Abstract
Purpose
The value of insulin-like growth factor 1 receptor (IGF-1R) for predicting survival of patients with breast cancer remains controversial. The purpose of this study was to perform a meta-analysis of the published data to attempt to clarify the impact of IGF-1R.
Methods
Studies published between January 1, 1990 and October 1, 2014 were identified using an electronic search to aggregate the available survival results. Studies were included if they reported detecting IGF-1R expression in the primary breast cancer and analyzed patient survival data according to IGF-1R status. The principal outcome measures were hazard ratios (HRs) for survival of IGF-1R-positive patients. Combined HRs and 95% confidence intervals (CIs) were estimated using fixed- or random-effects models according to between-study heterogeneity.
Results
Ten studies, involving 5,406 patients, satisfied our inclusion criteria. Data from five studies provided the impact of IGF-1R on overall survival (OS), three studies the impact on breast cancer-specific survival (BCSS), and seven studies the impact on disease-free survival (DFS). The results of meta-analysis showed that for DFS, membranous IGF-1R positivity was not a significant predictor. The combined HR for OS/BCSS was 0.63 (95% CI: 0.42–0.95, P=0.03), indicating that membranous IGF-1R positivity was a significant predictor of better survival. IGF-1R cytoplasmic positivity was significantly associated with longer DFS and OS/BCSS (combined HR: 0.56, 95% CI: 0.35–0.89, P=0.01; combined HR: 0.55, 95% CI: 0.35–0.85, P=0.008, respectively). The results of subgroup analysis suggested that membranous IGF-1R positivity in hormone-receptor-positive breast cancer was correlated with favorable DFS (combined HR: 0.61, 95% CI: 0.41–0.92, P=0.02) and OS/BCSS (combined HR: 0.73, 95% CI: 0.57–0.93, P=0.01). Membranous IGF-1R positivity in triple-negative breast cancer predicted worse DFS (combined HR: 1.86, 95% CI: 1.03–3.34, P=0.04). Membranous IGF-1R positivity in Her-2-positive or ER (estrogen receptor)-negative breast cancer was not found to be a significant prognostic indicator.
Conclusion
The results of this meta-analysis suggest that IGF-1R expression has different prognostic values for patients with breast cancers of different molecular subtypes. It was a favorable prognostic indicator in unselected breast cancers and hormone-receptor-positive cancers, but indicated poor survival in triple-negative breast cancers.
Keywords: IGF-1R, breast cancer, meta-analysis, prognosis, hazard ratio, overall survival, disease-free survival
Introduction
The World Health Organization GLOBOCAN project has reported that breast cancer is the most frequently diagnosed cancer in females, as well as the leading cause of cancer death.1 Breast cancer is currently classified into five molecular subtypes based on the expression of estrogen receptor (ER), progesterone receptor (PR), Her-2, and Ki67. Different combinations of therapeutic modalities have been utilized for the treatment of breast cancer based on the clinical staging and the molecular subtype. However, it is heartbreaking that worldwide more than 400,000 people still die yearly from breast cancer.2 The identification and validation of additional prognostic factors have the potential to improve the quality of individualized treatments for breast cancer patients.
Insulin-like growth factor 1 receptor (IGF-1R) is overexpressed in a variety of cancers, especially breast cancer.3 It has attracted increasing attention because of its role in enhancing cancer progression. IGF-1R has been investigated as a potential target for novel anticancer therapeutics.4 It has been detected in 50%–93% of patients with breast cancer;5–8 however, the prognostic value of IGF-1R for breast cancer remains controversial.8,9 To clarify the value of IGF-1R expression in breast cancer currently requires a combined analysis of the published data. In our study, we combined all eligible studies on the relationship of IGF-1R expression in breast cancers with disease-free survival (DFS) and/or overall survival (OS)/breast cancer-specific survival (BCSS). We aimed to obtain a precise conclusion on the relationship between IGF-1R expression and the outcomes of breast cancer patients.
Emerging experimental and clinical data show that IGF-1R interacts with ER/PR and Her-2 signaling pathways.10 To evaluate the association and interaction of ER/PR and Her-2 with IGF-1R at the clinical level, we performed additional subgroup analysis of the prognostic value of IGF-1R expression in different molecular subtypes of breast cancer.
Materials and methods
Search strategy
We searched the PubMed, Embase, and Web of Science databases using the keywords “breast cancer”, “type 1 insulin-like growth factor receptor”, “insulin-like growth factor 1 receptor”, “IGF-1R”, and “prognosis” to search for studies published between January 1, 1990 and October 1, 2014. The titles and abstracts of papers were first scanned to exclude irrelevant studies. The studies were finally chosen for inclusion by reading the complete text of the remaining papers. The bibliographies of all eligible papers were checked for other potentially relevant publications.
Selection criteria
The studies from the database were selected using the following criteria: 1) the expression of IGF-1R protein was assessed by immunohistochemistry or radioimmunoassay; 2) the study was published in English; 3) the study was limited to research on human primary breast cancer; 4) the study patients were female; 5) the end point of the study was DFS, OS, or BCSS; 6) the study provided the hazard ratios (HRs) and 95% confidence intervals (CIs), or data that could be used to calculate the HRs and 95% CIs, or Kaplan–Meier survival curves that provided sufficient data to extract HRs and 95% CIs.
Data extraction
Two reviewers, Yan SC and Jiao X, independently searched and assessed the studies, and the inclusion of a study was reached by consensus. The following items were recorded from each study: the first author’s name, year of publication, number of patients, assessment methods for IGF-1R expression, methods of estimating HRs, and HR values with 95% CIs. The studies were assessed for quality using REMARK (reporting recommendations for tumor MARKer prognostic studies)11 and the 18 items for reporting study quality, as defined by Chen et al.12
Statistical analysis
HRs with 95% CIs were combined to determine the effective value. Data, including Kaplan–Meier survival curves, from studies not directly reporting HRs and 95% CIs were used to calculate the HRs and 95% CIs according to the methods described by Parmar et al13 and Tierney et al.14 By convention, an observed HR of <1 implied better survival for IGF-1R-positive tumors. The χ2-square test was used to assess heterogeneity. A P-value less than 0.05 was considered significant. If the test of heterogeneity was significant, a combined HR was calculated using the random-effects model; otherwise, the fixed-effects model was used. Engauge Digitizer version 2.11 (free software downloaded from http://sourceforge.net) was used to extract data from Kaplan–Meier curves. All statistical tests, except those for publication bias, were performed using RevMan version 5.2 (free software downloaded from http://www.cochrane.org). Begg and Egger tests were used to assess publication bias, and these tests were performed using Stata SE 12.0 (StataCorp LP, College Station, TX, USA).
Results
Description of studies
A total of 345 published studies were identified. Scanning identified 325 unsuitable papers because they were case reports, review papers, laboratory studies, on male breast cancer, or were otherwise irrelevant to this study. Of the remaining 20 papers, 4 did not provide survival data; 2 did not provide HRs, and the provided survival data was not sufficient for calculating HRs; and 4 papers detected IGF-1R expression using polymerase chain reaction (PCR). Finally, there were ten studies published between 1990 and 2014 that satisfied the criteria for our meta-analysis5,8,9,15–21 (Figure 1). As shown in Table 1, two studies detected IGF-1R protein using radioimmunoassay, and eight studies detected IGF-1R protein using immunohistochemistry. In addition, when immunohistochemistry was used, most investigators defined IGF-1R positivity as membranous staining. However, three studies investigated positive membranous and cytoplasmic staining separately (Table 1). Three other reports did not specify if membranous or cytoplasmic staining for IGF-1R was considered positive. We queried the authors for their criteria for IGF-1R positivity. Two authors replied and described their criteria,17,20 and 1 did not.9 All of the ten eligible studies were retrospective. Table 1 lists the characteristics of these studies. The number of patients ranged from 72 to 2,871, with a total number of 5,406.
Figure 1.
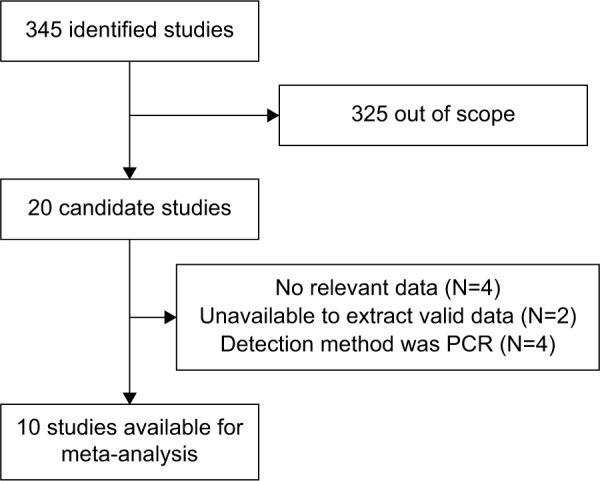
Flow diagram of study selection.
Abbreviations: N, number of studies; PCR, polymerase chain reaction.
Table 1.
Characteristics of the ten studies included in the meta-analysis of the prognostic value of insulin-like growth factor 1 receptor in breast cancers
| Reference | No of patients | Patients source | Technique | Cutoff survival | HR estimation | HR (95% CI) of OS/BCSS | HR (95% CI) of DFS |
|---|---|---|---|---|---|---|---|
| Bonneterre et al5 | 297 | France | RIA | ≥1% Mem | Survival curve | OS: 0.29 (0.17–0.49) | 0.29 (0.18–0.47) |
| Railo et al8 | 126 | Finland | RIA | ≥4% Mem | Survival curve | No | 1.86 (0.79–4.38) |
| Ueda et al15 | 150 | Japan | IHC | ≥10% weak or moderate complete Mem staining | Given by author | No | 0.52 (0.23–1.18) |
| Köstler et al16 | 72 | Austria | IHC | At least moderate Mem and/or Cyto staining | Given by author | OS: Mem: 0.76 (0.37–1.54) Cyto: 0.82 (0.42–1.60) |
No |
| Law et al17 | 112 | Canada | IHC | At least moderate Mem staining | Survival curve | OS: 1.47 (0.81–2.67) | No |
| Kim et al9 | 283 | South Korea | IHC | ≥26% (no mention) | Given by author | OS: 0.22 (0.06–0.79) | 0.46 (0.23–0.91) |
| Fu et al18 | 297 | Japan | IHC | ≥10% Mem and/or Cyto staining | Given by author | BCSS: Mem: 0.36 (0.06–2.05) Cyto: 0.49 (0.14–1.74) |
Mem: 0.47 (0.19–1.14) Cyto: 0.48 (0.16–1.14) |
| Hartog et al19 | 368 | The Netherlands | IHC | >10% Mem and/or Cyto staining | Given by author | BCSS: Mem: 1.19 (0.61–2.34) Cyto: 0.38 (0.20–0.74) |
Mem: 1.42 (0.08, 2.49) Cyto: 0.61 (0.34–1.09) |
| Yerushalmi et al20 | 2,871 | Canada | IHC | Strong Mem staining | Survival curve | BCSS: 0.50 (0.40–0.63) | No |
| Shin et al21 | 968 | South Korea | IHC | >10% Mem staining | Survival curve | OS: 0.78 (0.50–1.21) | 1.07 (0.74–1.55) |
Abbreviations: BCSS, breast cancer-specific survival; Cyto, cytoplasmic; CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; IHC, immunohistochemistry; Mem, membranous; OS, overall survival; RIA, radioimmunoassay.
Impact of IGF-1R on the outcome of patients with unselected breast cancer
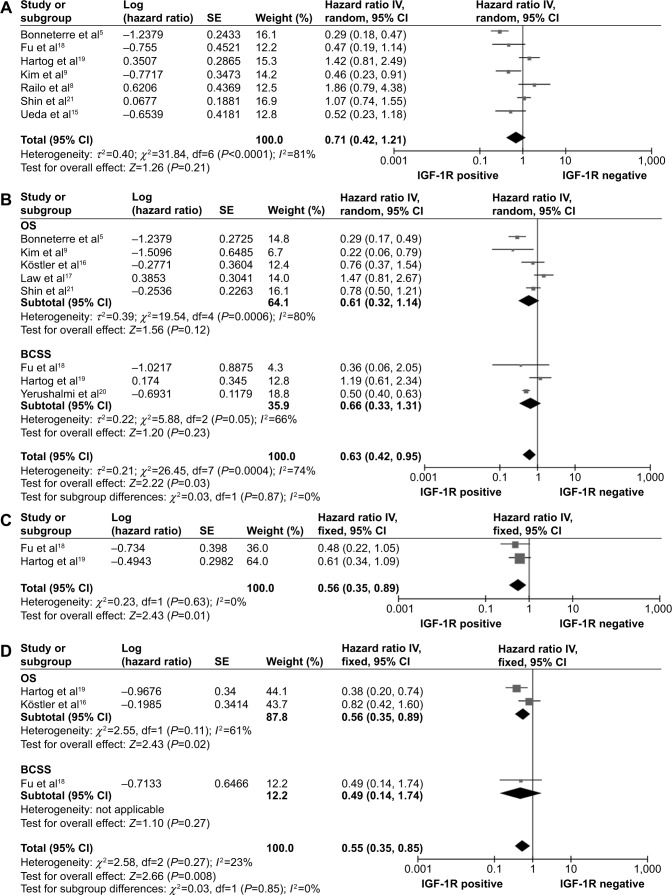
Seven studies investigated DFS for a total of 2,489 unselected breast cancer cases.5,8,9,15,18,19,21 Because of significant heterogeneity among the studies (P<0.0001; I2=81%), a random-effects model was used. The risk of membranous IGF-1R positivity in breast cancers was not statistically significant (combined HR: 0.71, 95% CI: 0.42–1.21; P=0.21) (Figure 2A). Eight studies, with a total of 5,196 cases, were evaluated for the effect of membranous IGF-1R expression on OS/BCSS (Figure 2B).5,9,16–21 A random-effects model was used to combine HRs because of the heterogeneity observed among the studies (P=0.0004; I2=74%). The combined HR of OS was 0.61 (95% CI, 0.32–1.14; P=0.12); the combined HR of BCSS was 0.66 (95% CI, 0.33–1.31; P=0.23); and the combined HR of OS/CSS was 0.63 (95% CI, 0.42–0.95; P=0.03). The results demonstrated that membranous IGF-1R positivity was a favorable prognostic indicator in breast cancer patients.
Figure 2.
Forest plot of hazard ratios (HRs) for disease-free survival (DFS) (A) and overall survival (OS)/breast cancer-specific survival (BCSS) (B) based on membranous insulin-like growth factor 1 receptor (IGF-1R) positivity, and DFS (C) and OS/BCSS (D) based on cytoplasmic IGF-1R positivity in the ten studies.
Three studies assessed membranous positivity and cytoplasmic positivity separately.16,18,19 A subcategory meta-analysis was performed to analyze the data on cytoplasmic IGF-1R expression. Two studies provided DFS data on 665 cases.18,19 Between-study heterogeneity was not significant (P=0.63, I2=0%); therefore, a fixed-effects model was used for analysis. Cytoplasmic IGF-1R positivity was associated with favorable DFS (combined HR: 0.56, 95% CI: 0.35–0.89; P=0.01) (Figure 2C). Three studies provided OS or BCSS data on 737 cases,16,18,19 and the meta-analysis showed that cytoplasmic IGF-1R positivity was associated with favorable OS/BCSS (combined HR: 0.55, 95% CI: 0.35–0.85; P=0.008) (Figure 2D).
Impact of IGF-1R on the outcome of patients with hormone-receptor-positive breast cancer
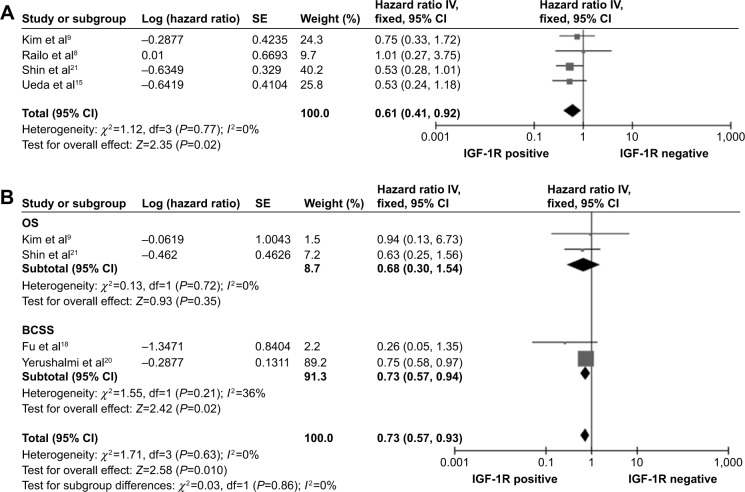
A subcategory meta-analysis was performed to analyze four eligible studies that contained ER- and/or PR-positive subgroups. The four subgroups in these studies provided DFS data on 863 cases with hormone-receptor-positive breast cancer.8,9,15,21 A fixed-effects model was used to combine the HR values (P=0.77, I2=0%). The results showed that IGF-1R positivity was significantly associated with favorable DFS (combined HR: 0.61, 95% CI: 0.41–0.92, P=0.02) (Figure 3A). Four subgroups in these studies provided data on OS/BCSS in 3,241 cases with ER- and/or PR-positive breast cancer.9,18,20,21 There was no heterogeneity (P=0.63, I2=0%) among these subgroups, and a fixed-effects model was used. The results showed that IGF-IR positivity was significantly associated with favorable OS/BCSS (combined HR: 0.73, 95% CI: 0.57–0.93, P=0.01) (Figure 3B).
Figure 3.
Forest plots of hazard ratios for disease-free survival (A) and overall survival (OS)/breast cancer-specific survival (BCSS) (B) of patients with hormone-receptor-positive breast cancers.
Impact of IGF-1R on the outcome of patients with ER-negative, triple-negative, and Her-2-positive breast cancer
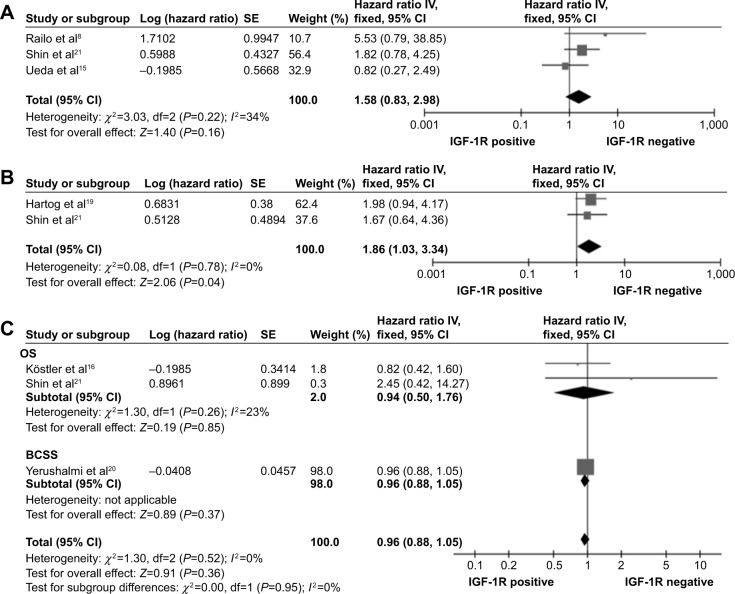
There were three studies that provided DFS data on 435 ER-negative cases.8,15,21 There was no heterogeneity (P=0.22, I2=34%), and the fixed-effects model was used. IGF-1R positivity was associated with a poor DFS, but the risk was not statistically significant (combined HR: 1.58, 95% CI: 0.83–2.98; P=0.22) (Figure 4A). Two studies provided DFS data on 337 triple-negative breast cancer (TNBC) cases.19,21 There was no heterogeneity (P=0.78, I2=0%), and the fixed-effects model was used. The results showed that IGF-1R positivity was significantly associated with poor DFS (combined HR: 1.86, 95% CI: 1.03–3.34; P=0.04) (Figure 4B). There were three studies that provided OS/BCSS data on 614 Her-2-positive cases.16,20,21 There was no heterogeneity (P=0.95, I2=0%), and the fixed-effects model was used. The combined HR was 0.96 (95% CI: 0.88–1.05; P=0.36) (Figure 4C), suggesting that IGF-1R positivity was not significant in Her-2 positive breast cancer.
Figure 4.
Forest plots of hazard ratios for disease-free survival (DFS) of patients with ER-negative breast cancers (A), for DFS of patients with triple-negative breast cancers (B), and for overall survival (OS)/breast cancer-specific survival (BCSS) of patients with Her-2-positive breast cancers (C).
Publication bias
Seven studies evaluating DFS yielded Begg and Egger P-values of 1.00 and 0.235, respectively. Figure 5A shows a Begg funnel plot. Eight studies evaluating OS/BCSS yielded Begg and Egger P-values of 0.089 and 0.209, respectively. Figure 5B shows a Begg funnel plot. Moreover, publication bias was not detected in subgroup meta-analyses.
Figure 5.
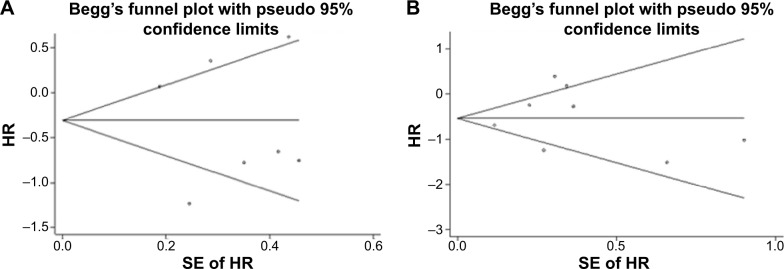
Funnel plots for the disease-free survival (A) and overall survival (OS)/breast cancer-specific survival (BCSS) (B) of unspecified breast cancers.
Abbreviations: SE, standard error; HR, hazard ratio.
Discussion
IGF-1R is a transmembrane tyrosine kinase receptor that is activated by binding with IGF Types 1 and 2.22 Activated IGF-1R has several effects on cell behavior. Signals originating from IGF-1R are involved in regulating cell proliferation, survival, differentiation, and transformation.23 IGF-1R is commonly expressed in primary breast cancers. Nielsen et al24 studied a cohort of 930 primary breast cancer patients and found that IGF-1R was expressed in 87% of the cases, but the prognostic significance of IGF-1R expression was unclear. In our meta-analysis, we compared the survival outcomes of breast cancer patients according to IGF-1R protein expression. The results showed that membranous IGF-1R positivity in breast cancers was a favorable prognostic indicator, with statistical significance for OS/BCSS (HR=0.63, 95% CI: 0.42–0.95, P=0.03); but it was not conclusive for DFS. However, OS is the most widely used end point in oncology trials. Additional meta-analysis was performed to evaluate the survival data with regard to cytoplasmic IGF-1R status. The HRs were found to be statistically significant for both OS/BCSS and DFS. Our data on cytoplasmic and membranous IGF-1R positivity predict favorable outcomes of patients with unselected breast cancer.
Breast cancer has been divided into five molecular subtypes according to the status of ER, PR, Her-2, and Ki67.25 In vitro and in vivo data have shown that IGF-1R is involved in regulating the ER/PR signaling pathway in breast cancer.10 It was reported that IGF-1R and ER synergistically enhance the proliferation of cancer cells,26 but IGF-1R activation fails to induce mitogenesis in the absence of ER.27,28 The strong connection between IGF-1R, ER, and PR implies that IGF-1R may particularly affect the outcome of hormone-receptor-positive patients. Four studies in our meta-analysis provided survival data on hormone-receptor-positive subgroups. The pooled HRs showed that IGF-1R positivity was a favorable prognostic indicator in hormone-receptor-positive breast cancers. Furthermore, several clinical studies have shown correlations between IGF-1R expression, ER expression, and low-grade malignancy. These results contradict the in vitro results showing that IGF-1R can enhance the proliferation of breast cancer cells. It has been speculated that the prognostic significance of IGF-1R expression is similar to that of ER.29 Although ER can provide survival signals for ER-positive breast cancer cells, its expression generally indicates a favorable outcome. Tamoxifen, which blocks ERs, has been effective for the treatment of premenopausal hormone-receptor-positive breast cancer. IGF-1R expression also reflects a relatively well differentiated tumor that may grow in an IGF-1R-dependent manner. By analogy with ER, IGF-1R may be a valuable target. It can be speculated that ER and PR are the predominant targets, and IGF-1R is a supplementary target for breast cancer.
It has been reported that signals from IGF-1R lead to opposite effects on cell proliferation in ER-negative tumors vs ER-positive tumors.27–29 The prognostic significance of IGF-1R for ER-negative breast cancer remains unclear. Three studies in our meta-analysis provided DFS data on ER-negative subgroups. Results showed that IGF-1R positivity was associated with an unfavorable outcome, but the risk was not statistically significant (combined HR: 1.58, 95% CI: 0.83–2.98; P=0.16). The results indicated that IGF-1R expression in ER-negative breast cancer may confer more malignant potential.
TNBC is a special subgroup of breast cancers that is characterized by absence of ER, PR, and Her-2 expression. There is no molecular target for the successful treatment of TNBC. Recently, Lehmann et al30 suggested that TNBC subtyping is needed to enhance the design and effectiveness of molecular-based therapies. They identified six triple-negative subtypes using cluster analysis, including the basal-like two and mesenchymal subtypes, which express IGF-1R. But the prognostic significance of IGF-1R expression in TNBC is not clear. There were only two studies in our meta-analysis that provided DFS data in TNBCs. The meta-analysis showed that IGF-1R positivity was an unfavorable prognostic indicator in TNBC (combined HR: 1.86, 95% CI: 1.03–3.34, P=0.04). The TNBC subtype has been reported to be more aggressive than other types. Although TNBC patients did not have an increased rate of local relapse, they had a high rate of distant metastasis, even among early-stage patients, which led to a 5-year distant metastasis-free survival rate of 71%.31 It has been reported that the IGF-1R signaling pathway contributes to migratory and invasive behavior of breast cancer cells.32 In addition, excessive IGF-1R signaling can lead to the activation of androgen receptors (ARs) downstream signaling in prostate cancers in the absence of androgens, which leads to IGF-1R-dependent cell survival and migration.33–35 Positive AR expression has been associated with favorable outcomes in breast cancer. Decreased AR expression is associated with distant metastases in patients with androgen-receptor-expressing TNBC.36 Consequently, there is a belief that IGF-1R may contribute to the tendency of TNBC to produce distant metastasis by downregulating AR expression and overriding AR and other survival pathways, leading to poor outcome. The results demonstrate that IGF-1R may be a therapeutic target, which might provide new treatment options for TNBC.
Several studies have described the signaling interactions between Her-2 and IGF-1R, and implicated the IGF-1R pathway in the development of resistance to trastuzumab.37,38 However, the clinical interaction of IGF-1R and Her-2 is unclear. There were three studies in the meta-analysis that did not find prognostic significance for IGF-1R expression in Her-2-positive cancers (combined HR: 0.96, 95% CI: 0.88–1.05. P=0.36). It has been reported that when Her-2 is overexpressed, other receptor tyrosine kinases exhibit ligand-independent activity because of Her-2 homodimerization and constitutive activation. Law et al17 have reported that phosphorylated IGF-1R can be found in Her-2-positive breast cancer and predicts unfavorable outcome. Whether phosphorylated IGF-1R outperforms IGF-1R as a biomarker needs further study.
Quality assessment according to REMARK guidelines was conducted for the ten studies in the meta-analysis. Sensitivity and subgroup analyses were performed to ensure that the results were reliable and valid. However, our meta-analysis has other limitations. First, the results of the subgroup analysis were less powerful because the combined HR was based on a relatively small number of patients. Second, there were different criteria for IGF-1R positivity. The cutoff values ranged from 1% to 26% of examined cells that stained positive, and most studies had a cutoff value of 10% (four studies). Two studies detected IGF-1R using radioimmunoassay. Third, two studies in our meta-analysis, which were the most recent (Yerushalmi et al20 and Shin et al21), had larger numbers of patients (2,871 and 968, respectively) compared to the rest of the studies (N ranged from 72 to 368). These two studies had a relatively high impact on the statistical results, which may reduce the reliability of this study for determining the role of IGF-1R in breast cancers. Therefore, we urgently need high-quality data to draw more accurate conclusions.
In conclusion, this study has demonstrated that IGF-1R positivity is significantly associated with better outcomes for patients with unselected breast cancer and the subgroups with hormone-receptor-positive cancers. IGF-1R positivity in TNBCs is associated with unfavorable outcome. IGF-1R is a valuable therapeutic target that may lead to the optimization of individualized treatment for breast cancer patients.
Acknowledgments
This research was supported by grants from the National Science Foundation of China (81302313, 81472806).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v20 Cancer Incidence and Mortality Worldwide: IARC CancerBase No 10 (Internet) Lyon, France: International Agency for Research on Cancer; 2010. [Accessed December 2, 2014]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Singh P, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1(IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol. 2014;31(1):805. doi: 10.1007/s12032-013-0805-3. [DOI] [PubMed] [Google Scholar]
- 4.Negi A, Ramarao P, Kumar R. Recent advancements in small molecule inhibitors of insulin-like growth factor-1 receptor (IGF-1R) tyrosine kinase as anticancer agents. Mini Rev Med Chem. 2013;13(5):653–681. doi: 10.2174/1389557511313050004. [DOI] [PubMed] [Google Scholar]
- 5.Bonneterre J, Peyrat JP, Beuscart R, et al. Prognostic significance of insulin-like growth factor 1 receptors in human breast cancer. Cancer Res. 1990;50(21):6931–6935. [PubMed] [Google Scholar]
- 6.Foekens JA, Portengen H, Janssen M, et al. Insulin-like growth factor-1 receptors and insulin-like growth factor-1-like activity in human primary breast cancer. Cancer. 1989;63(11):2139–2147. doi: 10.1002/1097-0142(19890601)63:11<2139::aid-cncr2820631112>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Papa V, Gliozzo B, Clark GM, et al. Insulin-like growth factor-I receptors are overexpressed and predict a low risk in human breast cancer. Cancer Res. 1993;53(16):3736–3740. [PubMed] [Google Scholar]
- 8.Railo MJ, von Smitten K, Pekonen F. The prognostic value of insulin-like growth factor-I in breast cancer patients. Results of a follow-up study on 126 patients. Eur J Cancer. 1994;30A(3):307–311. doi: 10.1016/0959-8049(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Cho YH, Park YL, et al. Prognostic significance of insulin growth factor-I receptor and insulin growth factor binding protein-3 expression in primary breast cancer. Oncol Rep. 2010;23(4):989–995. doi: 10.3892/or_00000724. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Zhang Z, Tang H, et al. Crosstalk between IGF-1R and other tumor promoting pathways. Curr Pharm Des. 2014;20(17):2912–2921. doi: 10.2174/13816128113199990596. [DOI] [PubMed] [Google Scholar]
- 11.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumour MARKer prognostic studies (REMARK) Eur J Cancer. 2005;41(12):1690–1696. doi: 10.1016/j.ejca.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Chen M, Cai E, Huang J, et al. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013;21(7):1126–1134. doi: 10.1158/1055-9965.EPI-12-0020. [DOI] [PubMed] [Google Scholar]
- 13.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda S, Tsuda H, Sato K, et al. Alternative tyrosine phosphorylation of signaling kinases according to hormone receptor status in breast cancer overexpressing the insulin-like growth factor receptor type 1. Cancer Sci. 2006;97:597–604. doi: 10.1111/j.1349-7006.2006.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Köstler WJ, Hudelist G, Rabitsch W, et al. Insulin-like growth factor-1 receptor (IGF-1R) expression does not predict for resistance to trastuzumab-based treatment in patients with Her-2/neu overexpressing metastatic breast cancer. J Cancer Res Clin Oncol. 2006;132(1):9–18. doi: 10.1007/s00432-005-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law JH, Habibi G, Hu K, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68(24):10238–10246. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 18.Fu P, Ibusuki M, Yamamoto Y, et al. Insulin-like growth factor-1 receptor gene expression is associated with survival in breast cancer: a comprehensive analysis of gene copy number, mRNA and protein expression. Breast Cancer Res Treat. 2011;130(1):307–317. doi: 10.1007/s10549-011-1605-0. [DOI] [PubMed] [Google Scholar]
- 19.Hartog H, Horlings HM, van der Vegt B, et al. Divergent effects of insulin-like growth factor-1 receptor expression on prognosis of estrogen receptor positive versus triple negative invasive ductal breast carcinoma. Breast Cancer Res Treat. 2011;129(3):725–736. doi: 10.1007/s10549-010-1256-6. [DOI] [PubMed] [Google Scholar]
- 20.Yerushalmi R, Gelmon KA, Leung S, et al. Insulin-like growth factor receptor (IGF-1R) in breast cancer subtypes. Breast Cancer Res Treat. 2012;132(1):131–142. doi: 10.1007/s10549-011-1529-8. [DOI] [PubMed] [Google Scholar]
- 21.Shin SJ, Gong G, Lee HJ, et al. Positive expression of insulin-like growth factor-1 receptor is associated with a positive hormone receptor status and a favorable prognosis in breast cancer. J Breast Cancer. 2014;17(2):113–120. doi: 10.4048/jbc.2014.17.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubbard SR, Miller WT. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol. 2007;19(2):117–123. doi: 10.1016/j.ceb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen TO, Andrews HN, Cheang M, et al. Expression of the insulin-like growth factor I receptor and urokinase plasminogen activator in breast cancer is associated with poor survival: potential for intervention with 17-allylamino geldanamycin. Cancer Res. 2004;64(1):286–291. doi: 10.1158/0008-5472.can-03-1242. [DOI] [PubMed] [Google Scholar]
- 25.Norum JH, Andersen K, Sørlie T. Lessons learned from the intrinsic subtypes of breast cancer in the quest for precision therapy. Br J Surg. 2014;101(8):925–938. doi: 10.1002/bjs.9562. [DOI] [PubMed] [Google Scholar]
- 26.Yee D, Lee AV. Crosstalk between the insulin-like growth factors and estrogens in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5(1):107–115. doi: 10.1023/a:1009575518338. [DOI] [PubMed] [Google Scholar]
- 27.Bartucci M, Morelli C, Mauro L, et al. Differential insulin-like growth factor I receptor signaling and function in estrogen receptor (ER)-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells. Cancer Res. 2001;61(18):6747–6754. [PubMed] [Google Scholar]
- 28.Oesterreich S, Zhang P, Guler RL, et al. Re-expression of estrogen receptor alpha in estrogen receptor alpha-negative MCF-7 cells restores both estrogen and insulin-like growth factor-mediated signaling and growth. Cancer Res. 2001;61(15):5771–5777. [PubMed] [Google Scholar]
- 29.Lee AV, Hilsenbeck SG, Yee D. IGF system components as prognostic markers in breast cancer. Breast Cancer Res Treat. 1998;47(3):295–302. doi: 10.1023/a:1005915420341. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24(36):5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 32.Kucab JE, Dunn SE. Role of IGF-1R in mediating breast cancer invasion and metastasis. Breast Dis. 2003;17:41–47. doi: 10.3233/bd-2003-17105. [DOI] [PubMed] [Google Scholar]
- 33.Chi KN, Bjartell A, Dearnaley D, et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment targets. Eur Urol. 2009;56(4):594–605. doi: 10.1016/j.eururo.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 34.Antonarakis ES, Carducci MA, Eisenberger MA. Novel targeted therapeutics for metastatic castration-resistant prostate cancer. Cancer Lett. 2010;291(1):1–13. doi: 10.1016/j.canlet.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krueckl SL1, Sikes RA, Edlund NM, et al. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64(23):8620–8629. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- 36.Sutton LM, Cao D, Sarode V, et al. Decreased androgen receptor expression is associated with distant metastases in patients with androgen receptor expressing triple-negative breast carcinoma. Am J Clin Pathol. 2012;138(4):511–516. doi: 10.1309/AJCP8AVF8FDPTZLH. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Zi X, Zhao Y, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93(24):1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 38.Nahta R. Deciphering the role of insulin-like growth factor-I receptor in trastuzumab resistance. Chemother Res Pract. 2012;2012:648965. doi: 10.1155/2012/648965. [DOI] [PMC free article] [PubMed] [Google Scholar]