Abstract
Objective
To evaluate the safety and efficacy of epidermal growth factor receptor (EGFR)–targeted therapy in patients with advanced penile or scrotal cancer.
Patients and Methods
We retrospectively reviewed charts of patients with penile or scrotal squamous cell carcinoma who had visited our tertiary cancer center from 2002 through 2009, including their subsequent treatment and follow-up. We collected details of EGFR-targeted therapy and clinical outcomes. Treatment-associated time to disease progression (TTP), overall survival (OS), responses to therapy, and toxicity were evaluated.
Results
Twenty-four patients had received EGFR-targeted therapies, including cetuximab, erlotinib, and gefitinib. The most common treatment given (67% of patients) was cetuximab combined with one or more cytotoxic drugs. The most common adverse effect was skin rash (71%); median TTP and OS were 11.3 weeks (1–40 weeks) and 29.6 weeks (2–205 weeks), respectively. OS for patients with visceral or bone metastases was significantly less than it was for those without (24.7 weeks vs. 49.9 weeks, P = .013). Among 17 patients treated with cetuximab alone or in combination with cisplatin, there were four partial responses (23.5%) including two patients with seemingly chemo-resistant tumor.
Conclusion
Our results suggest that cetuximab has antitumor activity in metastatic penile cancer, and may enhance the effect of cisplatin-based chemotherapy. Prospective studies of EGFR-targeted therapies in men with these tumors are warranted.
Keywords: Anti-epidermal growth factor monoclonal antibody, Penile Neoplasms, Squamous Cell Carcinoma, Survival
Introduction
Squamous cell carcinoma (SCC) is the most common malignant tumor affecting the penis.1 Its prevalence is less in developed Western countries than it is in Africa, India, China, and parts of South America. Gold-standard therapy for localized disease includes a variety of surgical techniques, such as glans-sparing partial penectomy, glansectomy, total or partial penectomy, and lymphadenectomy.2 Locally advanced or metastatic SCC of the penis or scrotum (herein termed PSCC) is defined as either a bulky primary tumor (clinical T4) or the presence of metastases. Effective treatment for PSCC often requires a multidisciplinary approach, employing both surgery and systemic therapy.3–5 Unfortunately, despite recent additions to the chemotherapy arsenal, survival rates for patients with tumor dissemination remain poor.6–8
PSCC primary tumors and metastases highly express epidermal growth factor receptor (EGFR).9,10 Published series have indicated a 91%–100% frequency of elevated EGFR expression in penile cancer. This high expression level suggests that targeting EGFR can yield advances in safe and effective therapy. In one published case report, objective response was described in a PSCC patient treated with panitumumab, an anti-EGFR monoclonal antibody.11 Very little is known, however, regarding the frequency of such responses or the activity of other anti-EGFR agents.
In this study, we retrospectively analyzed data from patients treated for PSCC with EGFR-targeted therapy at our tertiary cancer care center, The University of Texas MD Anderson Cancer Center. We sought to determine the time to disease progression (TTP), overall survival (OS), response characteristics, and toxicity associated with EGFR-targeted therapy in these patients.
Patients and Methods
Eligibility and Objectives
We obtained institutional review board approval for this study before reviewing patients’ charts, reports, and images. We initially screened for patients with clinical stage T4 or with any T stage and clinical N2, N3, or M1 PSCC seen at MD Anderson from January 1, 2002, through January 1, 2009. The SCC had to have been histologically proven. Patients from this group who had received EGFR-targeted therapy at any time were included in our analysis. Results of tumor immunostaining for EFGR protein, if available, were also recorded.
The median TTP and OS for all 24 patients we included in our study were calculated from the start of EGFR-targeted therapy. Progression was documented by reviewing available radiographic reports and images or by finding chart notes about evident clinical progression. Toxic events were identified through documentation in notes from clinic visits.
Statistical Methods
Statistical analysis and graphing was done with GraphPad Prism version 5.04 software (GraphPad Software, Inc.). TTP and OS were estimated by using the Kaplan-Meier method, and between-group comparisons were made by log-rank testing. Consolidative surgery was a censoring event for TTP in the three patients in whom it had been done. We chose P = .05 to indicate statistical significance.
Results
Patients’ Characteristics
No patients with PSCC had received EGFR-targeted therapies in 2002, 2003, and the first eight months of 2004. However, 24 patients had started treatment with one or more EGFR-targeted therapies in the period from September 29, 2004, through June 1, 2009. They were 36–71 years old (median, 59 years). The primary disease site was the penis in 23 patients (96%); the other’s was the scrotum. They had been moderately pretreated: 91.7% (22/24) had received at least one prior line of systemic chemotherapy, and one third (8/24) had received at least two lines (range, 0–4). Three patients were treated in the neoadjuvant setting after having demonstrated progression or lack of response to paclitaxel, ifosfamide, and cisplatin (TIP)7; the rest had visceral metastases or had been inoperable for other reasons.
All patients had biopsy-proven SCC. Half (12/24) had distant soft tissue, visceral, or bony metastases at the time of treatment with EGFR inhibition. The remainder had at least locally advanced disease, including inguinal, scrotal, or pelvic nodal masses. Tumor specimens from 13 of the patients had been immunostained for EGFR protein in the course of routine clinical care, and all had been positive. Specimens from the other 11 patients’ tumors had not been tested.
Treatment
Eight patients had received an EGFR-targeted drug alone (cetuximab, erlotinib, or gefitinib) (Table 1), 13 had received cetuximab plus a platinum drug (cisplatin [n = 12] or carboplatin [n = 1]) (Table 2), and three patients had received TIP plus cetuximab (Table 3). Several patients had gone on to receive additional EGFR-targeted therapies, which were not included in our analysis. All treatments had been given as off-label use of commercially available drugs, and the patients had not been participating in a clinical trial. Patient selection and choice of treatments were entirely at the discretion of the treating physicians.
Table 1.
Penile cancer patients treated with an EGFR-targeted agent alone and outcomes
| Patienta | Age (years) |
TNM clinical stage |
EGFR- targeted therapy |
Best response |
TTP (days) |
OS (days) |
Reason for discontinuing |
|---|---|---|---|---|---|---|---|
| 3 | 69 | TxN3M0 | Cetuximab | Partial response | 281 | 403 | Tumor progression |
| 4 | 59 | TxNxM1 | Erlotinib | Progression | 58 | 86 | Tumor progression |
| 5 | 69 | TxN3M1 | Cetuximab | Progression | 72 | 151 | Tumor progression |
| 12 | 70 | TxN2M1 | Cetuximab | Progression | 57 | 356 | Tumor progression |
| 14 | 48 | TxNxM1 | Gefitinib | Progression | 30 | 47 | Tumor progression |
| 16 | 48 | TxN3M0 | Erlotinib | Progression | 125 | 181 | Tumor progression |
| 22 | 71 | T4N3M1 | Cetuximab | Stable | 64 | 222 | Declining performance status |
| 24 | 61 | T4N3M1 | Cetuximab | Progression | 47 | 252 | Tumor progression |
All patients had received prior treatment. Patients 14 and 16 had received two prior chemotherapy regimens, patient 22 had received three regimens, and the others had received only one prior chemotherapy regimen or chemoradiotherapy. Patient 16 had also received one partial dose of cetuximab, experienced a hypersensitivity reaction, and then was treated with erlotinib.
EGFR, epidermal growth factor receptor; OS, overall survival; TTP, time to disease progression.
Table 2.
Penile cancer patients treated with cetuximab plus a platinum drug and treatment outcomes
| Patienta | Age (years) |
TNM clinical stage |
No. of prior chemotherapy regimens |
Best response |
TTP (days) |
OS (days) |
Reason for discontinuing |
|---|---|---|---|---|---|---|---|
| 1 | 58 | TxNxM1 | 2 | Progression | 11 | 14 | Tumor progression |
| 2 | 45 | TxN3M1 | 4 | Improvement | 96 | 153 | Tumor progression |
| 6 | 58 | TxN3M0 | 2 | Stable | 179 | 179 | Tumor hemorrhage |
| 9 | 62 | TxN3M0 | 1 | Partial response | 107b | 455 | Patient underwent surgical consolidation |
| 10 | 59 | T4N0M0 | 1 | Partial response | 94b | 1441 | Patient underwent surgical consolidation |
| 11 | 58 | TxNxM1 | 2 | Progression | 35 | 294 | Tumor progression |
| 15 | 67 | TxNxM1 | 2 | Stable | 31 | 31 | Death |
| 17 | 63 | TxN3M1 | 0 | Partial response | 135 | 243 | Tumor progression |
| 18 | 45 | T3N3M0 | 2 | Progression | 23 | 102 | Tumor progression |
| 19 | 71 | T2N3M0 | 2 | Progression | 29 | 52 | Tumor progression |
| 20 | 72 | TxNxM1 | 1 | Stable | 189 | 1332c | Tumor progression |
| 21 | 51 | TxN3M0 | 1 | Stable | 116 | 163 | Tumor progression |
| 23 | 43 | TxN3M0 | 1 | Progression | 46 | 920 | Tumor progression |
Patient 15 received cetuximab and carboplatin; all others received cetuximab and cisplatin. Patients 11 and 20 had metastasis to skin only.
TTP censored at time of consolidative surgery.
Alive at last follow-up.
OS, overall survival; TTP, time to disease progression.
Table 3.
Patients treated with cetuximab in combination with paclitaxel, ifosfamide, and cisplatin
| Patienta | Age (years) |
TNM clinical stage |
No. prior chemotherapy regimens |
Best response |
TTP (days) |
OS (days) |
Reason for discontinuing |
|---|---|---|---|---|---|---|---|
| 7 | 36 | T4N3M1 | 0 | Mixed response | 86 | 192 | Tumor progression |
| 8 | 59 | TxN3M1 | 1 | Partial response | 191 | 323 | Tumor progression |
| 13 | 68 | TxN2M0 | 1 | Partial response | 73b | 1131c | Patient underwent surgical consolidation |
Primary tumors were in the penis (patients 7 and 8) and scrotum (patient 13).
TTP censored at time of consolidative surgery.
Alive at last follow-up.
OS, overall survival; TTP, time to disease progression.
Cetuximab had been given with a loading dose of 400 mg/m2 on day 1 and then at 250 mg/m2 weekly. Four patients received erlotinib at some point during their treatment (150 mg by mouth [PO] daily), and one patient received gefitinib (250 mg PO daily). Dosage adjustments for all EGFR-targeted therapies had been made according to the agents’ approved labeling.
The patients who had received cetuximab in combination with a platinum drug (Table 2) had received cisplatin at a typical dosage of 25 mg/m2 on days 1, 2, and 3, or carboplatin (area under the curve = 5) in a 3-week dosing schedule. Additional chemotherapeutic regimens were given either in standard dosing regimens or as published previously.7,8,12
Five patients (20.8%) subsequently received additional systemic therapy after the EGFR-based therapies. Three patients (12.5%) had undergone chemoradiotherapy as part of their subsequent treatment, with one patient receiving 5-fluorouracil plus radiation, another receiving capecitabine plus radiation, and one continuing cetuximab–cisplatin during radiotherapy.
Toxicity
The EGFR-targeted therapies had been well tolerated: rash (grades 1 and 2) was the most common toxic effect, occurring in 17 of the 24 (70.8%) patients. Grade 3 or 4 adverse events that had occurred after cetuximab treatment included cellulitis, thrombocytopenia, and bronchospasm in one patient each, and one additional patient’s tumor hemorrhaged after cetuximab treatment, although the hemorrhage was thought to be unrelated to therapy. One patient died of undetermined cause 31 days after treatment with cetuximab and carboplatin; his death was thought to be unrelated to the treatment.
TTP and OS
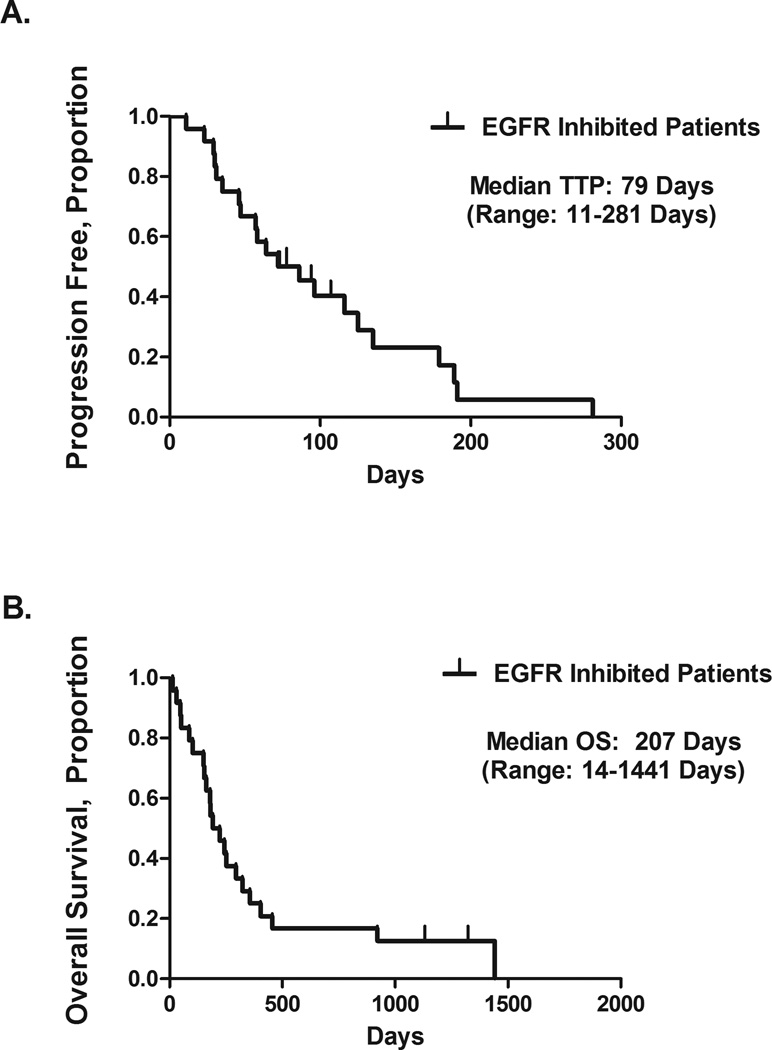
Patients had been followed for a median of 207 days (range, 14–1441 days). The 24 patients had an overall median TTP of 79 days (11.3 weeks), ranging from 11 to 281 days (Figure 1A). The median OS time was 207 days (29.6 weeks), ranging from 14 to 1441 days (Figure 1B).
Figure 1.
Time to disease progression (TTP) (A) and overall survival (OS) (B) in 24 patients treated with epidermal growth factor receptor (EGFR)–targeted therapy.
We divided the 24 patients into several subgroups for post hoc analyses to determine the effect of visceral metastases and consolidative surgery on their TTP and OS. The presence of visceral metastases at the start of EGFR-based therapy was associated with poor TTP and OS. Patients who had had visceral or bone metastases had a median TTP of 61 days, compared with 107 days in patients who had had only locally advanced or lymph node–metastatic disease (P = .15, log-rank analysis). Overall survival time of the patients with visceral, soft tissue, or bone metastases was 173 days (24.7 weeks), whereas for patients without, it was 349 days (49.9 weeks) (P = .013, log-rank analysis).
Neoadjuvant Therapy
We then examined outcomes following consolidative surgery in PSCC patients who had received EGFR-targeted therapy in the neoadjuvant setting. Owing to the overall advanced disease stage in our cohort, only three of the 24 patients (12.5%) had been selected for consolidative surgery after having demonstrated a response to cetuximab plus chemotherapy (patients 9, 10, and 13). The OS times for those three patients were 1441 days, 455 days, and 1131 days (alive), respectively, compared with a median OS of 181 days for those who had not undergone consolidation surgery (P = .028, log-rank analysis); two of those three (patients 10 and 13) experienced long-term disease-free survival and are described below.
Patient 10 had clinical T4 penile cancer replacing the penis and involving the scrotum and testicles. There was no lymphadenopathy by physical examination or on computed tomography (CT) imaging. After two courses of neoadjuvant TIP chemotherapy without response, the treatment had been changed to cisplatin plus cetuximab. During this treatment the tumor had improved sufficiently to permit surgery. Total penectomy, scrotectomy, and bilateral orchiectomies revealed residual SCC with negative margins. Bilateral groin dissection revealed one involved inguinal lymph node on each side. He received no further therapy and remained disease free for 44 months after the surgery, when he died of an undetermined cause.
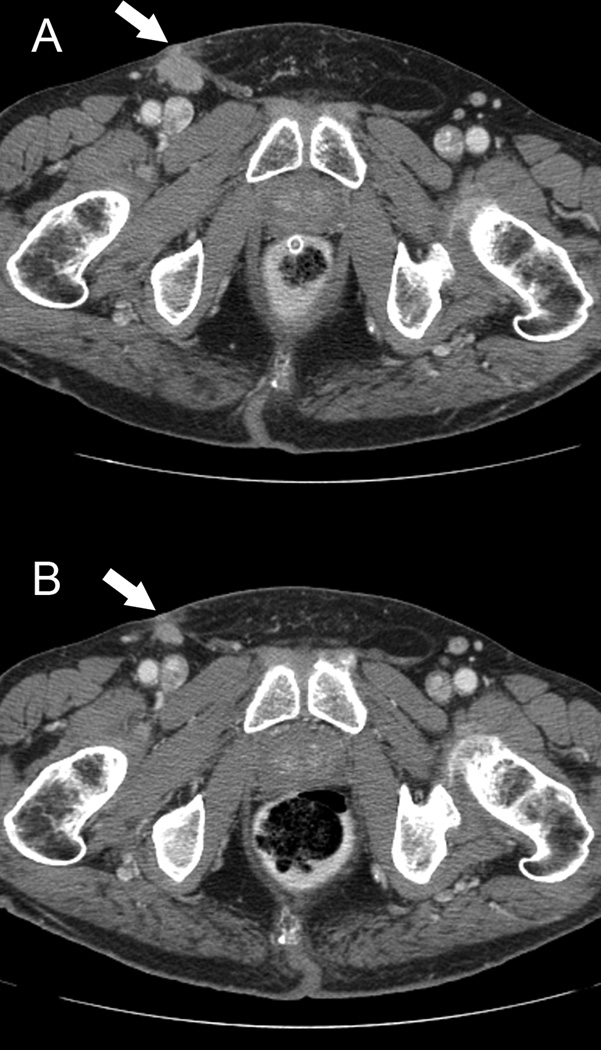
Patient 13 had had primary scrotal SCC metastatic to the right groin’s subcutaneous tissue, which had been excised. Two months later, he had had recurrent palpable tumor in the right groin, measuring 2.4 × 1.4 cm on CT imaging. He had been given two courses of neoadjuvant TIP chemotherapy during which there was progression. A 400-mg/m2 loading dose of cetuximab had been added to his chemotherapy on day 2 of the third cycle, followed by subsequent weekly doses of 250 mg/m2. Within two weeks of cetuximab’s addition, there had been visible and palpable improvement in the right groin mass (Figure 2). After two cycles of TIP plus cetuximab, he had undergone resection of the the residual groin mass, bilateral inguinal lymph node dissections, and ipsilateral pelvic lymph node dissection. Multifocal metastatic SCC had been identified in the subcutaneous fibroadipose tissue of the right groin, with negative margins and no lymph node involvement. On his latest follow-up, he had been disease free 35 months after the surgery (38 months after EGFR-targeted therapy).
Figure 2.
Patient 13 received cetuximab plus combination chemotherapy with paclitaxel, ifosfamide, and cisplatin (TIP) in the neoadjuvant setting. Computed tomographic scans show the appearance of right inguinal metastasis before the addition of cetuximab (A) and after 2 cycles of cetuximab plus TIP (B). Arrows indicate metastatic tumors.
Objective Tumor Response
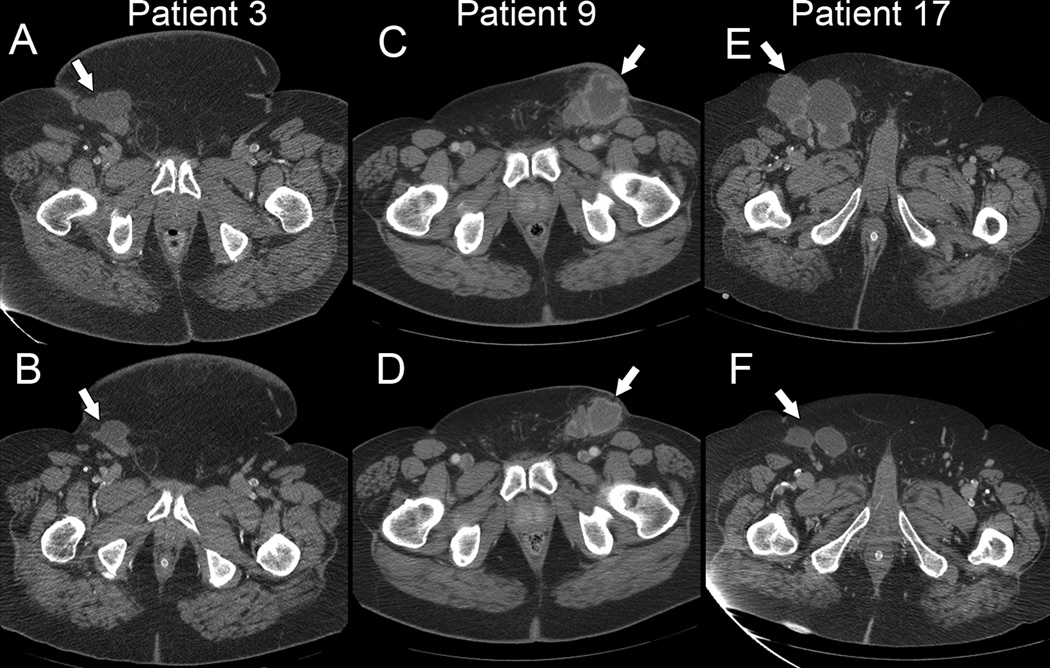
Because this was a retrospective cohort analysis, an objective response “rate” would not be meaningful. However, over their treatment course, several of the patients had shown radiographic responses involving regression of predominantly inguinal and pelvic metastatic tumors (Figure 3). The responding patients had received either cetuximab alone or cetuximab plus chemotherapy. Partial responses were seen in 1/5 patients (20%) who had received cetuximab alone, and in 3/12 patients (25%) who had received cetuximab plus cisplatin. Partial responses were also seen in 2/3 patients who had received cetuximab plus TIP. There were no objective responses to the small-molecule inhibitors gefitinib or erlotinib.
Figure 3.
Pretreatment (A, C, E) and post-treatment (B, D, F) computed tomographic scans from selected patients. Patient 3 received cetuximab alone. Patients 9 and 17 received cetuximab plus cisplatin. Time between scans was six months (A, B), six weeks (C, D), and four months (E, F). Arrows indicate metastatic tumors.
Discussion
To our knowledge, this is the first retrospective collection of data from penile cancer patients treated with EGFR-targeted therapies with and without additional systemic therapies. Our findings introduce a new paradigm for treating PSCC. Historically, the largest prospective experience with systemic therapy for metastatic penile cancer was a multicenter Southwest Oncology Group trial, in which patients who had had no prior systemic therapy were given bleomycin, methotrexate, and cisplatin (BMP).12 The response rate was 32.5%, and the median OS was 28 weeks. By comparison, our median OS of 29.6 weeks with EGFR-targeted therapy was impressive considering that most of our patients had been previously treated. Among the 17 patients who had received cetuximab alone or in combination with cisplatin, four (23.5%) had responded, including two patients whose tumors had been refractory to TIP.
We reported the results of neoadjuvant TIP given to patients with metastatic penile cancer confined to inguinal and pelvic lymph nodes.7 In that phase II study, 50% exhibited a response and 10%, a complete pathological response. Eighteen patients (75%) in the study we report here had also been given TIP either before or combined with EGFR-targeted therapy. The combination of cetuximab and TIP was well tolerated, as was the combination of cetuximab and cisplatin. Three patients had undergone consolidative surgery after neoadjuvant cetuximab plus cisplatin or TIP. Their relatively long survival time (and apparent cure in two patients) aligns with the results of our prior analyses,7,8 highlighting the important role of surgery after systemic therapy for a subgroup of PSCC patients, and suggesting a potential role for EGFR-targeted therapy in this setting.
Limitations of our study include its retrospective design and variation in the treatment. Patient selection factors may have influenced the likelihood of response and survival in this cohort. The clinical context ranged from patients who were treated in the neoadjuvant setting to those with advanced, seemingly chemo-resistant PSCC. Data on EGFR expression were missing for some of the patients because we relied on the existing pathology reports that were generated in the course of patient care. From the tumor samples that were tested, however, and from other published results, the evidence suggests that the vast majority of PSCC have strong expression of EGFR. Despite its limitations, our study provides unprecedented clinical data on the potential use of a targeted therapy in this rare disease.
With any targeted agent, obtaining proof that it exerted its effect on the intended target has utmost importance. One known side effect of EGFR inhibition is a characteristic acneiform rash, noted in up to 75% of patients with SCC of the skin treated with agents such as cetuximab.13 Indeed, this effect occurred in 71% of the patients in our series, confirming functional EGFR inhibition in vivo. Multi-institutional and multinational collaborations would now be helpful in elucidating the biology of EGFR-targeted therapies in PSCC. Further, the role of other biologic factors, e.g., human papilloma virus infection, EGFR mutations, and K-ras mutations, must be considered in predicting response to EGFR blockade.14–16
In conclusion, patients with PSCC tolerated treatment with the EGFR-targeted agents cetuximab, erlotinib, and gefitinib, and also cetuximab in combination with chemotherapy. The median OS in this cohort of mostly pretreated patients was as good as that previously reported with BMP chemotherapy in a group of patients with similar stage of disease and no prior therapy. These results and the responses that were seen suggest that further prospective study of EGFR-targeted therapy in patients with PSCC is warranted. Patients who are most likely to benefit appear to be those without visceral or bone metastases and those treated in the neoadjuvant setting.
Acknowledgements
We thank Dr. Sijin Wen for statistical analysis, and Karen F. Phillips, ELS(D), of the Department of Genitourinary Medical Oncology, for manuscript editing.
Funding: This work was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, 5 P30 CA016672.
References
- 1.Misra S, Chaturvedi A, Misra NC. Penile carcinoma: a challenge for the developing world. Lancet Oncol. 2004;5:240–247. doi: 10.1016/S1470-2045(04)01427-5. [DOI] [PubMed] [Google Scholar]
- 2.Horenblas S, van Tinteren H, Delemarre JF, et al. Squamous cell carcinoma of the penis. III. Treatment of regional lymph nodes. J Urol. 1993;149:492–497. doi: 10.1016/s0022-5347(17)36126-8. [DOI] [PubMed] [Google Scholar]
- 3.Pagliaro LC, Crook J. Multimodality therapy in penile cancer: when and which treatments? World J Urol. 2009;27:221–225. doi: 10.1007/s00345-008-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pettaway CA, Horenblas S. Penile cancer: incremental insights into etiology, diagnosis, staging, and management. World J Urol. 2009;27:139–140. doi: 10.1007/s00345-008-0364-y. [DOI] [PubMed] [Google Scholar]
- 5.Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. 2006;93:133–138. doi: 10.1002/jso.20414. [DOI] [PubMed] [Google Scholar]
- 6.Theodore C, Skoneczna I, Bodrogi I, et al. for the EORTC Genito-Urinary Tract Cancer Group. A phase II multicentre study of irinotecan (CPT 11) in combination with cisplatin (CDDP) in metastatic or locally advanced penile carcinoma (EORTC PROTOCOL 30992) Ann Oncol. 2008;19:1304–1307. doi: 10.1093/annonc/mdn149. [DOI] [PubMed] [Google Scholar]
- 7.Pagliaro LC, Williams DL, Daliani D, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol. 2010;28:3851–3857. doi: 10.1200/JCO.2010.29.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bermejo C, Busby JE, Spiess PE, et al. Neoadjuvant chemotherapy followed by aggressive surgical consolidation for metastatic penile squamous cell carcinoma. J Urol. 2007;177:1335–1338. doi: 10.1016/j.juro.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Börgermann C, Schmitz KJ, Sommer S, et al. Characterization of the EGF receptor status in penile cancer: retrospective analysis of the course of the disease in 45 patients. [In German] Urologe A. 2009;48:1483–1489. doi: 10.1007/s00120-009-2101-6. [DOI] [PubMed] [Google Scholar]
- 10.Lavens N, Gupta R, Wood LA. EGFR overexpression in squamous cell carcinoma of the penis. Curr Oncol. 2010;17(1):4–6. doi: 10.3747/co.v17i1.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Necchi A, Nicolai A, Colecchia M, et al. Proof of activity of anti–epidermal growth factor receptor–targeted therapy for relapsed squamous cell carcinoma of the penis. J Clin Oncol. 2011;29:e650–e652. doi: 10.1200/JCO.2011.34.8367. [DOI] [PubMed] [Google Scholar]
- 12.Haas GP, Blumenstein BA, Gagliano RG, et al. Cisplatin, methotrexate and bleomycin for the treatment of carcinoma of the penis: a Southwest Oncology Group study. J Urol. 1999;161:1823–1825. [PubMed] [Google Scholar]
- 13.Maubec E, Petrow P, Scheer-Senyarich I, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29:3419–3426. doi: 10.1200/JCO.2010.34.1735. [DOI] [PubMed] [Google Scholar]
- 14.Chaux A, Cubilla AL. The role of human papillomavirus infection in the pathogenesis of penile squamous cell carcinomas. Semin Diagn Pathol. 2012;29:67–71. doi: 10.1053/j.semdp.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Di Lorenzo G, Buonerba C, Gaudioso G, et al. EGFR mutational status in penile cancer. Expert Opin Ther Targets. 2013;17:501–505. doi: 10.1517/14728222.2013.783571. [DOI] [PubMed] [Google Scholar]
- 16.Andersson P, Kolaric A, Windahl T. PIK3CA, HRAS and KRAS gene mutations in human penile cancer. J Urol. 2008;179:2030–2034. doi: 10.1016/j.juro.2007.12.040. [DOI] [PubMed] [Google Scholar]