Abstract
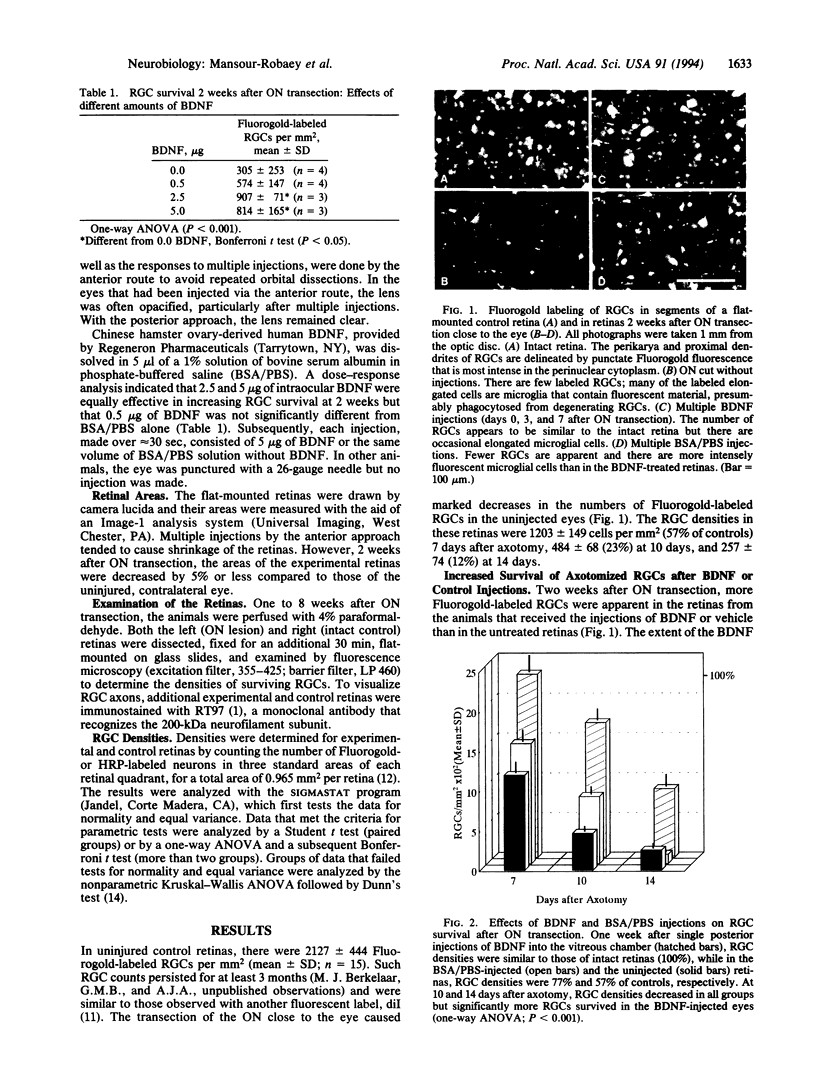
Optic nerve transection in adult rats results in the death of approximately 50% of the axotomized retinal ganglion cells (RGCs) by 1 week and nearly 90% by 2 weeks after injury. The capacity of brain-derived neurotrophic factor (BDNF) to prevent this early, severe loss of RGCs was investigated in vivo by intravitreal injections of BDNF [5 micrograms in 5 microliters of bovine serum albumin/phosphate-buffered saline (BSA/PBS)] or vehicle (5 microliters of BSA/PBS). Using quantitative anatomical techniques, we show that (i) all RGCs survived 1 week after a single injection of BDNF at the time of axotomy. (ii) RGC densities decreased in the BDNF-treated retinas by 2 weeks but remained significantly greater than in the untreated controls. (iii) An enhanced RGC survival was obtained with single injections of BDNF from 6 days before to 5 days after axotomy. (iv) Repeated injections resulted in greater numbers of surviving RGCs, an effect that declined to undetectable levels by 6 weeks. (v) There were indications for an endogenous local source of trophic support whose expression was triggered by ocular injury, particularly to the anterior part of the eye. (vi) With multiple BDNF injections, there was profuse axonal sprouting around the optic disc. This remarkable intraretinal growth was not, however, reflected in increased RGC innervation of the peripheral nerve grafts, which are known to facilitate regeneration when used as optic nerve substitutes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., Rasminsky M., Bray G. M., Carbonetto S., McKerracher L., Villegas-Pérez M. P., Vidal-Sanz M., Carter D. A. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond B Biol Sci. 1991 Mar 29;331(1261):337–343. doi: 10.1098/rstb.1991.0025. [DOI] [PubMed] [Google Scholar]
- Berkemeier L. R., Winslow J. W., Kaplan D. R., Nikolics K., Goeddel D. V., Rosenthal A. Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron. 1991 Nov;7(5):857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Ebendal T., Hedlund K. O., Norrgren G. Nerve growth factors in chick tissues. J Neurosci Res. 1982;8(2-3):153–164. doi: 10.1002/jnr.490080206. [DOI] [PubMed] [Google Scholar]
- Faktorovich E. G., Steinberg R. H., Yasumura D., Matthes M. T., LaVail M. M. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992 Sep;12(9):3554–3567. doi: 10.1523/JNEUROSCI.12-09-03554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faktorovich E. G., Steinberg R. H., Yasumura D., Matthes M. T., LaVail M. M. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990 Sep 6;347(6288):83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- Frisén J., Verge V. M., Cullheim S., Persson H., Fried K., Middlemas D. S., Hunter T., Hökfelt T., Risling M. Increased levels of trkB mRNA and trkB protein-like immunoreactivity in the injured rat and cat spinal cord. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11282–11286. doi: 10.1073/pnas.89.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F. H., Armstrong D. M., Williams L. R., Varon S. Morphological response of axotomized septal neurons to nerve growth factor. J Comp Neurol. 1988 Mar 1;269(1):147–155. doi: 10.1002/cne.902690112. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986 Aug;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R., Korsching S., Bandtlow C., Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987 Jun;104(6):1623–1631. doi: 10.1083/jcb.104.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M., Pagliusi S. R., Hohn A., Leibrock J., Barde Y. A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990 Aug;9(8):2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip N. Y., Ibáez C. F., Nye S. H., McClain J., Jones P. F., Gies D. R., Belluscio L., Le Beau M. M., Espinosa R., 3rd, Squinto S. P. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsma T. N., Friedman H. H., Berkelaar M., Bray G. M., Aguayo A. J. Different forms of the neurotrophin receptor trkB mRNA predominate in rat retina and optic nerve. J Neurobiol. 1993 Sep;24(9):1207–1214. doi: 10.1002/neu.480240907. [DOI] [PubMed] [Google Scholar]
- Johnson J. E., Barde Y. A., Schwab M., Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986 Oct;6(10):3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Parada L. F., Coulier F., Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989 Dec 1;8(12):3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer L. F. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987 Jan 9;235(4785):214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- LaVail M. M., Unoki K., Yasumura D., Matthes M. T., Yancopoulos G. D., Steinberg R. H. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A., Frautschy S. A., Gonzalez A. M., Baird A. A time course for the focal elevation of synthesis of basic fibroblast growth factor and one of its high-affinity receptors (flg) following a localized cortical brain injury. J Neurosci. 1992 Oct;12(10):3828–3837. doi: 10.1523/JNEUROSCI.12-10-03828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Friedman B., Alderson R. F., Wiegand S. J., Furth M. E., Lindsay R. M., Yancopoulos G. D. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990 Oct;5(4):501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Mey J., Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993 Feb 5;602(2):304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- Meyer M., Matsuoka I., Wetmore C., Olson L., Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992 Oct;119(1):45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero C. N., Hefti F. Rescue of lesioned septal cholinergic neurons by nerve growth factor: specificity and requirement for chronic treatment. J Neurosci. 1988 Aug;8(8):2986–2999. doi: 10.1523/JNEUROSCI.08-08-02986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöckli K. A., Lillien L. E., Näher-Noé M., Breitfeld G., Hughes R. A., Raff M. C., Thoenen H., Sendtner M. Regional distribution, developmental changes, and cellular localization of CNTF-mRNA and protein in the rat brain. J Cell Biol. 1991 Oct;115(2):447–459. doi: 10.1083/jcb.115.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos Solon, Bähr Mathias, Barde Yves-Alain, Vanselow Jens. Survival and Axonal Elongation of Adult Rat Retinal Ganglion Cells. Eur J Neurosci. 1989 Jan;1(1):19–26. doi: 10.1111/j.1460-9568.1989.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M., Bray G. M., Aguayo A. J. Regenerated synapses persist in the superior colliculus after the regrowth of retinal ganglion cell axons. J Neurocytol. 1991 Nov;20(11):940–952. doi: 10.1007/BF01190471. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M., Bray G. M., Villegas-Pérez M. P., Thanos S., Aguayo A. J. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci. 1987 Sep;7(9):2894–2909. doi: 10.1523/JNEUROSCI.07-09-02894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Sanz M., Villegas-Pérez M. P., Bray G. M., Aguayo A. J. Persistent retrograde labeling of adult rat retinal ganglion cells with the carbocyanine dye diI. Exp Neurol. 1988 Oct;102(1):92–101. doi: 10.1016/0014-4886(88)90081-7. [DOI] [PubMed] [Google Scholar]
- Villegas-Pérez M. P., Vidal-Sanz M., Bray G. M., Aguayo A. J. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988 Jan;8(1):265–280. doi: 10.1523/JNEUROSCI.08-01-00265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Pérez M. P., Vidal-Sanz M., Rasminsky M., Bray G. M., Aguayo A. J. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993 Jan;24(1):23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- Williams L. R., Varon S., Peterson G. M., Wictorin K., Fischer W., Bjorklund A., Gage F. H. Continuous infusion of nerve growth factor prevents basal forebrain neuronal death after fimbria fornix transection. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]




