Abstract
Objectives
We have previously demonstrated activity of Apo2L/TRAIL against patient pancreatic tumor xenografts. Here, we have examined the influence of the tumor implantation site on therapeutic response of orthotopic tumors and their metastases to Apo2L/TRAIL.
Methods
Sensitivity of six patient pancreatic tumor xenografts to Apo2L/TRAIL was determined in a subcutaneous model. To compare the response of orthotopic tumors, cells from subcutaneous xenografts were injected into the pancreas. Tumor growth was confirmed by histological examination of selected mice and then treatment was started. When all control mice developed externally palpable tumors, the experiment was terminated and pancreatic weights compared between control and treated groups. Magnetic resonance imaging was used to quantitate the response of orthotopic and metastatic tumors.
Results
The sensitivity to Apo2L/TRAIL observed in subcutaneous tumors was maintained in orthotopic tumors. Metastatic spread was observed with orthotopic tumor implantation. In an orthotopic model of a sensitive tumor, primary and metastatic tumor burden was significantly reduced and median survival significantly extended by Apo2L/TRAIL therapy.
Conclusions
Our data provide evidence that the site of tumor engraftment does not alter the inherent sensitivity of patient xenografts to Apo2L/TRAIL and these results highlight the potential of Apo2/TRAIL therapy against primary and metastatic pancreatic cancer.
Keywords: patient pancreatic tumor xenograft, implantation site, Apo2L/TRAIL, metastatic model
INTRODUCTION
The most common approach for preclinical evaluation of novel therapies in cancer research involves the conduct of studies in mice bearing subcutaneous human tumor xenografts.1,2 However, it is now well recognized that the therapeutic sensitivity of a tumor can be significantly influenced by the microenvironment. For example, the sensitivity of colon carcinoma cells to doxorubicin, but not 5-FU, varies depending on the implantation site3. Since tumors may respond to therapeutics in an agent and site dependent manner, it is important to determine the response of the tumor to a particular therapy in more than one site (e.g. orthotopic vs. subcutaneous) as well as to test the response of established metastases.
In this study we investigated the responses of six patient pancreatic tumor xenografts engrafted in two different sites (subcutaneous vs. orthotopic) to evaluate the influence of the implantation site on tumor response to the targeted therapy Apo2L/TRAIL. We also investigated the response of metastases derived from one of these tumors. Apo2L/TRAIL directly induces apoptosis through the cell surface death receptors DR4 and DR54 and we have previously shown that some patient tumors grown subcutaneously in SCID mice are sensitive to treatment with Apo2L/TRAIL while others are quite resistant.5 Recent clinical trials have shown that Apo2L/TRAIL has a good safety profile, although limited efficacy was observed.6 However, because Apo2L/TRAIL targets malignant cells without similar toxicity to normal cells4,7 there is still enthusiasm for therapies targeting death receptors and, in addition to Apo2L/TRAIL, antibodies to death receptors are also being investigated clinically.8,9 For this reason, the response of patient tumor xenografts to therapies targeting death receptors is clinically relevant and it is important in order to understand the degree to which the response of a tumor is inherent or influenced by different implantation sites.
Our results show that patient pancreatic xenografts responded to Apo2L/TRAIL in similarly in both implantation sites. Using a sensitive tumor, we developed a metastatic model and found that treated mice also had significantly reduced metastatic tumor burden compared to controls. Moreover, treatment of mice with confirmed metastases significantly improved survival, demonstrating that these metastatic tumors did not acquire resistance to Apo2L/TRAIL.
METHODS
Mice
Severe Combined Immunodeficient mice (SCID- strain C.B Igh-1bIcrTac-Prkdcscid/Ros) were obtained from the RPCI breeding colony. Experiments were approved by the Institute Animal Use and Care Committee (IACUC).
Surgical specimens and subcutaneous patient tumor xenografts
De-identified patient specimens were obtained through the Pathology Research Network and are referred to by a procurement number. The specimens were implanted subcutaneously in 2–4 mice5, engrafted tumors were passaged and subsequently used as donor tumors for experiments.
Apo2L/TRAIL treatment
Mice in the treatment groups received 500µg Apo2L/TRAIL/200µl saline (Apo2L/TRAIL was a gift from Genentech and Amgen) and mice in the control groups received 200µl saline daily. Characterization of the anti-tumor effect on subcutaneous tumors, was carried out as previously described5.
Orthotopic engraftment
Subcutaneous xenografted donor tumors were minced in Accutase (Innovative Cell Technologies, Inc., San Diego, CA cat. no. AT-104), incubated at room temperature for 20 minutes and filtered through sterile 40-µm nylon filters. Cell yield and viability was assessed by trypan blue. 2×106 cells were suspended in 25µl of RPMI media and mixed 1:1 with matrigel (BD Biosciences, cat. no. 356230). Surgical procedures were performed under sterile conditions in the LAR facility. Mice were anesthetized with Isoflurane, an incision in the skin of the left flank just inferior to the lowermost palpable rib was made, the peritoneum was incised and the spleen with the tail of the pancreas was exteriorized. 50 µl of cell suspension was injected into the tail of the pancreas by injecting parallel to the length of pancreas and observing the formation of a bleb. Alternatively, a 5mm3 piece of donor tumor was sutured to the surface of pancreas with 5-0 Vicryl. The spleen and pancreas were repositioned and the peritoneal cavity closed with 5-0 Vicryl suture. The skin was closed with Vetbond (3M Corp St Paul MN, No 1469SB). Buprenorphine (0.05 mg/kg SC) was given post-operatively for analgesia. In “survival” experiments, the mice were euthanized when they showed signs of distress including abdominal distension, jaundice or loss of more than 20% of body weight. The growth of orthotopic tumors and their metastases was evaluated by either assessing tumor burden (i.e. weight of the pancreas at the end of the experiment) or in vivo by magnetic resonance imaging.
Magnetic resonance imaging (MRI)
MRI was performed weekly beginning at 13 weeks post-implantation, using a 4.7 T/33-cm horizontal bore magnet (GE NMR Instruments, Fremont, CA) incorporating a removable gradient coil insert (G060; Bruker Medical Inc., Billerica, MA) generating maximum field strength of 950 mT/m and a custom-designed 35-mm RF transmit-receive coil. Animals were placed prone on an MR-compatible sled (Dazai Research Instruments, Toronto, Canada) within a carrier tube and positioned in the magnet. Induction and maintenance of anesthesia during imaging was achieved by inhalation of 2–3% Isoflurane in oxygen (Abbott Laboratories, IL). Coronal T2-weighted (T2W) images were acquired with the following parameters: FOV = 4.8 × 3.2 cm, matrix (MTX) = 256 × 192, number of slices = 21, slice thickness = 1.00 mm, TEeff = 41 ms, TR = 2424 ms, no. of averages = 4. Contrast-enhanced MRI was performed using the super paramagnetic iron oxide (SPIO) nanoparticle, FeREX (BioPal Inc., Worcester, MA). At the 15 week time point, coronal T2-weighted images were acquired before and after intravenous administration of FeREX (0.1cc of 0.5 mg/ml) for enhanced visualization of metastatic lesions. Following image acquisition, raw image sets were transferred to a processing workstation and processed using the medical imaging software, Analyze (AnalyzeDirect, version 7.0; Overland Park, KS). Primary tumor volumes and metastatic burden (mm3) were calculated from manually traced regions-of-interest (ROI). A two-tailed unpaired t test was used to compare primary tumor and metastatic burden between animals in the control and treatment group.
Histology
Tissues were formalin-fixed and processed for routine histology. Immunohistochemistry to identify cells of human origin was carried out with anti-human mitochondrial antibody (Chemicon International, Temecula CA, #MAB 1273) following antigen retrieval in citrate buffer.
Statistical Analysis
Significance was set at p=0.05. Graph Pad prism 5 analytical software was used for Student t-tests.
RESULTS
Tumor sensitivity to Apo2L/TRAIL in a subcutaneous site
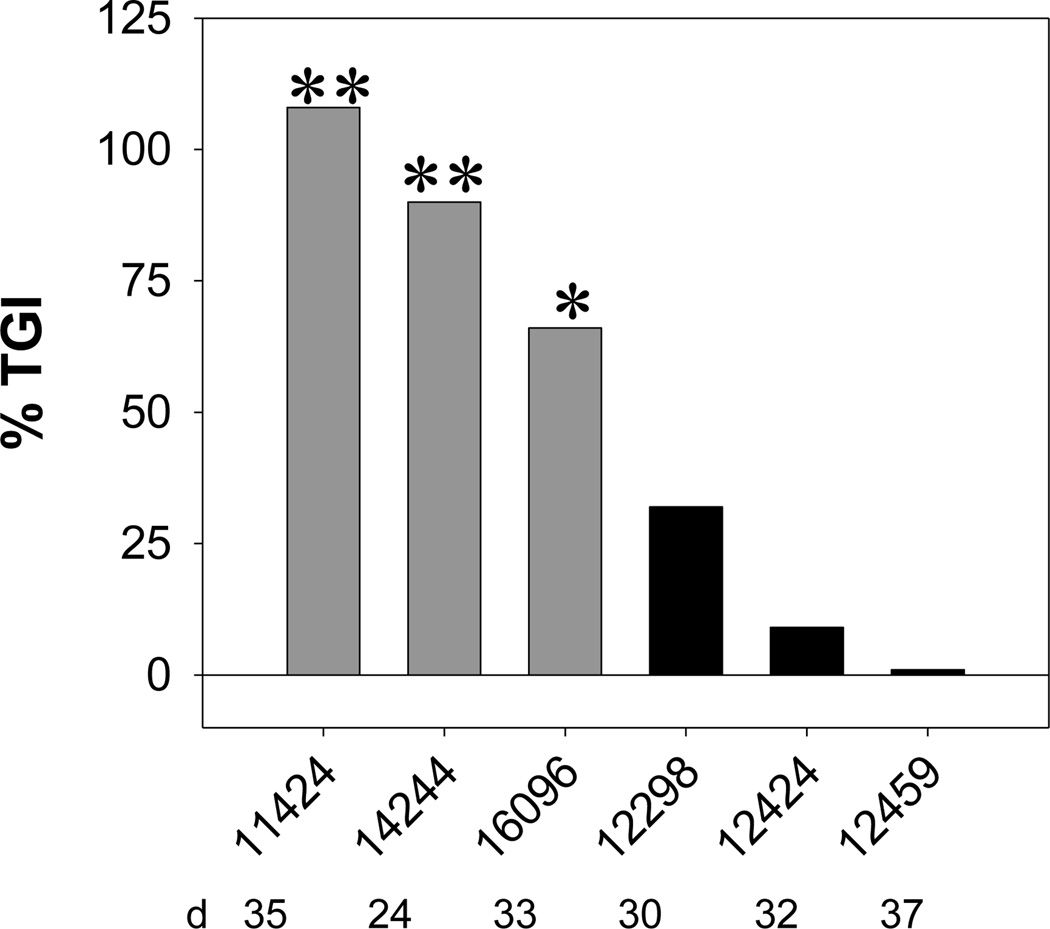
The response of several different patient pancreatic tumors to Apo2L/TRAIL was determined as previously described5. Mice were treated for either one or two 2-week cycles. If tumor growth was significantly inhibited by Apo2L/TRAIL treatment (Fig. 1, p< 0.05), tumors were designated sensitive (#11424, #14244, #16096) and, if not, they were designated resistant (#12298, #12424, #12459).
FIGURE 1.
Degree of tumor growth inhibition (TGI) of patient pancreatic tumors engrafted subcutaneously in SCID mice achieved by Apo2L/TRAIL treatment for 3 sensitive and 3 resistant xenografts. Tumor bearing mice were treated with 500µg/dose of Apo2L/TRAIL daily for approximately two 2-week cycles, separated by 1 week rest (the day on which the % tumor growth inhibition was calculated varied slightly between experiments as indicated). Sensitivity was designated based on the results of the Student t-test comparing average tumor size on day indicated (**p<0.005; *p<0.05). Gray bars- sensitive tumors; black bars- resistant tumors.
The orthotopic model
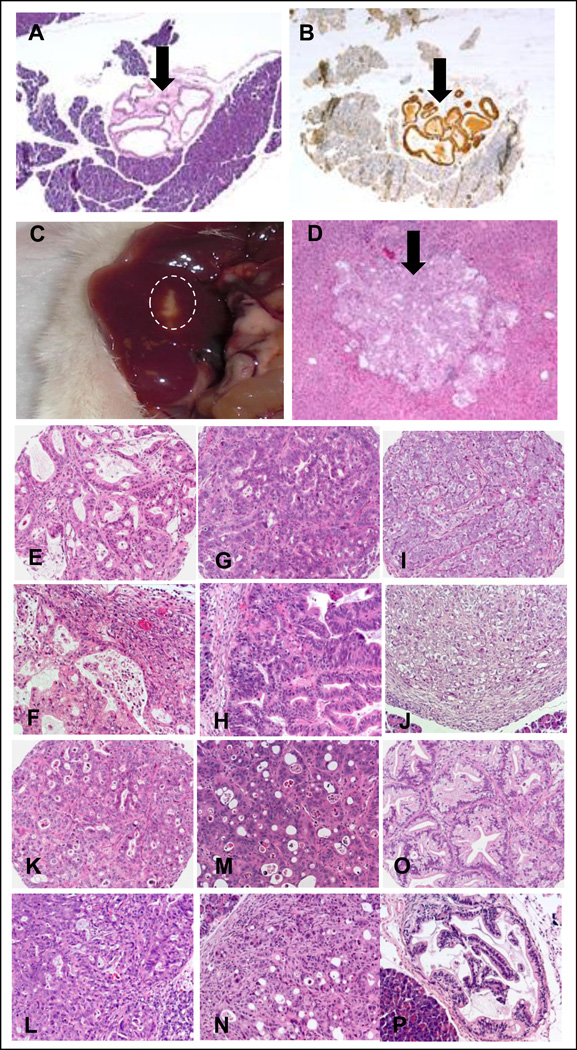
Two approaches for establishing orthotopic pancreatic xenografts, using either histologically intact tumors10 or a cell suspension from disaggregated tumors11 were evaluated. Both methods developed tumors at the primary site, but only tumors from the cell suspensions developed metastases (data not shown). The presence of primary tumor within the pancreas was confirmed by histology and immunohistochemistry for human mitochondria (Fig. 2A, B) and visible liver metastases (Fig. 2C) were confirmed histologically (Fig. 2D). Based on these observations, injection of a cell suspension was chosen to establish orthotopic tumors.
FIGURE 2.
Histology of engrafted pancreatic xenograft #11424. A. Viable tumor cells (arrow) can be identified in the SCID mouse pancreas. B. In a serial section, these cells are positive by IHC for human mitochondria (arrow) and thus confirmed to be of human origin. C. A metastatic lesion is grossly visible in the periphery of the liver (circled). D. A metastatic lesion in a section of the liver (arrow). E–P. Histological features of individual tumors are identical in subcutaneous and orthotopic sites: for each tumor the subcutaneous (SC) sample is shown above the orthotopic (Ortho) location: (#11424 E-SC, F-Ortho; #14244 G-SC, H-Ortho; #16096 I-SC, J-Ortho; #12298 K-SC, L-Ortho; #12424 M-SC, N-Ortho; #12459 O-SC, P-Ortho).
We then characterized the engraftment kinetics of the six tumors by engrafting groups of mice and necropsying one mouse weekly. Histologically identifiable tumors were seen in sections of pancreas in the three sensitive tumors between 2–5 weeks post-implantation (11424- 26 days, 14244- 39 days and 16096- 20 days). Development of the resistant tumors took longer (12298- 112 days, 12459- 68 days and 12424- 43 days). Liver metastases from 11424 and 14244 were found at 9 and 21 weeks respectively. Among the resistant tumors, only a single mouse with tumor 12298 developed a liver metastasis at 38 weeks. A comparison of tumors grown subcutaneously vs. orthotopically shows that the histologies are similar and distinctive histological features are retained in both sites (Fig. 2 E–P).
Tumor sensitivity or resistance to Apo2L/TRAIL is maintained in an orthotopic setting
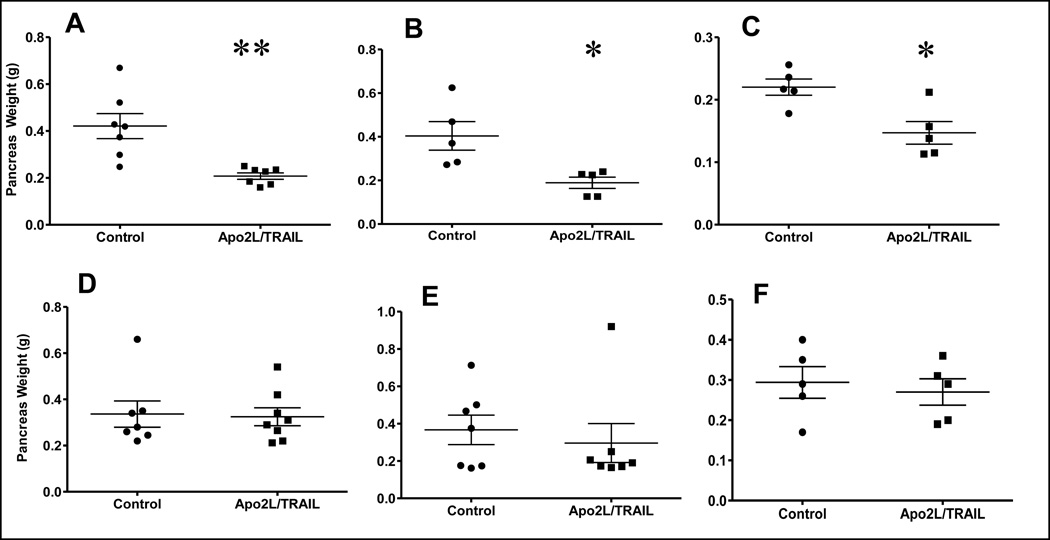
To investigate whether the sensitivity to Apo2L/TRAIL is influenced by tumor location, each sensitive tumor was engrafted orthotopically in groups of mice. After two weeks, one mouse per group was necropsied weekly until tumor engraftment was confirmed histologically. The remaining mice were divided into two groups and treatment initiated (see Table 1). Experiments were terminated when the majority of control mice developed large, externally palpable tumors (11424= 5/7, 14244= 7/7 and 16096= 4/5); upon necropsy tumors (too small to be palpated) were found in all control mice. In contrast, very few mice in the treated groups had large, palpable tumors (11424= 0/7; 14244= 0/7 and 16096= 2/5), although histological evidence of small tumor foci was found in all mice. Correspondingly, the average pancreatic weight was significantly higher in control vs. treated mice (Fig. 3A–C).
TABLE 1.
Response of patient orthotopic pancreatic xenografts to treatment with Apo2L/TRAIL. Tumors were engrafted, selected mice were removed and examined for histological evidence of tumor growth, the remaining mice experiment were divided into control and treatment groups and treatment was intitiated. The number of mice with large, externally palpable tumors was assessed at the end of treatment as indicated. Few treated mice bearing tumors 11424, 14244 and 16096 had palpable tumors compared to the controls, while similar numbers of control and treated mice with tumors 12298, 12424 and 12459 had large, palpable tumors.
| Tumor # | Day treatment began |
Day treatment ended |
Total days treated |
Control mice with externally palpable tumors |
Apo2L/TRAIL treated mice with externally palpable tumors |
|---|---|---|---|---|---|
| 11424 | 16 | 35 | 19 | 5/7 | 0/7 |
| 14244 | 27 | 55 | 28 | 5/7 | 0/7 |
| 16096 | 29 | 77 | 48 | 4/5 | 2/5 |
| 12298 | 108 | 136 | 28 | 7/7 | 8/8 |
| 12424 | 82 | 143 | 61 | 6/7 | 5/7 |
| 12459 | 35 | 55 | 55 | 2/5 | 3/5 |
FIGURE 3.
Responses of the six patient pancreatic xenografts engrafted orthotopically to Apo2L/TRAIL treatment. Mice were treated daily with Apo2L/TRAIL and tumor burden (as indicated by weight of the pancreas) was evaluated at the end of treatment. A–C. Mice with tumors 11424 (A), 14244 (B) and 16096 (C) all showed a significant differences in pancreatic weight between control and treated mice; D–E Mice with tumors 12298 (D), 12424 (E) and 12459 (F) did not. (**p<0.005; *p<0.05).
When similar experiments were done using resistant tumors, mice in both control and treated groups developed large, palpable tumors (Table 1) and the pancreatic weights were not significantly different (Fig. 3D–F). However, we observed a tendency towards increased sensitivity to Apo2L/TRAIL in tumor #12424 (Fig. 3E, p= 0.07). Additional experiments showed that subcutaneous tumors established from intact tumor pieces displayed resistance as originally observed (n=5, p= 0.89) while subcutaneous tumors established by injection of disaggregated cells in matrigel showed a trend towards increased sensitivity (n=5, p= 0.08) based on final tumor volume (data not shown). This suggests that it is the disaggregation of this tumor that slightly increases its sensitivity to Apo2L/TRAIL and not the difference in implantation site.
Because it has been reported that Apo2L/TRAIL treatment of tumors in which the apoptotic pathway is blocked can increase metastasis12, we examined mice bearing resistant tumors for signs of metastatic lesions after treatment. Neither gross nor histological examination of the liver, peritoneum, spleen, diaphragm, viscera, lymph, and portal lymph nodes revealed any evidence of increased metastases.
Apo2L/TRAIL inhibits growth of primary and metastatic disease and confers survival benefit
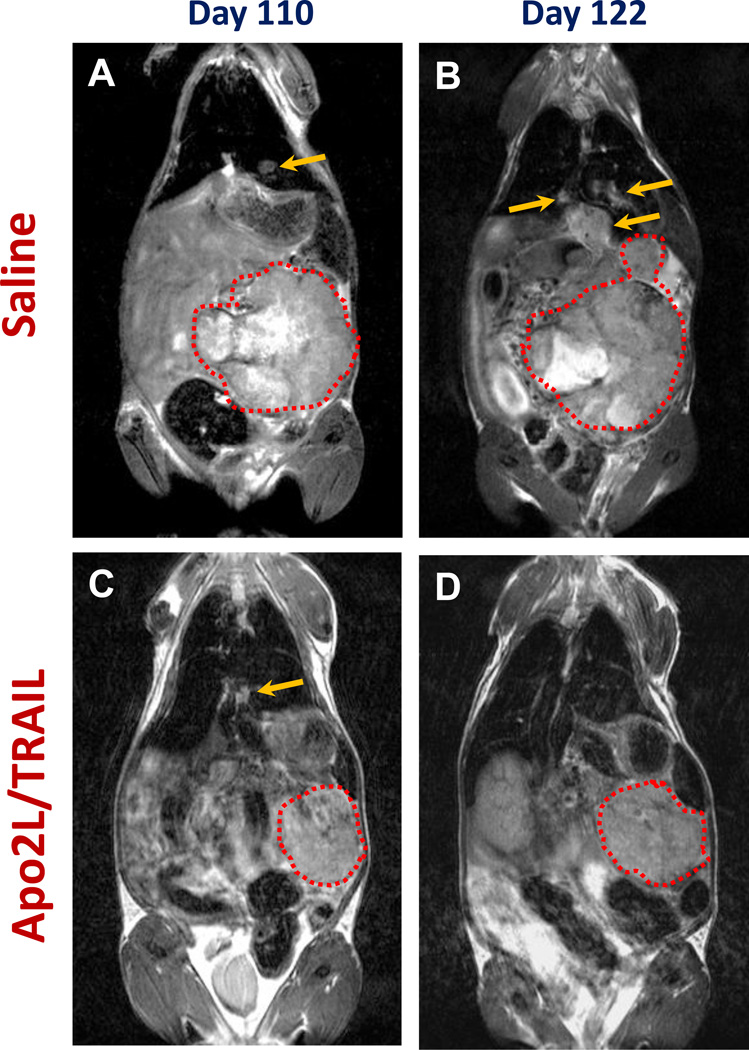
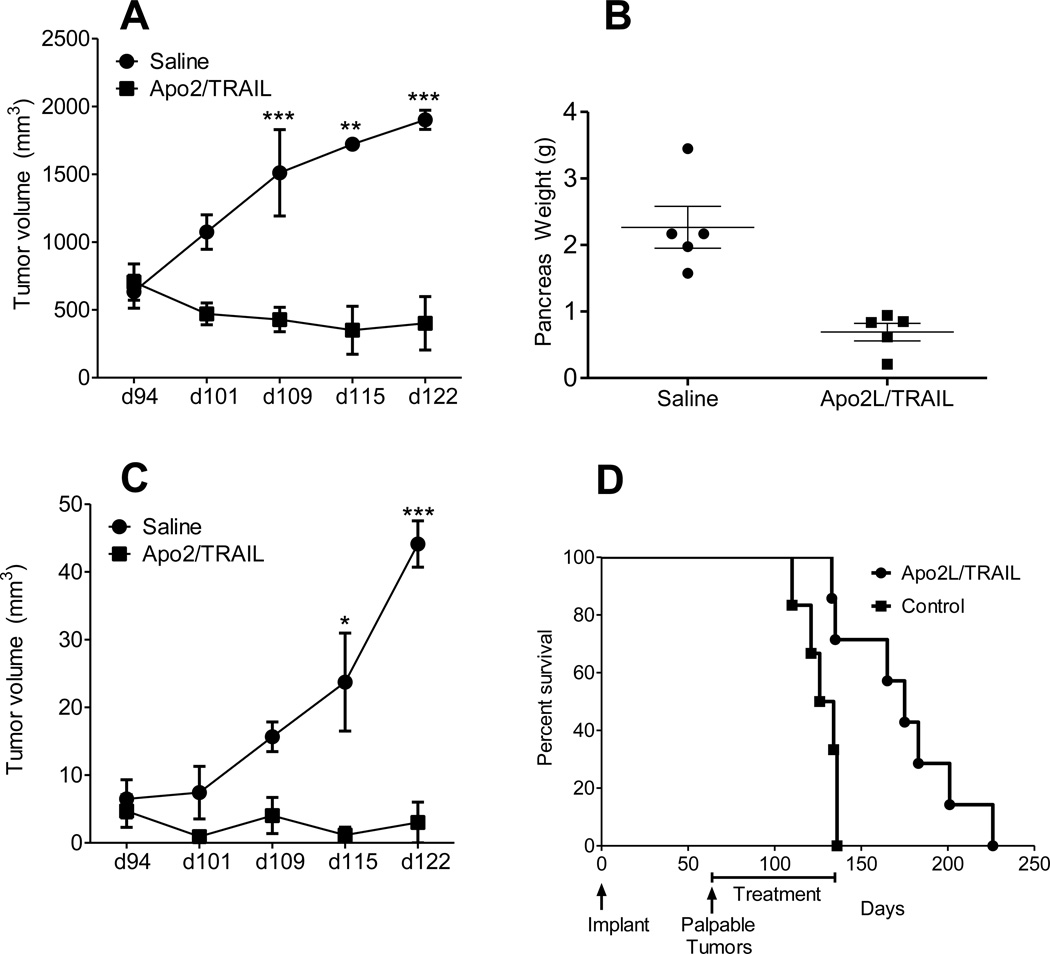
To examine the activity of Apo2/TRAIL against primary and metastatic pancreatic cancer, 13 mice were implanted orthotopically with a metastasizing tumor (#11424; Control, n=6, and Apo2L/TRAIL treated, n= 7) and treatment was initiated at week 13 because tumor #11424 was seen to develop metastases between 8–13 weeks. MRI imaging was performed weekly to monitor primary tumors and metastatic burden. Measured values are reported as mean ± standard error of the mean. Representative coronal T2-weighted MR images (primary tumor outlined in red) of a saline-treated mouse and a mouse treated with Apo2L/TRAIL at two different time points are shown in Fig. 4. Growth curves of primary tumors in saline treated animals and animals treated with Apo2/TRAIL are shown in Fig. 5A. Primary tumor volumes of animals in both groups were comparable at the time treatment was initiated (d94: saline= 634 ± 121 mm3, Apo2L/TRAIL= 705 ± 134 mm3; p>0.05). A significant increase (p<0.05) in primary tumor volume was observed in saline-treated controls on day 109 (1511± 318 mm3), day 115 (1722 ± 18 mm3) and day 122 (1902 ± 70 mm3). In contrast, a marked degree of tumor growth inhibition was observed in Apo2L/TRAIL treated animals. Differences in tumor volume between saline and TRAIL treatment groups were statistically significant on day 109, d115 and d122. In agreement with the MRI data, the pancreatic weights in the control mice were significantly higher than those of treated mice (Fig. 5B).
FIGURE 4.
Response of orthotopic and metastatic pancreatic cancer to Apo2L/TRAIL. The presence of both primary and metastatic tumors was confirmed prior to initiating treatment on day 94. Representative coronal T2-weighted MR images of a saline-treated mouse (A, B) and an Apo2L/TRAIL treated mouse (C, D) on day 110 (A, C) and on day 122 (B, D) in which the appearance of both primary and metastatic lesions are indicated (primary tumors are outlined in red; arrows point to metastatic lesions).
FIGURE 5.
Growth of primary orthotopic tumors and metastatic lesions is inhibited by Apo2L/TRAIL and survival is extended in treated mice. A. Growth curves of primary tumors in control and Apo2/TRAIL treated mice. B. Final pancreatic weights in the mice shown in (A). C. Metastatic burden (mm3) of control and Apo2/TRAIL treated animals at different time points post implantation. Differences in metastatic burden between animals in saline and treatment groups were statistically significant on day 115 (p<0.05) and d122 (p<0.001). D. Kaplan-Meier survival curves of control and Apo2/TRAIL treated animals. A significant increase in median survival was observed following Apo2/TRAIL treatment (175 days; p=0.01) compared to control animals (130 days).
The effect of Apo2L/TRAIL therapy on metastatic disease was also investigated using MRI. Non-contrast enhanced T2-weighted and SPIO-enhanced T2-weighted MRI was utilized to visualize metastatic lesions in mice. Metastatic lesions were visualized in the liver, spleen and diaphragm in 5/6 control animals and 5/7 Apo2L/TRAIL treated animals (Fig.4). Fig. 5C shows metastatic burden (mm3) of saline and Apo2/TRAIL treated animals at different time points post implantation. A significant increase (p<0.001) in metastatic burden was observed in saline-treated controls between day 94 (6.4± 2.8 mm3) and day 122 (44.1 ± 3.4 mm3). In comparison, Apo2L/TRAIL treated animals showed significant inhibition of metastatic burden over time. Differences in metastatic burden between animals in saline and treatment groups were statistically significant on day 115 (p<0.05) and d122 (p<0.001).
Finally, in a separate experiment, we investigated whether Apo2L/TRAIL therapy in the metastasizing model had survival benefit. Treatment was initiated at 8 weeks when the majority of mice developed palpable pancreatic tumors. Mice were monitored for signs of distress and the time of sacrifice was considered the survival end-point. All the mice in the control group reached this end point by week 20, whereas the majority of mice in the treatment group were still surviving. Therefore, the treatment was stopped and the remaining mice in the treatment group evaluated by MRI (n=5). MRI imaging revealed minimal tumor burden in pancreas and no evidence of metastases supporting the conclusion that inhibition of growth of both primary and metastatic tumors by Apo2L/TRAIL significantly improved the survival of these mice. Following cessation of treatment, the mice progressed and met the criteria for the survival end-point; all mice were sacrificed by d226. The mean survival of the groups was 130 days for the control group and 175 days for the treated group (Fig. 5D; p= 0.01).
DISCUSSION
Preclinical tumor models derived from primary patient specimens may be more representative of patient tumors than long- established cell lines and may better predict the response of patient tumors (see10,11,13). We have established patient tumor xenografts for several solid tumors including breast, colon, pancreas and head and neck.14–17 Using patient pancreatic and colon tumor xenografts implanted subcutaneously, we have previously characterized the response of these tumors to Apo2L/TRAIL.5,18,19 Since tumor biology and therapeutic response can be strongly influenced by the microenvironment, we have now examined the activity of Apo2/TRAIL against an orthotopic/metastatic model of pancreatic cancer. This study extends our previous work and shows that the response of six patient pancreatic tumor xenografts grown subcutaneously to Apo2L/TRAIL is maintained in a different (orthotopic) location as well as in metastases of a sensitive tumor.
There have been limited studies comparing the efficacy of targeting death receptors in subcutaneous and orthotopic locations. One study compared the response to Apo2L/TRAIL of NCI-H460 lung tumors engrafted subcutaneously to those engrafted orthotopically20 and observed inhibition of tumor growth (by Apo2L/TRAIL alone which was enhanced in combination with chemotherapy) in the two locations as well as improved survival of mice. A similar study was carried out evaluating the responses of NCI-H460 to a DR5 targeting antibody (drozitumab) with similar results.21 Also, in a study of breast cancer cell lines, it was shown that these lines are more sensitive to anti-DR5 (lexatumumab) than anti-DR4 antibodies (mapatumumab) and that antibody treatment of mice with orthotopic MDA-MB-231 tumors resulted in reduced growth of primary tumors as well as statistically significantly fewer metastases.22
Orthotopic models of pancreatic adenocarcinoma which metastasize to clinically relevant sites have been developed using cell lines and patient tumors.10,23–25 In several of these studies, inhibition of the primary tumor has been shown to correlate with inhibition of formation of metastases. However, instances in which the response of the metastases differed from that of the primary have also been observed, for example, inhibition of hedgehog signaling by cyclopamine did not significantly inhibit primary orthotopic pancreatic tumor growth, while it did significantly decrease the instance of metastases.26. In a clinical setting however, metastatic disease is often present at diagnosis and so a model in which the efficacy of a therapeutic can be evaluated against established metastases is valuable. Recently, using a metastatic melanoma model in which metastasis occurs within a predictable time frame, the ability of metronomic chemotherapy to improve survival and control established metastases in melanoma was demonstrated.27 In this study, we utilized MRI to non-invasively monitor primary and metastatic tumor burden in mice with orthotopic tumors. While optical imaging methods including bioluminescence and fluorescence imaging are widely utilized in preclinical studies, they require transfection of tumor cell lines with optically active reporters. This approach is not feasible with xenografts established from surgically derived tumor tissue. In comparison, MRI provides excellent soft tissue contrast and good spatial resolution in vivo that enables distinct delineation of primary and metastatic tumors without the need for manipulating tumor cells or tissues. The multislice imaging capabilities of MRI without the use of radioactive tracers makes it ideal for non-invasive longitudinal monitoring of tumor growth in preclinical model systems. The results of our experiments with the metastasizing tumor #11424 show that, for this tumor, treatment with Apo2L/TRAIL is able to inhibit the growth of both primary tumors in the pancreas and documented metastatic lesions and, furthermore, that this treatment significantly improved survival in the treated animals.
While there are many reports of selective apoptosis in tumor cells induced by Apo2L/TRAIL, there have also been cautionary reports of increased metastasis by cells that are resistant to apoptosis.12 In the present study we examined the response of three different resistant patient tumor xenografts and did not observe any increase in instances of metastasis.
Originally, investigations of the anti-tumor effects of Apo2L/TRAIL focused on the fact that a large number of malignant human cell lines underwent apoptosis in response to engagement of the death receptors DR4 and DR5, both in vitro and in immunodeficient mouse xenograft models, while normal cells did not.28,29
Walczak et al29 reported that while leucine zipper trimerized-human TRAIL (LZ-huTRAIL) and mouse LZ-msTRAIL are equally effective against the mouse tumor LB27.4 in a chromium release assay, LZ-huTRAIL is 300× more effective against the human Jurkat cell line than was the LZ-msTRAIL. To specifically examine the question of cross-reactivity, Bossen et al30 set up an expression system where the extracellular domains of the human and mouse ligands and receptors were expressed on the cell surface and their interactions could be evaluated. They found species cross-reactivity in which both the huTRAIL and msTRAIL interacted with all four human receptors and the msDR5 and msDcR2. In those assays, they stated that the ligands were predicted to be hexamers and therefore could exhibit higher avidity than the natural trimers. In fact, a recent report by Wilson et al31 demonstrated that an oligomeric form of huApo2L/TRAIL induced apoptosis in the murine endothelial cells within xenografted human tumors whereas the trimeric form did not. Therefore, in terms of examining mechanisms of Apo2L/TRAIL induced apoptosis in tumors vs. normal cells in xenografts, species cross-reactivity with mouse stromal elements may occur with some forms of ligands and antibodies targeting death receptors, but not with the trimerized form used in this study. In terms of what information different models may provide, the patient xenografts such as used in this study have several advantages. Since they are derived from freshly obtained surgical specimens acquired at time of patient diagnosis, they are uniquely representative of the heterogeneity of the patient population and should provide evidence of how the diverse patient population will respond to therapies (either traditional or targeted). However, three drawbacks of these patient xenograft models derive from the necessity of using SCID mice: 1) the stroma in the tumor microenvironment is derived from the murine host and therefore therapy induced effects on the stroma may not occur, 2) cells of the adaptive immune system are lacking and 3) the engrafted tumors are often at advanced stages of malignant development.
Genetically engineered mouse models (GEM models) are now being developed that recapitulate many features of human cancer initiation and progression in the autochthonous setting in immunocompetent mice (see recent reviews32,33). These GEM models provide for the development of malignancy in the presence of a functional immune system which is important in understanding the role of TRAIL in endogenous tumor control because TRAIL is expressed by activated immune cells and has been shown to play a role in immunosurveillance.34 Furthermore, the histological features of these tumor models are architecturally representative of human tumors and when tumors develop in the appropriate cells with genetic alterations found in patient tumors, they could be expected to act like patient tumors in response to therapies.32 Although GEM models are not yet used routinely in preclinical therapeutic studies, two recent reports demonstrate the potential of these models. Singh and colleagues carried out an extensive study with GEM models that phenocopy human NSCLC and pancreatic cancer and successfully showed that results obtained in these preclinical trials correlated with results from actual clinical trials.35,36 In a second example, Olive and colleagues37 used a GEM model of pancreatic cancer which developed extensive desmoplasia to show that inhibition of stromal elements could facilitate delivery of chemotherapy and significantly improve anti-tumor efficacy. GEM models are based on a limited number of relevant genetic mutations, however, and will be unlikely to reproduce all the genetic changes occurring in diverse patient derived tumor models.
The fact that tumor development is not synchronous in GEM models, though, creates experimental challenges in carrying out therapeutic studies requiring cohorts of mice with tumors at comparable stages and size. Also, in testing targeted therapies, it may be necessary to use “surrogate” molecules whose activity in the murine system is specifically designed to mimic the affinity of the human molecules in human tumor models. Clearly, different models have different strengths and weaknesses and preclinical studies using both types of models should provide complementary information about novel therapeutics thus improving chances of accurately predicting which will be successful in the clinic.
In summary, our results demonstrate potent antitumor activity of Apo2/TRAIL therapy against a patient tumor xenograft in several different sites (subcutaneous, primary orthotopic and metastatic pancreatic cancer) in vivo. Overall, these results support the idea that initial pre-clinical evaluations of some therapeutics (in this case Apo2L/TRAIL) using patient tumors in a subcutaneous location can be predictive for response in orthostatic and metastatic sites. Although it might be predicted that patient tumors have already “escaped” from immune surveillance molecules, this is clearly not true for all tumors. Our findings concerning the response of pancreatic tumor xenografts to Apo2L/TRAIL support the further development of death receptor targeting therapeutics for the treatment of pancreatic malignancies and demonstrate the need to develop methods for identifying those tumors which are sensitive to these apoptosis inducing therapies.
ACKNOWLEDGMENT
We thank Genentech for the generous gift of Apo2L/TRAIL.
Support: This work was supported by the National Institutes of Health R01 CA10888801A1 (EAR), NIH 5T32CA108456 T32 and utilized Shared Resources supported by the RPCI’s Comprehensive Cancer Center Support grant (NIH/NCI CA16056).
Footnotes
Disclosure: The authors have no conflicts of interest.
REFERENCES
- 1.Richmond A, Su Y. Mouse xenograft models vs GEM models for human cancer therapeutics. Dis Model Mech. 2008;1:78–82. doi: 10.1242/dmm.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francia G, Kerbel RS. Raising the bar for cancer therapy models. Nat Biotechnol. 2010;28:561–562. doi: 10.1038/nbt0610-561. [DOI] [PubMed] [Google Scholar]
- 3.Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1999;17:279–284. doi: 10.1023/a:1006140513233. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 5.Hylander BL, Pitoniak R, Penetrante RB, et al. The anti-tumor effect of Apo2L/TRAIL on patient pancreatic adenocarcinomas grown as xenografts in SCID mice. J Transl Med. 2005;3:22. doi: 10.1186/1479-5876-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Eckhardt SG, Kurzrock R, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;28:2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 8.Hotte SJ, Hirte HW, Chen EX, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 9.Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res. 2010;16:1701–1708. doi: 10.1158/1078-0432.CCR-09-1692. [DOI] [PubMed] [Google Scholar]
- 10.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89:5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MP, Evans DB, Wang H, et al. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4:1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trauzold A, Siegmund D, Schniewind B, et al. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25:7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- 13.Rubio-Viqueira B, Jimeno A, Cusatis G, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 14.Sakakibara T, Xu Y, Bumpers HL, et al. Growth and metastasis of surgical specimens of human breast carcinomas in SCID mice. Cancer J Sci. Am. 1996;2:291–300. [PubMed] [Google Scholar]
- 15.Seshadri M, Merzianu M, Tang H, et al. Establishment and characterization of patient tumor-derived head and neck squamous cell carcinoma xenografts. Cancer Biol Ther. 2009 Dec;8:2275–2283. doi: 10.4161/cbt.8.23.10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver DF, Hempling RE, Piver MS, et al. Effects of IL-12 on human ovarian tumors engrafted into SCID mice. Gynecol Oncol. 1999;72:154–160. doi: 10.1006/gyno.1998.5239. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Silver DF, Yang NP, et al. Characterization of human ovarian carcinomas in a SCID mouse model. Gynecol Oncol. 1999;72:161–170. doi: 10.1006/gyno.1998.5238. [DOI] [PubMed] [Google Scholar]
- 18.Naka T, Sugamura K, Hylander BL, et al. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients' colon tumors grown in SCID mice. Cancer Res. 2002;62:5800–5806. [PubMed] [Google Scholar]
- 19.Sugamura K, Gibbs JF, Belicha-Villanueva A, et al. Synergism of CPT-11 and Apo2L/TRAIL against two differentially sensitive human colon tumor xenografts. Oncology. 2008;74:188–197. doi: 10.1159/000151366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin H, Yang R, Fong S, et al. Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand cooperates with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Cancer Res. 2004;64:4900–4905. doi: 10.1158/0008-5472.CAN-04-0408. [DOI] [PubMed] [Google Scholar]
- 21.Jin H, Yang R, Ross J, et al. Cooperation of the agonistic DR5 antibody apomab with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Clin Cancer Res. 2008;14:7733–7740. doi: 10.1158/1078-0432.CCR-08-0670. [DOI] [PubMed] [Google Scholar]
- 22.Malin D, Chen F, Schiller C, et al. Enhanced metastasis suppression by targeting TRAIL receptor 2 in a murine model of triple-negative breast cancer. Clin Cancer Res. 2011;17:5005–5015. doi: 10.1158/1078-0432.CCR-11-0099. [DOI] [PubMed] [Google Scholar]
- 23.Bruns CJ, Harbison MT, Kuniyasu H, et al. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee NC, Bouvet M, Nardin S, et al. Antimetastatic efficacy of adjuvant gemcitabine in a pancreatic cancer orthotopic model. Clin Exp Metastasis. 2000;18:379–384. doi: 10.1023/a:1010831823004. [DOI] [PubMed] [Google Scholar]
- 25.Loukopoulos P, Kanetaka K, Takamura M, et al. Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity. Pancreas. 2004;29:193–203. doi: 10.1097/00006676-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz-Munoz W, Man S, Kerbel RS. Effective treatment of advanced human melanoma metastasis in immunodeficient mice using combination metronomic chemotherapy regimens. Clin Cancer Res. 2009;15:4867–4874. doi: 10.1158/1078-0432.CCR-08-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 30.Bossen C, Ingold K, Tardivel A, et al. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- 31.Wilson NS, Yang A, Yang B, et al. Proapoptotic activation of death receptor 5 on tumor endothelial cells disrupts the vasculature and reduces tumor growth. Cancer Cell. 2012;22:80–90. doi: 10.1016/j.ccr.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Cook N, Jodrell DI, Tuveson DA. Predictive in vivo animal models and translation to clinical trials. Drug Discov Today. 2012;17:253–260. doi: 10.1016/j.drudis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Mazur PK, Siveke JT. Genetically engineered mouse models of pancreatic cancer: unravelling tumour biology and progressing translational oncology. Gut. 2012;61:1488–1500. doi: 10.1136/gutjnl-2011-300756. [DOI] [PubMed] [Google Scholar]
- 34.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 35.Singh M, Lima A, Molina R, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol. 2010;28:585–593. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- 36.Singh M, Murriel CL, Johnson L. Genetically engineered mouse models: closing the gap between preclinical data and trial outcomes. Cancer Res. 2012;72:2695–2700. doi: 10.1158/0008-5472.CAN-11-2786. [DOI] [PubMed] [Google Scholar]
- 37.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]