Abstract
Individuals with autism spectrum disorder (ASD) are thought to lack self-awareness and to experience difficulty empathizing with others. Although these deficits have been demonstrated in previous studies, most of the target stimuli were constructed for typically developing (TD) individuals. We employed judgment tasks capable of indexing self-relevant processing in individuals with and without ASD. Fourteen Japanese men and 1 Japanese women with high-functioning ASD (17–41 years of age) and 13 Japanese men and 2 TD Japanese women (22–40 years of age), all of whom were matched for age and full and verbal intelligence quotient scores with the ASD participants, were enrolled in this study. The results demonstrated that the ventromedial prefrontal cortex was significantly activated in individuals with ASD in response to autistic characters and in TD individuals in response to non-autistic characters. Although the frontal–posterior network between the ventromedial prefrontal cortex and superior temporal gyrus participated in the processing of non-autistic characters in TD individuals, an alternative network was involved when individuals with ASD processed autistic characters. This suggests an atypical form of empathy in individuals with ASD toward others with ASD.
Keywords: autism spectrum disorder, empathy, self, similarity, ventromedial prefrontal cortex
INTRODUCTION
As suggested by the term ‘autism’, which comes from the Greek word autós, meaning self, a lack of self-awareness is a central element of autism spectrum disorder (ASD) (Toichi et al., 2002; Lombardo et al., 2010). Deficits in self-related processing lead to difficulties in empathizing with others (Lombardo et al., 2007). Individuals with ASD also show deficits in reciprocal social interactions and impairment in verbal communication, such as difficulties in understanding humor, irony and sarcasm (Frith, 2003). These pragmatic language impairments are thought to be based on deficits in theory of mind, the ability to attribute mental states to oneself and to others. This ability to make inferences about what other people think allows one to predict their behaviors (Baron-Cohen et al., 1985). During the process involved in making inferences, the theory of mind network, including the medial prefrontal cortex, precuneus (and posterior cingulate cortex) and temporoparietal junction (and adjunct superior temporal sulcus), is recruited when individuals reflect on themselves and others (Amodio and Frith, 2006; Frith and Frith, 2006; Mitchell et al., 2006a,b; Saxe et al., 2006; Lombardo et al., 2010). Several brain imaging studies have investigated the neural basis of theory of mind in TD individuals (Fletcher et al., 1995; Baron-Cohen et al., 1999; Castelli et al., 2000; Gallagher et al., 2000, 2002; Vogeley et al., 2001; Ferstl and von Cramon, 2002). The theory of mind network is altered in ASD (Mason et al., 2008; Mizuno et al., 2011; Morita et al., 2012).
Observations in ASD groups lacking theory of mind and/or empathy as well as recent neuroimaging research have provided empirical evidence of a neural basis for theory of mind and empathy (Völlm et al., 2006; Bird et al., 2010). Impairment in theory of mind has been implicated in neurodevelopmental disorders in ASD (Lombardo et al., 2007). Additionally, previous studies on brain connectivity have demonstrated that the degree of synchronization in activation (i.e. functional connectivity) between frontal and posterior brain regions is lower in ASD. The first report of this nature was in the context of a language comprehension task (Just et al., 2004); undersynchronization of activation during cognitive tasks has been reported between the frontal lobe and more posterior regions in several other paradigms (Just et al., 2004, 2012; Kana et al., 2006, 2009).
Although deficits in ASD have been demonstrated in previous studies, most of the target stimuli used in those studies were constructed for typically developing (TD) individuals. However, it may be difficult for individuals with ASD to understand TD individuals, just as it is difficult for TD individuals to understand those with ASD. Concerning the similarity between self and other brain regions, ventral parts of the medial prefrontal cortex (mPFC) respond both during self-referential processing (Kelley et al., 2002; Northoff et al., 2006) and during mental state inferences concerned with others (Gallagher et al., 2000, 2002; Frith and Frith, 2006). The neural substrates underlying self-referential thought and theory of mind are characterized by overlap (Mitchell et al., 2005, 2006b; Jenkins et al., 2008; Tamir and Mitchell, 2010).
Previous studies demonstrated a lack of preferential responsiveness to self-information in the ventromedial prefrontal cortex (vmPFC) in individuals with ASD (Lombardo et al., 2010; Pfeifer et al., 2013). Compared with those with ASD, TD individuals recruited the vmPFC to a significantly greater extent for self vs. other in the reflective mentalizing task and in tasks pertaining to physical self-judgments and the British Queen. Previous behavioral studies have demonstrated that individuals with ASD do not benefit from self-referential elaboration (Toichi et al., 2002; Lombardo et al., 2007) and display a lack of ‘neural self-reference effect’ in the vmPFC (Lombardo et al., 2010).
When readers and characters are matched in personality, TD readers empathize with story characters similar to themselves (Komeda et al., 2013b). If this were also the case for individuals with ASD, these individuals may show empathy, a process in which one identifies with similar others. Additionally, the self-related brain network of individuals with ASD may participate in interactions with targets who have autistic traits. We used self- and other judgment tasks to test these hypotheses (Figure 1). Participants read sentences and responded to questions about them using two buttons (Yes and No). On the basis of the items in the Social Responsiveness Scale (SRS; Constantino and Todd, 2005; Kamio et al., 2009, 2013), each sentence described the behavior of a target character with traits identified as autistic or non-autistic. For example, self-judgments and other judgments about autistic and non-autistic characters involved participants’ reading a description about a character (e.g. ‘I would rather be alone than with others’ and ‘Yuya would rather be with others than be alone’, respectively) and evaluating their identification with this description (i.e. ‘Do you agree with the sentence?’ and ‘Do you think you are similar to him?’). Sex was matched between participant and character.
Fig. 1.
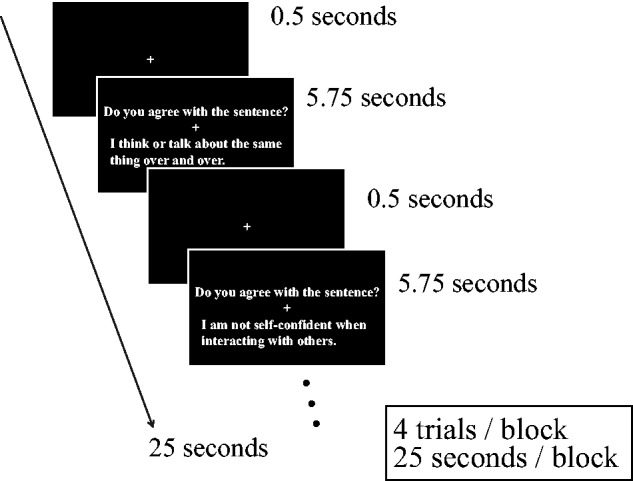
We employed a block design for the experiment. Schematic depiction of stimuli and task design of each block in the fMRI study. First, a fixation crosshair was presented, followed by the experimental stimuli, which were displayed for 5.75 s. The top line in each stimulus-containing rectangle presented a question (‘Do you agree with the sentence?’ for a self-task and ‘Do you think you are similar to him/her for an other task’), and the bottom line described a response with or without autistic traits.
We predicted that similarities between perceivers and targets would facilitate empathy, leading to selective responses toward targets similar to themselves. This prediction is known as the similarity hypothesis (Komeda et al., 2013a,b). Recent studies on TD adults have demonstrated that similarities between readers and characters play a critical role in cognitive tasks such as story comprehension and memory. For example, personality is an important contributor to similarities between readers and characters (Komeda et al., 2009, 2013b). Indeed, it is easier for highly extraverted than for less extraverted participants to understand stories about a highly extraverted story character (Komeda et al., 2009). Additionally, highly extraverted readers judge the behavioral outcomes of highly extraverted fictional characters more rapidly than do less extraverted readers, and highly neurotic readers judge the outcomes of highly neurotic characters more rapidly than do less neurotic readers (Komeda et al., 2013b).
Individuals with ASD provide specific responses to autistic fictional characters (Komeda et al., 2013a). For example, in the case of episodes about ASD characters, individuals with ASD more effectively retrieved consistent outcomes than inconsistent outcomes, and TD individuals retrieved stories with TD characters more effectively than stories with autistic characters. Thus, similarity between reader and fictional character had different effects on the memory retrieval of individuals with and without ASD.
In this study, we used functional magnetic resonance imaging (fMRI) to investigate whether activation of the vmPFC, known to be involved in self-related information processing (Lombardo et al., 2007; Tamir and Mitchell, 2010; Pfeifer et al., 2013) and empathy (Shamay-Tsoory et al., 2009; Schulte-Rüther et al., 2011; Shamay-Tsoory, 2011), was observed when participants made judgments about characters similar to themselves. We also examined whether seed-to-voxel functional connectivity differed among groups.
METHODS
Participants
Fourteen Japanese men and 1 Japanese women with high-functioning ASD (between 17 and 41 years of age) were recruited at the Department of Neuropsychiatry of the University of Fukui Hospital, Japan, and the Department of Psychiatry and Neurobiology of the Kanazawa University Hospital, Japan. Psychiatrists (i.e. H.K. and T.M.) diagnosed participants based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; American Psychiatric Association, 2000) and on the standardized criteria of the Diagnostic Interview for Social and Communication Disorders (Wing et al., 2002), which reportedly possesses good psychometric properties (Nygren et al., 2009). This instrument also contains items on early development and a section on activities of daily living, which provide data about functioning in areas other than social and communication-related domains (Wing et al., 2002). The ASD group consisted of 12 participants with autistic disorder and 3 with Asperger’s disorder. Thirteen TD Japanese men and two TD Japanese women (between 22 and 40 years of age), matched for age and full and verbal intelligence quotient (IQ) scores, were recruited from the local community (Table 1). Participants were excluded if they had a history of major medical or neurological illness, including epilepsy or significant head trauma, or a lifetime history of alcohol or drug dependence. Participants with a first-degree relative with a DSM-IV Axis I disorder were also excluded. IQ assessments were performed using the Wechsler Adult Intelligence Scale III (Wechsler, 1997). All participants had full-scale IQ scores >85. Although there was a significant difference in performance IQ scores1 between the ASD and the TD groups, there were no significant group differences for age or full-scale and verbal IQs (P > 0.05). To quantify autistic traits, we used the Autism-Spectrum Quotient (AQ) (Baron-Cohen et al., 2001), which consists of the following five subscales: social skills, attention switching, attention to detail, communication and imagination.
Table 1.
Mean chronological age, full-scale IQ, verbal IQ, performance IQ and AQ scores in individuals with ASD and TD adults
| ASD group (n = 15) | TD group (n = 15) | T | P | |
|---|---|---|---|---|
| Age (years) | 26.7 (5.8) | 26.1 (5.2) | −0.30 | 0.77 |
| Full-scale IQ | 99.7 (12.0) | 107.4 (9.8) | −1.9 | 0.06 |
| Verbal IQ | 104.6 (14.8) | 108.5 (10.8) | −0.82 | 0.42 |
| Performance IQ | 92.1 (16.1) | 104.3 (8.3) | −2.6* | 0.01 |
| AQ | 32.0 (8.5) | 14.6 (5.5) | 6.6* | 0.00 |
Note: Data are expressed as mean (s.d.).
*P < .05.
The protocol used for this study was approved by the ethics committee of the University of Fukui. After a complete explanation of the study, all the participants gave written informed consent prior to participation.
Stimuli
Each sentence described the behavior of a target character with autistic or non-autistic traits, as determined using the SRS (Figure 1). The use of the Japanese version of the SRS was permitted by Western Psychological Services. Making self-judgments involving an autistic character involved reading a sentence (e.g. ‘I would rather be alone than with others’) and answering the following question: ‘Do you agree with the sentence?’ Making other judgments involving a non-autistic character involved reading a sentence [e.g. ‘Yuya (Japanese male name) would rather be with others than alone’] and answering a different question: ‘Do you think you are similar to him?’ The subject of the sentence was ‘I’ in the self-judgment task, whereas the subject was the name of a Japanese character in the other judgment task. Four experimental conditions were used: autistic character in self-judgments, autistic character in other judgments, non-autistic character in self-judgments and non-autistic character in other judgments. Sex was matched between the participant and the character.
Experimental procedure
During the fMRI scan, participants read the sentences and made judgments about them by pressing a button with the right index (for Yes responses) or middle finger (for No responses). In each trial (5.75 s), subjects were presented with a self-judgment (‘Do you agree with the sentence?’) or an other judgment (‘Do you think you are similar to him/her’) in the top line, followed by a sentence describing a character with or without autistic traits in the bottom line (Figure 1).
We used a block design, which was the most efficient means of detecting activation (Friston et al., 1999; Handwerker et al., 2004; Meltzer et al., 2008). During scanning, the subjects performed a total of 6 sessions (each lasting 4 min 22.5 s, with 10 blocks for each of the 5 conditions, including fixation rest blocks). Eight trials were conducted under each experimental condition (autistic character in self, autistic character in other, non-autistic character in self and non-autistic character in other judgment), and each sessions included 32 experimental trials. A total of 6 sessions (192 experimental trials) were conducted, yielding 48 trials for each experimental condition (192 trials/4 conditions). Within each session, the blocks were ordered differently, and the order of the six sessions was counterbalanced across subjects.
Imaging parameters
Functional images were acquired with T2*-weighted gradient-echo echo-planar imaging (EPI) sequences with a 3-T MR imager (Signa Excite; General Electric Medical Systems, Milwaukee, WI) and a standard birdcage head coil. There were 6 fMRI runs; during each run, 105 volumes were acquired. Each volume consisted of 40 slices with a thickness of 3 mm and a 0.5 mm gap to cover the entire brain. The time interval between two successive acquisitions of the same slice (TR: Repetition time) was 2500 ms, with an echo time (TE: Echo time) of 25 ms and a flip angle (FA) of 80°. The field of view was 192 × 192 mm and the matrix size was 64 × 64, giving voxel dimensions of 3 × 3 mm. Three-dimensional, inversion recovery-prepared spoiled gradient echo images (TR = 7.12 ms, TE = 3.06 ms, FA = 8°, matrix size = 256 × 256, slice thickness = 1 mm; in total, 166 transaxial images) were obtained as a high-resolution anatomical reference for each subject.
Imaging processing and statistical analysis
The first 5 volumes of each fMRI session were discarded because of unsteady magnetization, and the remaining 100 volumes per session were used for analysis. Image and statistical analyses were performed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab R2014a (Mathworks, Sherborn, MA). The images were realigned to correct for dislocations caused by head motion. The realigned images were normalized to the Montreal Neurological Institute (MNI) atlas (Shorvon et al., 1994). Finally, the anatomically normalized fMRI images were filtered using a Gaussian kernel with a full width at half-maximum of 8 mm in the x, y and z axes.
Functional connectivity analyses are affected by the head motion of participants during fMRI scanning (Müller et al., 2011; Power et al., 2012; Jung et al., 2014; Tyszka et al., 2014). To assess the effects of head motion and motion artefacts during functional connectivity analyses, the root mean square of six movement parameters obtained during the realignment process (x, y, z translations and x, y, z rotations), mean frame-to-frame root mean square motion (Van Dijk et al., 2012) and frame-wise displacement (FD) (Power et al., 2012) were calculated for each participant. There were no significant group differences in mean frame-to-frame root mean square motion (P > 0.05) or FD (P > 0.05).
After preprocessing, task-related activation was evaluated with the general linear model (Friston et al., 1995; Worsley and Friston, 1995). The design matrix for the single-subject analyses contained four task-related regressors (self-judgments for an autistic character, other judgments for an autistic character, self-judgments for a non-autistic character and other judgments for a non-autistic character). Regressors of interest (condition effects) were generated using a boxcar function, convolved with a hemodynamic response function. Regressors that were of no interest, such as the session effect and high-pass filtering (128 s), were also included to eliminate the low-frequency trend. To exclude the effects of head motion, motion regressors were included in single-subject models. Motion regressors based on realignment estimates were included as nuisance regressors during general linear model estimation.
In the second-level analysis, a three-way analysis of variance (ANOVA) with group (ASD or TD) as a between-subject factor and character (autistic or non-autistic) and judgment (self or other) as within-subject factors was performed for the mean response using the contrast images specified above. The analyses searched for brain regions showing a significant interaction between group and character. We employed a statistical threshold of P < 0.001 and a spatial extent of at least 10 voxels for these whole-brain analyses. Next, a correlation analysis was performed with the individual psychological measurements. We identified regions of interest (ROIs) as spheres with 12 mm radii centered on the maximal foci of activation, using an interaction contrast between group and character for the whole-brain analyses, and extracted the volume of images. We assessed the correlation between autistic traits (AQ scores) and ROIs as follows: correlations involving the vmPFC were based on the interaction between group and character.
Finally, we conducted functional connectivity analyses by a seed-driven approach with the ‘Conn toolbox’ software (Whitfield-Gabrieli and Nieto-Castanon, 2012). The toolbox removes confounding effects related to white matter or cerebrospinal fluid signal as well as motion parameters. It analyses the connectivity between one or multiple seed areas and the whole brain. We defined the vmPFC as a seed (the coordinates (4, 48, − 8) in MNI space). This location was defined by brain activation results based on the interaction between group and character in self- and other judgments. The main area of interest in connectivity analyses was the network between the vmPFC (4, 48, − 8) and other brain areas.
RESULTS
Behavioral results
We conducted a three-way ANOVA based on the number of ‘Yes’ responses with group (ASD or TD) as a between-subjects factor and character type (autistic or non-autistic) and judgments (self or other) as within-subject factors (Figure 2, Table 2). The behavioral results revealed a significant interaction between group and character (F(1, 28) = 23.58, P < 0.05, MSe (mean squared error) = 265.02, Prep = 0.99, = 0.46). Post hoc analyses showed that the ASD group gave Yes responses for autistic characters more than the TD group did (F(1, 28) = 14.60, P < 0.05, MSe = 185.40, Prep = 0.99, = 0.34), whereas the TD group gave Yes responses for non-autistic characters more than the ASD participants did (F(1, 28) = 29.76, P < 0.05, MSe = 120.05, Prep = 0.99, = 0.52).
Fig. 2.
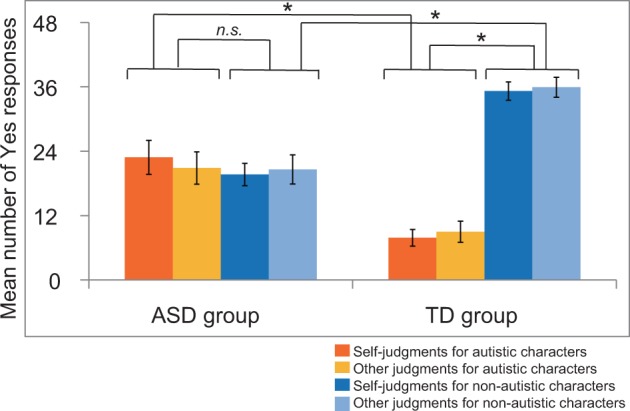
Behavioral results for self- and other judgments. The number of Yes responses is shown in ASD and TD groups. Because 48 trials were conducted under each condition, scores ranged between 0 and 48. Dark orange bars denote self-judgments for autistic characters; light orange bars denote other judgments for autistic characters; dark blue bars denote self-judgments for non-autistic characters; and light blue bars denote other judgments for non-autistic characters. Error bars indicate standard errors. *P < 0.05.
Table 2.
fMRI rating and reaction-time data
| Number of Yes responses |
Reaction times (ms) |
|||
|---|---|---|---|---|
| ASD group | TD group | ASD group | TD group | |
| Self-judgments for autistic characters | 22.9 (12.2) | 7.9 (6.0) | 3607.7 (501.9) | 3338.3 (709.1) |
| Other judgments for autistic characters | 20.9 (11.7) | 9.0 (7.6) | 3788.0 (585.2) | 3540.1 (677.1) |
| Self-judgments for non-autistic characters | 19.7 (8.0) | 35.2 (6.6) | 3714.1 (461.4) | 3426.1 (715.3) |
| Other judgments for non-autistic characters | 20.6 (10.5) | 35.9 (7.2) | 4047.2 (576.4) | 3696.1 (708.8) |
Note: Data are expressed as mean (s.d.).
The ASD group showed no significant difference in the frequency of Yes responses for autistic characters vs. non-autistic characters (F(1, 14) = 0.12, P > 0.05, MSe = 364.17, Prep = 0.60, = 0.01), whereas the TD group gave Yes responses more frequently for non-autistic characters than for autistic characters (F(1, 14) = 66.58, P < 0.05, MSe = 165.87, Prep = 0.99, = 0.83).
We conducted a three-way ANOVA based on reaction times with group (ASD or TD) as a between-subjects factor and character type (autistic or non-autistic) and judgments (self or other) as within-subject factors (Table 2). The main effect of group was not significant (F(1, 28) = 1.67, P > 0.05, MSe = 1 498 512.22, Prep = 0.81, = 0.06). The three-way interaction (F(1, 28) = 0.76, P > 0.05, MSe = 17 738.61, Prep = 0.73, = 0.03), the two-way interaction between group and character (F(1, 28) = 1.44, P > 0.05, MSe = 19 355.85, Prep = 0.80, = 0.05) and the two-way interaction between group and judgment (F(1, 28) = 0.15, P > 0.05, MSe = 21 739.98, Prep = 0.61, = 0.01) were not significant. However, the interaction between character and judgment was significant (F(1, 28) = 5.16, P < 0.05, MSe = 17 738.61, Prep = 0.94, = 0.16). Post hoc analyses showed that self-judgments for autistic characters were faster than self-judgments for non-autistic characters (F(1, 28) = 11.19, P < 0.05, MSe = 12 650.43, Prep = 0.98, = 0.29), and the other judgments for autistic characters were faster than other judgments for non-autistic characters (F(1, 28) = 26.44, P < 0.05, MSe = 24 444.03, Prep = 0.99, = 0.49). Post hoc analyses also showed that self-judgments were faster than other judgments for both autistic (F(1, 28) = 26.88, P < 0.05, MSe = 20 373.67, Prep = 0.99, = 0.49) and non-autistic (F(1, 28) = 71.38, P < 0.05, MSe = 19 104.91, Prep = 0.99, = 0.72) characters.
Brain activation results
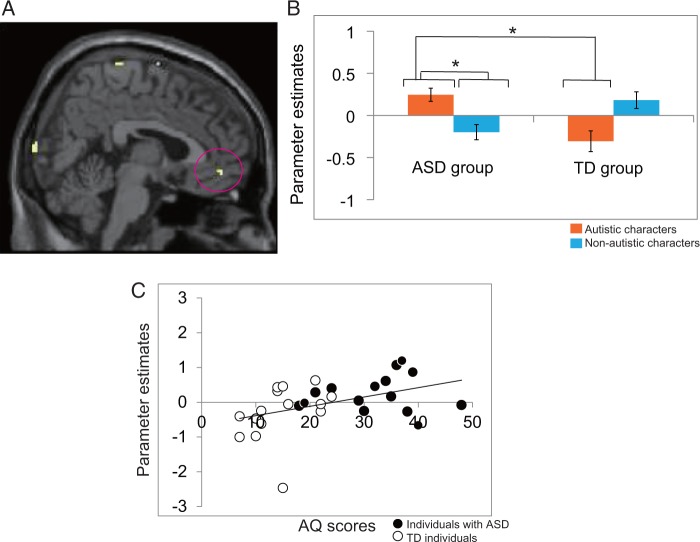
We investigated the brain activation associated with the interaction between group and character (Table 3). Results were thresholded at P < 0.001 (uncorrected) for a spatial extent of at least 10 voxels. The inferior frontal gyrus (IFG), postcentral gyrus, paracentral lobule, precuneus, cuneus, lingual gyrus, cerebellum, fusiform and superior frontal gyrus and vmPFC were activated in both groups when the ASD group judged characters with and the TD group judged characters without autistic traits (Figure 3A and B). Post hoc tests were performed on the parameter estimates.
Table 3.
Contrast tables and regions of activation for the interaction between group and character
| Region | BA | Cluster size | T | MNI coordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| IFG | 47 | 38 | 3.95 | −26 | 26 | −20 |
| Postcentral gyrus | 5 | 61 | 3.78 | −6 | −50 | 74 |
| Paracentral lobule | 4 | 61 | 3.30 | −6 | −40 | 76 |
| Precuneus | 7 | 61 | 3.25 | −8 | −66 | 64 |
| Cuneus | 18 | 14 | 3.56 | 4 | −100 | 10 |
| Lingual gyrus | 18 | 80 | 3.54 | 14 | −82 | −16 |
| Cerebellum | 80 | 3.52 | 20 | −76 | −20 | |
| Fusiform gyrus | 37 | 18 | 3.47 | 48 | −54 | −22 |
| Paracentral lobule | 6 | 11 | 3.41 | 2 | −34 | 76 |
| SFG | 6 | 14 | 3.41 | 0 | −2 | 74 |
| vmPFC | 10 | 10 | 3.39 | 4 | 48 | −8 |
BA = Brodmann area; SFG = superior frontal gyrus.
Self ASD = self-judgments for autistic characters; other ASD = other judgments for autistic characters; self non-ASD = self-judgments for non-autistic characters; other non-ASD = other judgments for non-autistic characters.
P < 0.001, uncorrected at the voxel level for a spatial extent of at least 10 voxels.
Fig. 3.
(A) Brain activation in self- and other judgments. P < 0.001, uncorrected at the voxel level for a spatial extent of at least 10 voxels. vmPFC (4, 48, −8) activation based on the interaction between group and character. (B) The mean for parameter estimates at the cluster denoting vmPFC activation based on the interaction between group and character (autistic characters for the ASD group and non-autistic characters for the TD group). Dark orange bars denote judgments for autistic characters; light blue bars denote judgments for non-autistic characters. Error bars indicate standard errors. *P < 0.05. (C) Plots of correlations (r = 0.43, P < 0.05) between AQ scores and vmPFC activation during judgments for autistic characters in the interaction between group and character. Black circles indicate individuals with ASD (n = 15); white circles indicate TD individuals (n = 15).
Functional connectivity results
In individuals with ASD as well as TD individuals, the vmPFC and other areas were activated during self-processing. However, this leaves the question of whether there are differences between individuals with ASD and TD individuals in terms of network connectivity in these brain areas. To address this, group differences in functional connectivity were assessed (Table 4). Results were thresholded at P < 0.001 (uncorrected) for a spatial extent of at least 10 voxels. Compared with TD participants, those with ASD showed greater functional connectivity between the vmPFC and anterior cingulate, the vmPFC and thalamus and the vmPFC and middle cingulate during autistic character judgments.
Table 4.
Seed to voxel functional connectivity analyses based on vmPFC as seed region
| Region | BA | Cluster size | T | MNI coordinates |
|||
|---|---|---|---|---|---|---|---|
| X | y | z | |||||
| ASD group > TD group | |||||||
| Self ASD and other ASD | Anterior cingulate | 32 | 86 | 5.50 | −14 | 20 | 34 |
| Thalamus | 20 | 4.10 | −10 | −2 | 6 | ||
| Middle cingulate | 24 | 12 | 3.83 | 12 | 8 | 36 | |
| Self non-ASD and | IFG | 47 | 54 | 4.27 | −30 | 38 | −10 |
| other non-ASD | Middle occipital | 19 | 24 | 4.01 | 36 | −70 | 8 |
| Claustrum | 16 | 3.93 | 30 | 14 | 6 | ||
| TD group > ASD group | |||||||
| Self ASD and other ASD | IFG | 45 | 235 | 5.06 | −46 | 24 | 20 |
| dlPFC | 46 | 235 | 4.63 | −44 | 32 | 20 | |
| IFG | 44 | 27 | 4.82 | 64 | 10 | 10 | |
| MFG | 6 | 62 | 4.57 | −26 | 12 | 68 | |
| MFG | 6 | 27 | 4.56 | 30 | 14 | 56 | |
| Cerebellum | 18 | 4.23 | −54 | −60 | −38 | ||
| IFG | 44 | 15 | 3.66 | −56 | 10 | 6 | |
| Self non-ASD and | IFG | 44 | 146 | 5.10 | −60 | 12 | 16 |
| other non-ASD | Precentral gyrus | 6 | 146 | 4.00 | −64 | 0 | 12 |
| dlPFC | 9 | 86 | 4.68 | 48 | 14 | 38 | |
| STG | 22 | 14 | 4.04 | −68 | −20 | 0 | |
| dmPFC | 8 | 12 | 3.93 | 2 | 28 | 44 | |
| MFG | 6 | 20 | 3.74 | 32 | 12 | 60 | |
| dlPFC | 46 | 13 | 3.68 | 46 | 28 | 24 | |
BA = Brodmann area; dlPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex; MFG = middle frontal gyrus.
Self ASD = self-judgments for autistic characters; other ASD = other judgments for autistic characters; self non-ASD = self-judgments for non-autistic characters; other non-ASD = other judgments for non-autistic characters.
P < 0.001, uncorrected at the voxel level for a spatial extent of at least 10 voxels.
In contrast, compared with ASD participants, TD participants showed greater functional connectivity between the vmPFC and IFG, the vmPFC and precentral gyrus, the vmPFC and dorsolateral prefrontal cortex, the vmPFC and superior temporal gyrus (STG), the vmPFC and dorsomedial prefrontal cortex and the vmPFC and middle frontal gyrus during non-autistic character judgments.
DISCUSSION
Rating data for self- and other judgments
According to the behavioral results, the ASD group provided Yes responses for autistic characters more than the TD group did, whereas the TD group provided Yes responses for non-autistic characters more than the ASD group did. Thus, ASD and TD groups responded with affirmative answers to characters similar to themselves. However, the ASD group did not answer Yes for autistic characters more frequently than they did for non-autistic characters, whereas the TD group answered Yes for non-autistic characters more frequently than they did for autistic characters.
Subjective measurement may not be suitable for individuals with ASD. SRS can provide ratings from parents, teachers, spouses, other relatives or friends; it is difficult for individuals with ASD to monitor themselves using self-report scales.2 These results reflect a relative lack of self-awareness in ASD (Toichi et al., 2002). For example, children with ASD exhibit less self-consciousness; furthermore, autobiographical memories, which are experienced by the self, are remembered less well compared with events happening to others (Millward et al., 2000; Bruck et al., 2007; Lind, 2010; Williams and Happé, 2010). Additionally, the self-reference effect in memory is reduced in adults with ASD (Toichi et al., 2002; Lombardo et al., 2007). Taken together, these findings indicate that the strong differentiation observed in the TD group and the total lack of differentiation observed in the ASD group are both well supported.
We found no significant differences between the ASD and TD groups with regard to reaction times, and reaction times for other judgments were longer than those for self-judgments in both groups. Thus, for both groups, the cognitive load was greater for other judgments than for self-judgments. Our similarity hypothesis was not supported by the behavioral data in that selective responses toward similar targets were not observed.
Interaction between group and character
To address the question of whether individuals with ASD show specific responses for others with ASD, the interaction between group (with or without ASD) and character (autistic or non-autistic) must be examined.3 The two-way interaction (group × character) was evaluated. According to the activation data for the interaction between group and character, the vmPFC was activated in the ASD and TD groups during self- and other judgments when the ASD group judged characters with autistic traits and the TD group judged characters without autistic traits. The findings of this study suggest that both individuals with ASD and TD individuals make selective neural responses toward others who are similar to themselves. Although individuals with ASD showed a relative lack of self-consciousness in their explicit subjective ratings, the selective activation in response to similar others with ASD reflected in the brain imaging data may suggest an implicit identification with similar others.
According to previous studies with ASD and TD participants, the vmPFC distinguished between self- and other evaluations in TD adults but not in individuals with ASD (Lombardo et al., 2010a; Pfeifer et al., 2013). However, these previous studies used a fictional character (Harry Potter) as the other target, and this character was not similar to the participants. In this study, the vmPFC activations in both ASD and TD groups were significantly greater when judging matched (autistic characters for individuals with ASD and non-autistic characters for TD individuals) than mismatched targets (autistic characters for TD individuals and non-autistic characters for individuals with ASD). Thus, individuals with ASD did not have vmPFC dysfunction in terms of the ability to distinguish between the self and another person, and the vmPFC seemed to underpin the ability to make distinctions between ASD and TD targets.
Another previous study also found that vmPFC activation did not distinguish between self- and other judgments in adults with ASD (Kennedy and Courchesne, 2008), although the ‘other’ used in this design was someone with whom participants were likely to be very close: their mother. The vmPFC activation is related to processing similar others (Schmitz et al., 2004; Ochsner et al., 2005; Jenkins et al., 2008; Chen et al., 2010; Krienen et al., 2010), which is consistent with our finding that the vmPFC was activated in response to the self and similar others in both the TD group and the ASD group.
It is important to note that the IFG, postcentral gyrus, paracentral lobule, precuneus, cuneus, lingual gyrus, cerebellum, fusiform and SFG, as well as vmPFC were activated during this study. All these areas were larger clusters than the vmPFC. Although this study focused on the vmPFC funtion, future studies need to further investigate the functions of these areas.
Differences in connectivity
According to the functional connectivity results, frontal–posterior connectivity (Just et al., 2012) was observed in TD individuals, but not in individuals with ASD, during similar judgments (autistic judgments in the ASD group and non-autistic judgments in the TD group). The present findings reflect differences in the type of brain connectivity exhibited by the ASD and TD groups. Although the activation results for both groups revealed that the vmPFC was activated in response to similar others, the functional connectivity results reflected a specific network in each group, i.e. responses in individuals with ASD toward autistic characters and responses in TD individuals toward non-autistic characters. Although empathic responses in TD individuals are based on collaboration between frontal (the vmPFC as the area for self-representation) and posterior (the STG; Wernicke’s area for language processing) areas, empathic responses in individuals with ASD are based on collaboration within frontal areas.
Several previous studies have demonstrated a lack of empathy and deficits in self-related representation, in ASD (Greimel et al., 2010; Lombardo et al., 2010; Schulte-Rüther et al., 2011). However, previous studies also showed that it was difficult for those with ASD to assume the perspectives of TD others. To our knowledge, few studies have employed characters with and without autistic traits as target stimuli (Komeda et al., 2013a). It is difficult for TD individuals to understand others who are dissimilar from themselves (Komeda et al., 2013b). Thus, it is not surprising that it is also difficult for those with ASD to understand TD individuals. This is the first empirical study to investigate the empathy of ASD individuals for others with autistic traits (for a review, Dern, 2008). Individuals with ASD are likely to empathize with other people with ASD. Empathy varies as a function of similarity between participants and characters (Komeda et al., 2013b). Individuals with ASD and TD individuals with high levels of autistic traits, even if they have not been diagnosed with ASD, are likely to better understand others with autism. Interestingly, even when characters had autistic traits, individuals with ASD seemingly did not use the frontal–posterior network during self- and other judgments.
CONCLUSION
Individuals with ASD do not lack empathy toward others who are similar to themselves, just as TD individuals respond selectively to others who are similar than to those who are dissimilar. In contrast, the neural mechanisms underlying the responses of those with ASD to others differ from those underlying this process in TD individuals. Individuals with ASD do not employ the frontal–posterior network during cognitions pertaining to the behavior of other ASD individuals.
Because these findings explain the characteristics of individuals with ASD, they may also contribute to improving special needs education, educational interventions and developmental support for individuals with autism (Åsberg, 2010; Åsberg and Sandberg, 2010). In terms of clinical implications, the present findings suggest that people with ASD characteristics would be able to help people with ASD. In terms of education, this study carries implications for the development of curricula for special needs classes.
Conflict of Interest
None declared.
Acknowledgments
We express our sincere appreciation to the participants and their families, who generously contributed their time and had the courage to participate in this research. We are also grateful to Taisuke Shimada for data collection. This research was funded, in part, by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (26118505, 25293248, 21791120, 26590145) and by the Takeda Science Foundation. Part of this research is the result of ‘Integrated Research on Neuropsychiatric Disorders’ carried out under the Strategic Research Program for Brain Sciences by the MEXT of Japan. H.K.’s contribution was supported by a JSPS Postdoctoral Fellowship for Research Abroad.
Footnotes
1 Effects on performance IQ were not controlled in the subsequent analyses, as we found no significant correlation between performance IQs and parameter estimates for ROIs, including the vmPFC (r = −.27, P > 0.05).
2 Adults can also rate themselves using the optional Adult (self-report) Form in SRS 2 (Constantino and Gruber, 2012).
3 The regressors include both Yes and No responses. We conducted analyses based on participants’ individual responses. However, vmPFC activation was not observed because subjective measurement was not suitable for individuals with ASD.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) 4th edn. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Åsberg J. Patterns of language and discourse comprehension skills in school-aged children with autism spectrum disorders. Scandinavian Journal of Psychology. 2010;51:534–39. doi: 10.1111/j.1467-9450.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- Åsberg J, Sandberg AD. Discourse comprehension intervention for high-functioning students with autism spectrum disorders: preliminary findings from a school-based study. Journal of Research in Special Educational Needs. 2010;10:91–8. [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright SA, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in the insula are modulated by levels of alexithymia but not autism. Brain. 2010;133:1515–25. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck M, London K, Landa R, Goodman J. Autobiographical memory and suggestibility in children with autism spectrum disorder. Development and Psychopathology. 2007;19:73–95. doi: 10.1017/S0954579407070058. [DOI] [PubMed] [Google Scholar]
- Castelli F, Happé F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Chen AC, Welsh RC, Liberzon I, Taylor SF. “Do I like this person?” A network analysis of midline cortex during a social preference task. Neuroimage. 2010;51:930–39. doi: 10.1016/j.neuroimage.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. In: Social Responsiveness Scale, Second Edition (SRS-2) 2nd edn. Torrance CA, editor. Western Psychological Services; 2012. [Google Scholar]
- Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biological Psychiatry. 2005;57:655–60. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Dern S. Autistic intelligence, autistic perception and autistic patterns of thought that we all share in different degrees—an update. 2008 Available: http://www.awares.org/conferences/show_paper.asp?section=000100010001&conferenceCode=000200100012&id=191, Accessed August 23 2014. [Google Scholar]
- Ferstl EC, von Cramon DY. What does the frontomedian cortex contribute to language processing: coherence or theory of mind? Neuroimage. 2002;17:1599–612. doi: 10.1006/nimg.2002.1247. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC. Other minds in the brain: a functional imaging study of ‘‘theory of mind’’ in story comprehension. Cognition. 1995;57:109–21. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, et al. Analysis of fMRI time series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–19. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the Enigma. 2nd edn. Oxford: Blackwell; 2003. [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–4. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Jack AI, Roepstorff A, Frith CD. Imaging the intentional stance in a competitive game. Neuroimage. 2002;16:814–21. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- Greimel E, Schulte-Rüther M, Kircher T, et al. Neural mechanisms of empathy in adolescents with autism spectrum disorder and their fathers. Neuroimage. 2010;49:1055–65. doi: 10.1016/j.neuroimage.2009.07.057. [DOI] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D’Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage. 2004;21:1639–51. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Macrae CN, Mitchell JP. Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4507–12. doi: 10.1073/pnas.0708785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Kosaka H, Saito DN, et al. Default mode network in young male adults with autism spectrum disorder: relationship with autism spectrum traits. Molecular Autism. 2014;5:35. doi: 10.1186/2040-2392-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neuroscience and Biobehavioral Reviews. 2012;36:1292–313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y, Inada N, Moriwaki A, et al. Quantitative autistic traits ascertained in a national survey of 22 529 Japanese school children. Acta Psychiatrica Scandinavica. 2013;128:45–53. doi: 10.1111/acps.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y, Tsujii H, Inada N, et al. Validation of the Japanese version of the social responsiveness scale:comparison with PDD-Autism Society Japan Rating Scales (PARS) Seishin Igaku. 2009;51:1101–9. [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–93. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal–posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social Neuroscience. 2009;4:135–52. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Social Cognitive and Affective Neuroscience. 2008;3:177–90. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda H, Kawasaki M, Tsunemi K, Kusumi T. Differences between estimating protagonists’ emotions and evaluating readers’ emotions in narrative comprehension. Cognition & Emotion. 2009;23:135–51. [Google Scholar]
- Komeda H, Kosaka H, Saito DN, et al. Episodic memory retrieval for story characters in high-functioning autism. Molecular Autism. 2013a;4:20. doi: 10.1186/2040-2392-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda H, Tsunemi K, Inohara K, Kusumi T, Rapp DN. Beyond disposition: the processing consequences of explicit and implicit invocations of empathy. Acta Psychologica. 2013b;142:349–55. doi: 10.1016/j.actpsy.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Tu P-C, Buckner RL. Clan mentality: evidence that the medial prefrontal cortex responds to close others. Journal of Neuroscience. 2010;30:13906–15. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind SE. Memory and the self in autism: a review and theoretical framework. Autism. 2010;14:430–56. doi: 10.1177/1362361309358700. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PLoS One. 2007;2:e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, et al. Atypical neural self-representation in autism. Brain. 2010;133:611–24. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–80. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Constable RT. Biphasic hemodynamic responses influence deactivation and may mask activation in block-design fMRI paradigms. Human Brain Mapping. 2008;29:385–99. doi: 10.1002/hbm.20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward C, Powell S, Messer D, Jordan R. Recall for self and other in autism: children’s memory for events experienced by themselves and their peers. Journal of Autism and Developmental Disorders. 2000;30:15–28. doi: 10.1023/a:1005455926727. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Cloutier J, Banaji MR, Macrae CN. Medial prefrontal dissociations during processing of trait diagnostic and nondiagnostic person information. Social Cognitive and Affective Neuroscience. 2006a;1:49–55. doi: 10.1093/scan/nsl007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006b;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Liu Y, Williams DL, Keller TA, Minshew NJ, Just MA. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. 2011;134:2422–35. doi: 10.1093/brain/awr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Kosaka H, Saito DN, et al. Emotional responses associated with self-face processing in individuals with autism spectrum disorders: an fMRI study. Social Neuroscience. 2012;7:37–41. doi: 10.1080/17470919.2011.598945. [DOI] [PubMed] [Google Scholar]
- Müller R, Shih P, Keehn B. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cerebral Cortex. 2011;21:2233–43. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nygren G, Hagberg B, Billstedt E, Skoglund A, Gillberg C, Johansson M. The Swedish version of the Diagnostic Interview for Social and Communication Disorders (DISCO-10). Psychometric properties. Journal of Autism and Developmental Disorders. 2009;39:730–41. doi: 10.1007/s10803-008-0678-z. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Merchant JS, Colich NL, Hernandez LM, Rudie JD, Dapretto M. Neural and behavioral responses during self-evaluative processes differ in youth with and without autism. Journal of Autism and Developmental Disorders. 2013;43:272–85. doi: 10.1007/s10803-012-1563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self-reflection in individual subjects. Social Cognitive and Affective Neuroscience. 2006;1:229–34. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22:941–7. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M, Greimel E, Markowitsch HJ, Kamp-Becker I, Remschmidt H, Fink GR, et al. Dysfunctions in brain networks supporting empathy: anfMRI study in adults with autism spectrum disorders. Social Neuroscience. 2011;6:1–21. doi: 10.1080/17470911003708032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17:18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shorvon SD, Fish DR, Andermann F, Bydder GM, Stefan H. Magnetic Resonance Scanning and Epilepsy. New York: Plenum Press; 1994. [Google Scholar]
- Tamir DI, Mitchell JP. Neural correlates of anchoring-and-adjustment during mentalizing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10827–32. doi: 10.1073/pnas.1003242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toichi M, Kamio Y, Okada T, et al. A lack of self-consciousness in autism. American Journal of Psychiatry. 2002;159:1422–4. doi: 10.1176/appi.ajp.159.8.1422. [DOI] [PubMed] [Google Scholar]
- Tyszka JM, Kennedy DP, Paul LK, Adolphs R. Largely typical patterns of resting-state functional connectivity in high-functioning adults with autism. Cerebral Cortex. 2014;24:1894–905. doi: 10.1093/cercor/bht040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–38. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, et al. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–81. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Völlm BA, Taylor AN, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–8. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2:125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Williams D, Happé F. Recognising ‘social’ and ‘nonsocial’ emotions in self and others: a study of autism. Autism: The International Journal of Research and Practice. 2010;14:285–304. doi: 10.1177/1362361309344849. [DOI] [PubMed] [Google Scholar]
- Wing L, Leekam SR, Libby SJ, Gould J, Larcombe M. The Diagnostic Interview for Social and Communication Disorders: background, inter-rater reliability and clinical use. Journal of Child Psychology and Psychiatry. 2002;43:307–25. doi: 10.1111/1469-7610.00023. [DOI] [PubMed] [Google Scholar]
- Worsley K, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]