and synthetic double-stranded RNA. These observations suggest that extraction of ribosomes with 0.5 M KCl may release a ribosomal protein that fractionates with eIF-3. This protein may function in unwinding or melting the secondary structure of mRNA and thus facilitate translation.
Keywords: double-stranded RNA, globin mRNA, protein synthesis, helix coil transition
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Bear D. G., Ng R., Van Derveer D., Johnson N. P., Thomas G., Schleich T., Noller H. F. Alteration of polynucleotide secondary structure by ribosomal protein S1. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1824–1828. doi: 10.1073/pnas.73.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Hershey J. W. Purification and characterization of initiation factor IF-E3 from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3005–3009. doi: 10.1073/pnas.73.9.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtker H. The reaction of ribonucleic acid with formaldehyde. I. Optical absorbance studies. Biochemistry. 1967 Sep;6(9):2718–2727. doi: 10.1021/bi00861a011. [DOI] [PubMed] [Google Scholar]
- Cashion L. M., Stanley W. M., Jr Two eukaryotic initiation factors (IF-I and IF-II) of protein synthesis that are required to form an initiation complex with rabbit reticulocyte ribosomes. Proc Natl Acad Sci U S A. 1974 Feb;71(2):436–440. doi: 10.1073/pnas.71.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Safer B., Merrick W. C., Anderson W. F., London I. M. Inhibition of protein synthesis in rabbit reticulocyte lysates by double-stranded RNA and oxidized glutathione: indirect mode of action on polypeptide chain initiation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1286–1290. doi: 10.1073/pnas.72.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINTZIS H. M. Assembly of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1961 Mar 15;47:247–261. doi: 10.1073/pnas.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D. Role of subunits in the ribosome cycle. Nature. 1971 May 21;231(5299):153–157. doi: 10.1038/231153a0. [DOI] [PubMed] [Google Scholar]
- Doty P., Boedtker H., Fresco J. R., Haselkorn R., Litt M. SECONDARY STRUCTURE IN RIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1959 Apr;45(4):482–499. doi: 10.1073/pnas.45.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Ranu R. S., London I. M. Control of protein synthesis in reticulocyte lysates: effects of 3':5'-cyclic AMP, ATP, and GTP on inhibitions induced by hemedeficiency, double-stranded RNA, and a reticulocyte translationa inhibitor. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1112–1116. doi: 10.1073/pnas.73.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Lingrel J. B. Hemoglobin messenger ribonucleic acid. Distribution of the 9S ribonucleic acid in polysomes of different sizes. Biochemistry. 1969 Mar;8(3):829–831. doi: 10.1021/bi00831a010. [DOI] [PubMed] [Google Scholar]
- Falvey A. K., Staehelin T. Structure and function of mammalian ribosomes. I. Isolation and characterization of active liver ribosomal subunits. J Mol Biol. 1970 Oct 14;53(1):1–19. doi: 10.1016/0022-2836(70)90042-2. [DOI] [PubMed] [Google Scholar]
- Gralla J., DeLisi C. mRNA is expected to form stable secondary structures. Nature. 1974 Mar 22;248(446):330–332. doi: 10.1038/248330a0. [DOI] [PubMed] [Google Scholar]
- Herrick G., Alberts B. Nucleic acid helix-coil transitions mediated by helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2133–2141. [PubMed] [Google Scholar]
- Hoffman W. L., Ilan J. Purification on hydroxyapatite of liver ribosomes and polysomes from unfasted mice. Biochim Biophys Acta. 1974 Oct 11;366(2):199–214. doi: 10.1016/0005-2787(74)90334-7. [DOI] [PubMed] [Google Scholar]
- Holder J. W., Lingrel J. B. Determination of secondary structure in rabbit globin messenger RNA by thermal denaturation. Biochemistry. 1975 Sep 23;14(19):4209–4215. doi: 10.1021/bi00690a009. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Lee S. G. Isolation of nucleic acid-binding protein: stimulation of reverse transcriptase-catalysed DNA synthesis. Nature. 1976 Feb 12;259(5543):499–502. doi: 10.1038/259499a0. [DOI] [PubMed] [Google Scholar]
- Ilan J., Ilan J. A possible role of the AUG codon in the initiation of polypeptide synthesis in a eukaryotic orgamism. Biochim Biophys Acta. 1970 Dec 14;224(2):614–619. doi: 10.1016/0005-2787(70)90594-0. [DOI] [PubMed] [Google Scholar]
- Ilan J., Ilan J. An mRNA bound initiation factor and its role in translation of natural message. Nat New Biol. 1973 Feb 7;241(110):176–180. doi: 10.1038/newbio241176a0. [DOI] [PubMed] [Google Scholar]
- Ilan J., Ilan J. Requirement for homologous rabbit reticulocyte initiation factor 3 for initiation of alpha- and beta-globin mRNA translation in a crude protozoal cell-free system. J Biol Chem. 1976 Sep 25;251(18):5718–5725. [PubMed] [Google Scholar]
- Ilan J., Patel N. Mechanism of gene expression in Tenebrio molitor. Juvenile hormone determination of translational control through transfer ribonucleic acid and enzyme. J Biol Chem. 1970 Mar 25;245(6):1275–1281. [PubMed] [Google Scholar]
- Ilan J., Singer M. Sampling of the leucine pool from the growing peptide chain: difference in leucine specific activity of peptidyl-transfer RNA from free and membrane-bound polysomes. J Mol Biol. 1975 Jan 5;91(1):39–51. doi: 10.1016/0022-2836(75)90370-8. [DOI] [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Inhibition of cellular protein synthesis by double-stranded RNA: inactivation of an initiation factor. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1222–1226. doi: 10.1073/pnas.70.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard B., Salser W. Secondary structures formed by random RNA sequences. Biochem Biophys Res Commun. 1975 Apr 7;63(3):548–554. doi: 10.1016/s0006-291x(75)80419-0. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Mathews M. B. Double-stranded RNA as an inhibitor of protein synthesis and as a substrate for a nuclease in extracts of Krebs II ascites cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):225–229. doi: 10.1073/pnas.70.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson J., Mathews M. B., Osborn M., Borghetti A. F. Hemoglobin messenger ribonucleic acid translation in cell-free systems from rat and mouse liver and Landschutz ascites cells. Biochemistry. 1972 Sep 12;11(19):3636–3640. doi: 10.1021/bi00769a022. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of eukaryotic protein synthesis: (Met-tRNA f -40S ribosome) initiation complex catalysed by purified initiation factors in the absence of mRNA. Nat New Biol. 1973 Mar 14;242(115):35–38. doi: 10.1038/newbio242035a0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundkvist I. C., McKeehan W. L., Schreier M. H., Staehelin T. Initiation factor activity associated with free 40 S subunits from rat liver and rabbit reticulocytes. J Biol Chem. 1974 Oct 25;249(20):6512–6516. [PubMed] [Google Scholar]
- Sundkvist I. C., Staehelin T. Structure and function of free 40 S ribosome subunits: Characterization of initiation factors. J Mol Biol. 1975 Dec 15;99(3):401–418. doi: 10.1016/s0022-2836(75)80135-5. [DOI] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Boublik M. Destabilization of the secondary structure of RNA by ribosomal protein S1 from Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):957–964. doi: 10.1016/0006-291x(76)90685-9. [DOI] [PubMed] [Google Scholar]
- Van N. T., Holder J. W., Woo S. L., Means A. R., O'Malley B. W. Secondary structure of ovalbumin messenger RNA. Biochemistry. 1976 May 18;15(10):2054–2062. doi: 10.1021/bi00655a005. [DOI] [PubMed] [Google Scholar]
- Williamson R., Morrison M., Lanyon G., Eason R., Paul J. Properties of mouse globin messenger ribonucleic acid and its preparation in milligram quantities. Biochemistry. 1971 Aug 3;10(16):3014–3021. doi: 10.1021/bi00792a005. [DOI] [PubMed] [Google Scholar]


