Abstract
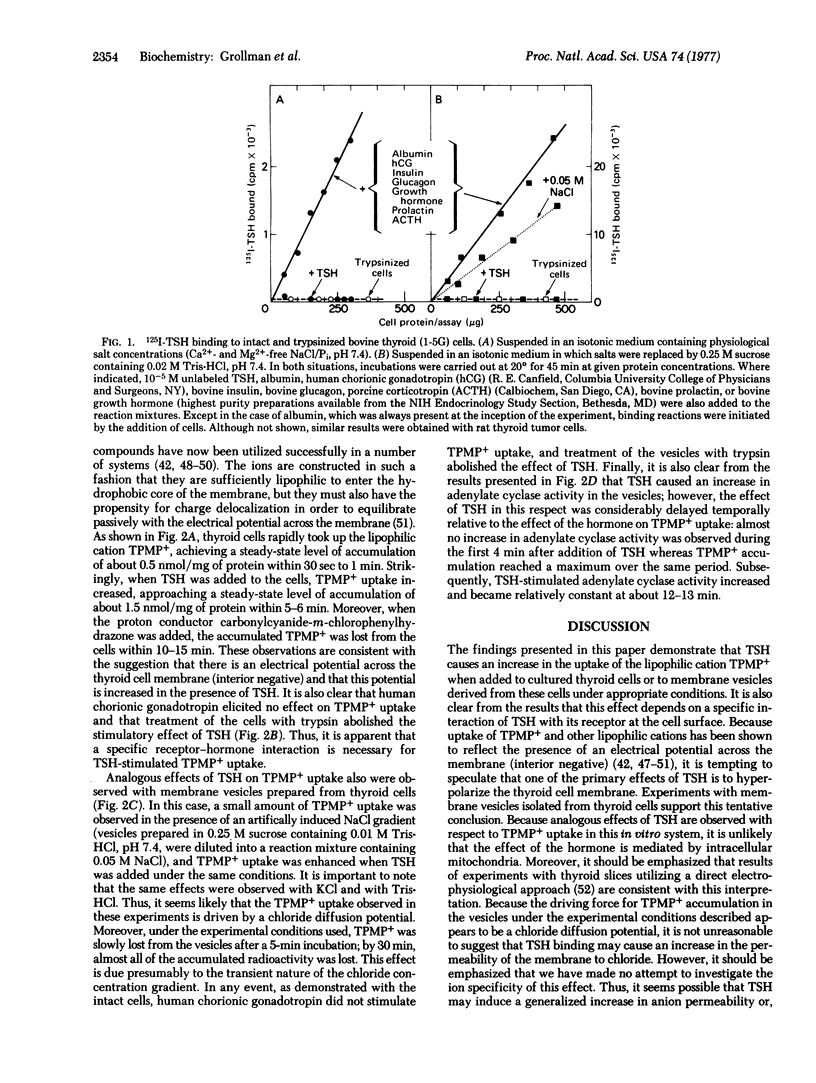
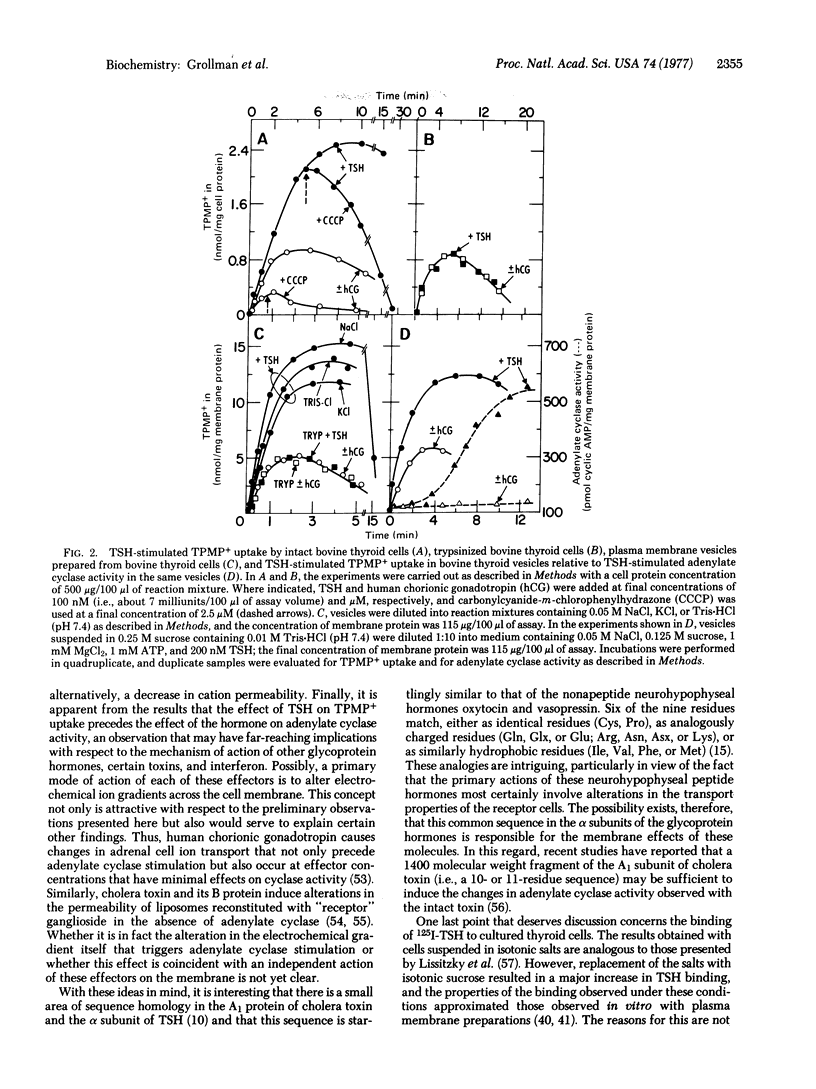
Cultured thyroid cells accumulate the lipophilic cation triphenylmethylphosphonium, indicating that there is an electrical potential (interior negative) across the plasma membrane. Thyrotropin stimulates the uptake of the lipophilic cation 3-fold, and the proton conductor carbonylcyanide-m-chlorophenylhydrazone causes efflux of triphenylmethylphosphonium accumulated in the presence or absence of thyrotropin. The stimulatory effect of thyrotropin on triphenylmethylphosphonium accumulation is not mimicked by human chorionic gonadotropin, a glycoprotein hormone with a similar structure whose target organ is not the thyroid, and the effect is abolished if the thyrotropin-receptor activity of the cells is destroyed by treatment with trypsin. Analogous effects are observed with thyroid plasma membrane vesicles which are essentially devoid of mitochondrial and soluble enzyme activities. Triphenylmethylphosphonium uptake and stimulation by thyrotropin occurs when NaCl, KCl, or Tris·HCl concentration gradients are artifically imposed across the vesicle membrane ([salt]out > [salt]in). It seems likely, therefore, that triphenylmethylphosphonium uptake is driven by a chloride diffusion potential (interior negative) and that thyrotropin either increases the permeability of the membrane to anions or decreases its permeability to cations. Thyrotropin-stimulated triphenylmethylphosphonium uptake in the vesicle preparations reaches a quasi steady-state within 3 min; in contrast, thyrotropin-stimulated adenylate cyclase activity is negligible during this period of time, becomes measurable after about 4 min, and is optimal after 12-15 min. Thus, a primary mode of action of thyrotropin on the thyroid cell may be an alteration in the electrical potential across the plasma membrane. The relevance of this observation to the mechanism of action of other glycoprotein hormones, certain bacterial toxins, and interferon is discussed.
Keywords: lipophilic ions, membrane vesicles, adenylate cyclase, toxins, interferon
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir S. M., Carraway T. F., Jr, Kohn L. D., Winand R. J. The binding of thyrotropin to isolated bovine thyroid plasma membranes. J Biol Chem. 1973 Jun 10;248(11):4092–4100. [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Bolonkin D., Tate R. L., Luber J. H., Kohn L. D., Winand R. J. Experimental exophthalmos. Binding of thyrotropin and an exophthalmogenic factor derived from thyrotropin to retro-orbital tissue plasma membranes. J Biol Chem. 1975 Aug 25;250(16):6516–6521. [PubMed] [Google Scholar]
- Coon H. G., Weiss M. C. A quantitative comparison of formation of spontaneous and virus-produced viable hybrids. Proc Natl Acad Sci U S A. 1969 Mar;62(3):852–859. doi: 10.1073/pnas.62.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cespedes C., Christensen H. N. Complexity in valinomycin effects on amino acid transport. Biochim Biophys Acta. 1974 Feb 26;339(1):139–145. doi: 10.1016/0005-2736(74)90339-3. [DOI] [PubMed] [Google Scholar]
- Ensor J. M., Munro D. S. A comparison of the in-vitro actions of thyroid-stimulating hormone and cyclic 3',5'-adenosine monophosphate on the mouse thyroid gland. J Endocrinol. 1969 Mar;43(3):477–485. doi: 10.1677/joe.0.0430477. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Aloj S. M., Kohn L. D., Brady R. O. Relationship of gangliosides to the structure and function of thyrotropin receptors: their absence on plasma membranes of a thyroid tumor defective in thyrotropin receptor activity. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4060–4064. doi: 10.1073/pnas.73.11.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Kohn L. D. Cholera toxin inhibits interferon action. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1078–1084. doi: 10.1016/0006-291x(76)91012-3. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., MII S., KOHOUT P. M. Studies on the terminal electron transport system. I. Succinic dehydrogenase. J Biol Chem. 1955 Dec;217(2):551–567. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPEL L. A., HILMORE R. J. Purification and properties of 5-nucleotidase. J Biol Chem. 1951 Feb;188(2):665–676. [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hladky S. B. Ion transport across thin lipid membranes: a critical discussion of mechanisms in selected systems. Q Rev Biophys. 1972 May;5(2):187–282. doi: 10.1017/s0033583500000883. [DOI] [PubMed] [Google Scholar]
- Heinz E., Geck P., Pietrzyk C. Driving forces of amino acid transport in animal cells. Ann N Y Acad Sci. 1975 Dec 30;264:428–441. doi: 10.1111/j.1749-6632.1975.tb31501.x. [DOI] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLAINER L. M., CHI Y. M., FREIDBERG S. L., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. IV. The effects of neurohormones on the formation of adenosine 3',5'-phosphate by preparations from brain and other tissues. J Biol Chem. 1962 Apr;237:1239–1243. [PubMed] [Google Scholar]
- Kohn L. D., Friedman R. M., Holmes J. M., Lee G. Use of thyrotropin and cholera toxin to probe the mechanism by which interferon initiates its antiviral activity. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3695–3699. doi: 10.1073/pnas.73.10.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L. D., Winand R. J. Relationship of thyrotropin to exophthalmos-producing substance. Formation of an exophthalmos-producing substance by pepsin digestion of pituitary glycoproteins containing both thyrotropic and exophthalmogenic activity. J Biol Chem. 1971 Nov;246(21):6570–6575. [PubMed] [Google Scholar]
- Kryzhanovsky G. N. The mechanism of action of tetanus toxin: effect on synaptic processes and some particular features of toxin binding by the nervous tissue. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(3-4):247–270. doi: 10.1007/BF00499880. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lebon P., Moreau M. C., Cohen L., Chany C. Different effect of ouabain on the interferon production and action. Proc Soc Exp Biol Med. 1975 May;149(1):108–112. doi: 10.3181/00379727-149-38753. [DOI] [PubMed] [Google Scholar]
- Ledley F. D., Mullin B. R., Lee G., Aloj S. M., Fishman P. H., Hunt L. T., Dayhoff M. O., Kohn L. D. Sequence similarity between cholera toxin and glycoprotein hormones: implications for structure activity relationship and mechanism of action. Biochem Biophys Res Commun. 1976 Apr 19;69(4):852–859. doi: 10.1016/0006-291x(76)90452-6. [DOI] [PubMed] [Google Scholar]
- Lee G., Aloj S. M., Brady R. O., Kohn L. D. The structure and function of glycoprotein hormone receptors: ganglioside interactions with human chorionic gonadotropin. Biochem Biophys Res Commun. 1976 Nov 22;73(2):370–377. doi: 10.1016/0006-291x(76)90717-8. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Active amino acid transport in plasma membrane vesicles from Simian virus 40-transformed mouse fibroblasts. Characteristics of electrochemical Na+ gradient-stimulated uptake. J Biol Chem. 1977 Mar 25;252(6):1990–1997. [PubMed] [Google Scholar]
- Lever J. E. Regulation of active alpha-aminoisobutyric acid transport expressed in membrane vesicles from mouse fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2614–2618. doi: 10.1073/pnas.73.8.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissitzky S., Fayet G., Verrier B., Hennen G., Jaquet P. Thyroid-stimulating hormone binding to cultured thyroid cells. FEBS Lett. 1973 Jan 1;29(1):20–24. doi: 10.1016/0014-5793(73)80006-7. [DOI] [PubMed] [Google Scholar]
- Macchia V., Meldolesi M. F., Chiariello M. Adenyl-cyclase in a transplantable thyroid tumor: loss of ability to respond to TSH. Endocrinology. 1972 Jun;90(6):1483–1491. doi: 10.1210/endo-90-6-1483. [DOI] [PubMed] [Google Scholar]
- Matuo Y., Wheeler M. A., Bitensky M. W. Small fragments from the A subunit of cholera toxin capable of activating adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2654–2658. doi: 10.1073/pnas.73.8.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKENZIE J. M. The bioassay of thyrotropin in serum. Endocrinology. 1958 Sep;63(3):372–382. doi: 10.1210/endo-63-3-372. [DOI] [PubMed] [Google Scholar]
- Mendelson C., Dufau M., Catt K. Gonadotropin binding and stimulation of cyclic adenosine 3':5'-monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975 Nov 25;250(22):8818–8823. [PubMed] [Google Scholar]
- Moss J., Fishman P. H., Richards R. L., Alving C. R., Vaughan M., Brady R. O. Choleragen-mediated release of trapped glucose from liposomes containing ganglioside GM1. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3480–3483. doi: 10.1073/pnas.73.10.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Richards R. L., Alving C. R., Fishman P. H. Effect of the A and B protomers of choleragen on release of trapped glucose from liposomes containing or lacking ganglioside GM1. J Biol Chem. 1977 Jan 25;252(2):797–798. [PubMed] [Google Scholar]
- Mullin B. R., Aloj S. M., Fishman P. H., Lee G., Kohn L. D., Brady R. O. Cholera toxin interactions with thyrotropin receptors on thyroid plasma membranes. Proc Natl Acad Sci U S A. 1976 May;73(5):1679–1683. doi: 10.1073/pnas.73.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin B. R., Fishman P. H., Lee G., Aloj S. M., Ledley F. D., Winand R. J., Kohn L. D., Brady R. O. Thyrotropin-ganglioside interactions and their relationship to the structure and function of thyrotropin receptors. Proc Natl Acad Sci U S A. 1976 Mar;73(3):842–846. doi: 10.1073/pnas.73.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I. The effect of dibutyryl cyclic 3',5'-AMP on the thyroid. Biochem Biophys Res Commun. 1966 Oct 5;25(1):14–16. doi: 10.1016/0006-291x(66)90632-2. [DOI] [PubMed] [Google Scholar]
- Pastan I., Wollman S. H. Colloid droplet formation in dog thyroid in vitro. Induction by dibutyrl cyclic-AMP. J Cell Biol. 1967 Oct;35(1):262–266. doi: 10.1083/jcb.35.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Tate R. L., Schwartz H. I., Holmes J. M., Kohn L. D. Thyrotropin receptors in thyroid plasma membranes. Characteristics of thyrotropin binding and solubilization of thyrotropin receptor activity by tryptic digestion. J Biol Chem. 1975 Aug 25;250(16):6509–6515. [PubMed] [Google Scholar]
- WOLLMAN S. H. PRODUCTION AND PROPERTIES OF TRANSPLANTABLE TUMORS OF THE THYROID GLAND IN THE FISCHER RAT. Recent Prog Horm Res. 1963;19:579–618. [PubMed] [Google Scholar]
- Williams J. A., Wolff J. Thyroid secretion in vitro: multiple actions of agents affecting secretion. Endocrinology. 1971 Jan;88(1):206–217. doi: 10.1210/endo-88-1-206. [DOI] [PubMed] [Google Scholar]
- Winand R. J., Kohn L. D. Relationships of thyrotropin to exophthalmic-producing substance. Purification of homogeneous glycoproteins containing both activities from [3H]-labeled pituitary extracts. J Biol Chem. 1970 Mar 10;245(5):967–975. [PubMed] [Google Scholar]
- Winand R. J., Kohn L. D. Thyrotropin effects on thyroid cells in culture. Effects of trypsin on the thyrotropin receptor and on thyrotropin-mediated cyclic 3':5'-AMP changes. J Biol Chem. 1975 Aug 25;250(16):6534–6540. [PubMed] [Google Scholar]
- Wolff J., Jones A. B. The purification of bovine thyroid plasma membranes and the properties of membrane-bound adenyl cyclase. J Biol Chem. 1971 Jun 25;246(12):3939–3947. [PubMed] [Google Scholar]
- Yamashita K., Field J. B. Preparation of thyroid plasma membranes containing a TSH-responsive adenyl cyclase. Biochem Biophys Res Commun. 1970 Jul 13;40(1):171–178. doi: 10.1016/0006-291x(70)91062-4. [DOI] [PubMed] [Google Scholar]


