Abstract
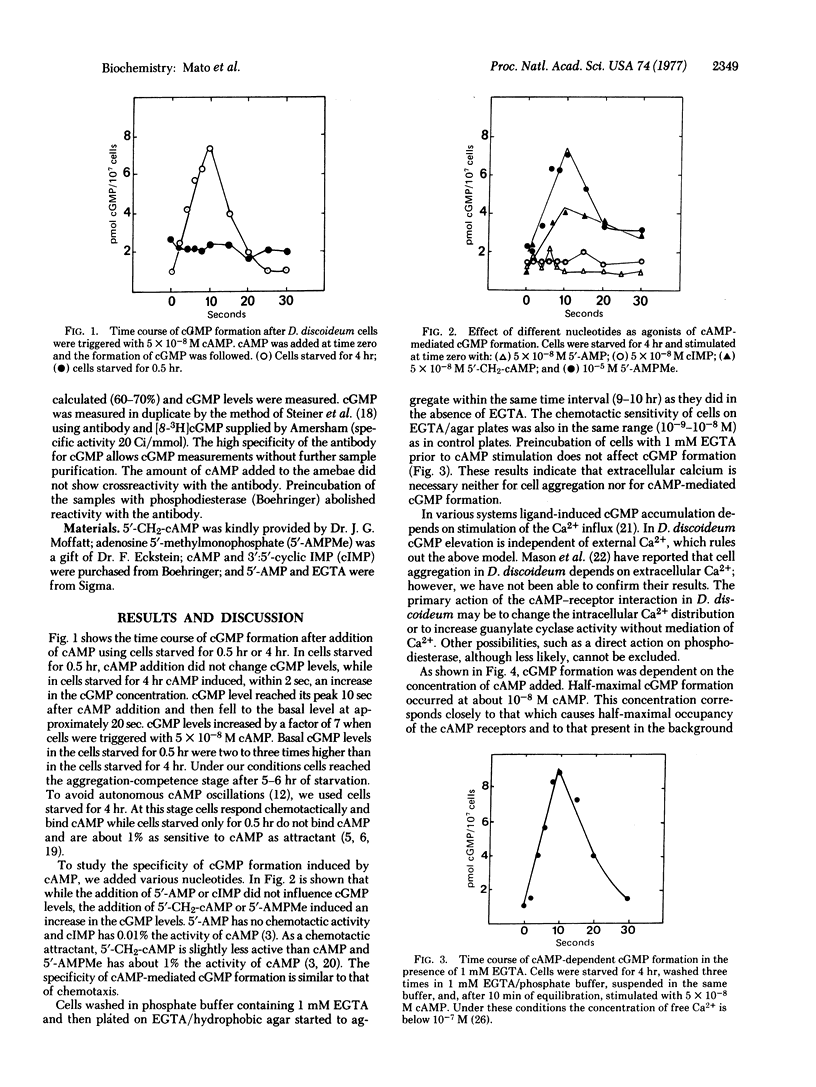
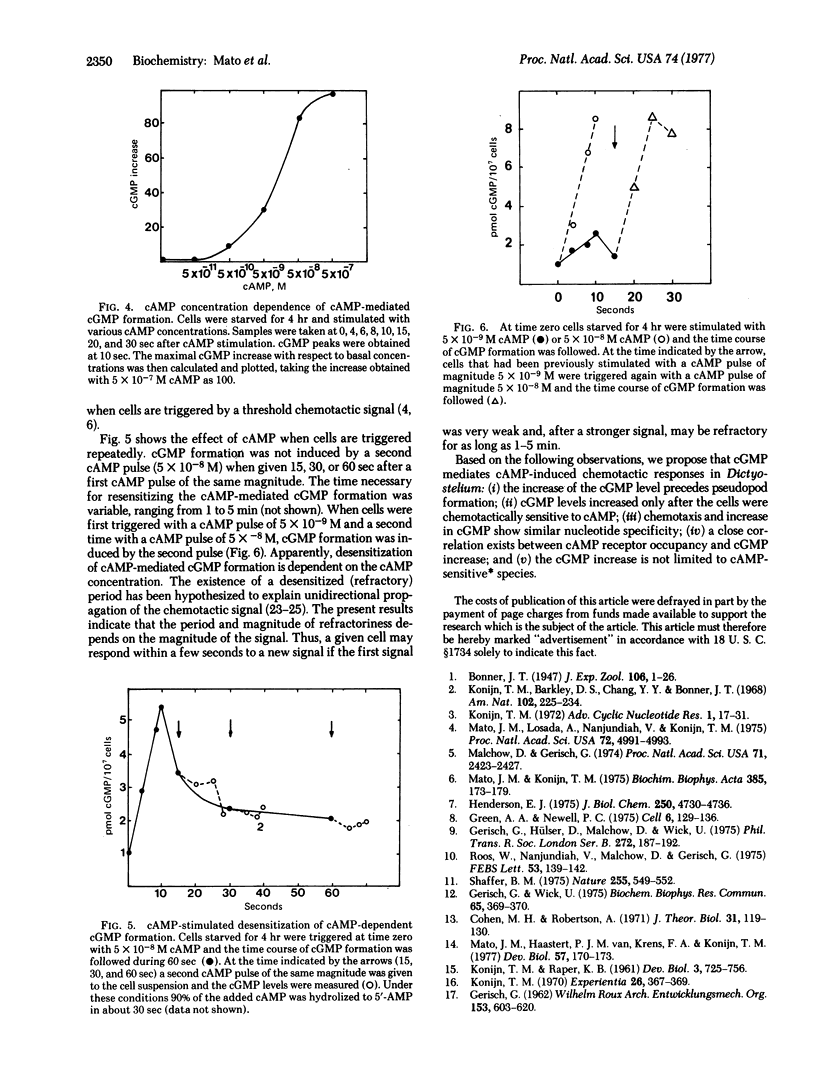
Suspensions of 3′:5′-cyclic AMP (cAMP)-sensitive cells of Dictyostelium discoideum responded to a cAMP pulse with increased 3′:5′-cyclic GMP (cGMP) levels. Under the assay conditions used (2 × 108 cells per ml in 10 mM phosphate buffer, pH 6.0) cAMP (5 × 10-8 M final concentration) increased cGMP levels from 1 pmol per 107 cells to 7 pmol per 107 cells in 10 sec and basal levels were recovered in 20-25 sec. cGMP accumulation did not occur when cells were in the cAMP-insensitive stage. cAMP-sensitive cells responded with increased cGMP levels when triggered by 5 × 10-8 M 5′-CH2-cAMP or 10-5 M adenosine-5′-methylmonophosphate (5′-AMPMe) but not after addition of 5 × 10-8 M 3′:5′-cyclic IMP (cIMP) or 5 × 10-8 M 5′-AMP. As agonists of cAMP, 5′-CH2-cAMP and 5′-AMPMe have, respectively, more than 10% and 1% the chemotactic activity of cAMP, while cIMP has 0.01% the activity of cAMP and 5′-AMP is inactive up to a concentration of 10-3 M. cAMP-mediated cGMP formation was dependent upon cAMP concentration, with a half-maximal cAMP concentration of about 10-8 M. This cAMP concentration agrees closely with that necessary for half-maximal receptor occupation. cAMP-mediated cGMP formation was independent of the presence of extracellular Ca2+; cell aggregation and chemotaxis were also independent of the presence of external Ca2+. Therefore, cAMP action does not depend on stimulation of the Ca2+ influx. cAMP was found to mediate desensitization of cAMP-dependent cGMP formation. Addition of 5 × 10-8 M cAMP to sensitive cells induced a desensitization period that lasted 1-5 min. Desensitization was dependent on the cAMP concentration. Finally, we propose that the translation of a chemotactic signal from the cell surface to pseudopod formation in Dictyostelium involves changes in the levels of cGMP.
Keywords: chemotaxis, desensitization, Ca2+, cellular slime molds, cell aggregation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcantara F., Monk M. Signal propagation during aggregation in the slime mould Dictyostelium discoideum. J Gen Microbiol. 1974 Dec;85(2):321–334. doi: 10.1099/00221287-85-2-321. [DOI] [PubMed] [Google Scholar]
- Bonner J. T., Barkley D. S., Hall E. M., Konijn T. M., Mason J. W., O'Keefe G., 3rd, Wolfe P. B. Acrasin, Acrasinase, and the sensitivity to acrasin in Dictyostelium discoideum. Dev Biol. 1969 Jul;20(1):72–87. doi: 10.1016/0012-1606(69)90005-0. [DOI] [PubMed] [Google Scholar]
- Cohen M. H., Robertson A. Chemotaxis and the early stages of aggregation in cellular slime molds. J Theor Biol. 1971 Apr;31(1):119–130. doi: 10.1016/0022-5193(71)90125-1. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Hülser D., Malchow D., Wick U. Cell communication by periodic cyclic-AMP pulses. Philos Trans R Soc Lond B Biol Sci. 1975 Nov 6;272(915):181–192. doi: 10.1098/rstb.1975.0080. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Wick U. Intracellular oscillations and release of cyclic AMP from Dictyostelium cells. Biochem Biophys Res Commun. 1975 Jul 8;65(1):364–370. doi: 10.1016/s0006-291x(75)80102-1. [DOI] [PubMed] [Google Scholar]
- Green A. A., Newell P. C. Evidence for the existence of two types of cAMP binding sites in aggregating cells of Dictyostelium discoideum. Cell. 1975 Oct;6(2):129–136. doi: 10.1016/0092-8674(75)90003-3. [DOI] [PubMed] [Google Scholar]
- Henderson E. J. The cyclic adenosine 3':5'-monophosphate receptor of Dictyostelium discoideum. Binding characteristics of aggregation-competent cells and variation of binding levels during the life cycle. J Biol Chem. 1975 Jun 25;250(12):4730–4736. [PubMed] [Google Scholar]
- KONIJN T. M., RAPER K. B. Cell aggregation in Dictyostelium discoideum. Dev Biol. 1961 Dec;3:725–756. doi: 10.1016/0012-1606(61)90038-0. [DOI] [PubMed] [Google Scholar]
- Konijn T. M. Cyclic AMP as a first messenger. Adv Cyclic Nucleotide Res. 1972;1:17–31. [PubMed] [Google Scholar]
- Konijn T. M. Microbiological assay of cyclic 3',5'-AMP. Experientia. 1970 Apr 15;26(4):367–369. doi: 10.1007/BF01896891. [DOI] [PubMed] [Google Scholar]
- Malchow D., Gerisch G. Short-term binding and hydrolysis of cyclic 3':5'-adenosine monophosphate by aggregating Dictyostelium cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2423–2427. doi: 10.1073/pnas.71.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. W., Rasmussen H., Dibella F. 3'5' AMP and Ca 2+ in slime mold aggregation. Exp Cell Res. 1971 Jul;67(1):156–160. doi: 10.1016/0014-4827(71)90631-8. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Konijn T. M. Chemotaxis and binding of cyclic AMP in cellular slime molds. Biochim Biophys Acta. 1975 Apr 7;385(2):173–179. doi: 10.1016/0304-4165(75)90345-1. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Konijn T. M. The chemotactic of cyclic AMP and AMP derivatives with substitutions in the phosphate moiety in Dictyostelium discoideum. FEBS Lett. 1977 Mar 15;75(1):173–176. doi: 10.1016/0014-5793(77)80079-3. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Losada A., Nanjundiah V., Konijn T. M. Signal input for a chemotactic response in the cellular slime mold Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4991–4993. doi: 10.1073/pnas.72.12.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Roos W., Nanjundiah V., Malchow D., Gerisch G. Amplification of cyclic-AMP signals in aggregating cells of Dictyostelium discoideum. FEBS Lett. 1975 May 1;53(2):139–142. doi: 10.1016/0014-5793(75)80005-6. [DOI] [PubMed] [Google Scholar]
- Schultz G., Hardman J. G. Regulation of cyclic GMP levels in the ductus deferens of the rat. Adv Cyclic Nucleotide Res. 1975;5:339–351. [PubMed] [Google Scholar]
- Shaffer B. M. Secretion of cyclic AMP induced by cyclic AMP in the cellular slime mould Dictyostelium discoideum. Nature. 1975 Jun 12;255(5509):549–552. doi: 10.1038/255549a0. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]


