Abstract
Drug withdrawal is often conceptualized as an aversive state that motivates drug-seeking and drug-taking behaviors in humans. Stress is more difficult to define, but is also frequently associated with aversive states. Here we describe evidence for the simple theory that drug withdrawal is a stress-like state, on the basis of common effects on behavioral, neurochemical, and molecular endpoints. We also describe data suggesting a more complex relationship between drug withdrawal and stress. As one example, we will highlight evidence that, depending on drug class, components of withdrawal can produce effects that have characteristics consistent with mood elevation. In addition, some stressors can act as positive reinforcers, defined as having the ability to increase the probability of a behavior that produces it. As such, accumulating evidence supports the general principles of opponent process theory, whereby processes that have an affective valence are followed in time by an opponent process that has the opposite valence. Throughout, we identify gaps in knowledge and propose future directions for research. A better understanding of the similarities, differences, and overlaps between drug withdrawal and stress will lead to the development of improved treatments for addiction, as well as for a vast array of neuropsychiatric conditions that are triggered or exacerbated by stress.
Keywords: addiction, corticotropin-releasing factor, cyclic AMP response element binding protein, dynorphin, glutamate, opiate, opponent process, psychostimulant
Introduction
Stress is a complex construct with interdependent biological and psychological components. By definition, it is the nonspecific response of an organism to any demand (stressor) placed upon it that requires adaptation (Selye, 1936; McEwen and Gianaros, 2011). Stressors typically engage the hypothalamic–pituitary–adrenal (HPA) axis to produce physiological and emotional responses. The biological effects of stress are relatively hardwired and can be measured objectively (changes in hormone release, heart rate), whereas emotional effects can depend on an individual’s perception of their ability to cope with the stressor (Coyne et al., 1981; Maier and Watkins, 2005) and are therefore more subjective. The same stressor may trigger pleasure in one individual but aversion in another. A popular conceptualization of stress is that it is a constellation of negative, depressive-like emotions (anhedonia, anxiety, irritability, feeling overwhelmed) that should be avoided, although stimuli that would seem to qualify as stressors can be experienced as euphoric and thrilling (e.g. skydiving). In laboratory animals, some forms of stress can produce ‘anomalous’ responses that are not easily conceptualized as reflecting depressive-like effects (Willner, 2005). Key to our understanding of how stressors affect emotion is the concept of allostasis, in which stability of homeostatic systems is maintained through adaptive plasticity (i.e. opponent processes). Here we will discuss how both the onset and the offset of drugs of abuse can be considered stressors that trigger adaptive plasticity within neural circuits that regulate motivation and emotion. These neuroadaptive responses contribute to the pathophysiology of drug addiction, defined here as a chronic, relapsing disease characterized by compulsive drug seeking and use despite harmful consequences (Koob and Volkow, 2010).
Reinforcement is a process by which a response-contingent delivery or removal of a stimulus increases the probability of a subsequent response. There are two forms: positive reinforcement, which is associated with euphoria, and negative reinforcement, which is associated with cessation of an aversive state. Both forms contribute to the addiction process (Wise and Koob, 2014), although positive reinforcement is often associated with the development of addiction and negative reinforcement is associated with the maintenance of addiction. As an individual repeatedly uses a drug, a shift occurs such that cessation of withdrawal-induced dysphoria (a stressful state) drives continued drug use and relapse (Koob and Le Moal, 2008; Wise and Koob, 2014). The neurobiological basis of this shift, which has been conceptualized as an opponent process theory (Solomon and Corbit, 1974; below), is the focus of considerable research. This framework holds true for all drugs considered to be addictive, although each class of drug engenders unique patterns of use and progression to addiction. Here we will focus on the similarities and differences between stress and dependence on opioids and psychostimulants. With opioids (e.g. heroin, prescription painkillers), the progression from impulsive to compulsive intake often starts with misuse to get high and relieve tension (i.e. self-medication) followed by development of tolerance (Chartoff and Connery, 2014). Only 3–13% of first-time users of prescription painkillers or heroin, respectively, develop dependence within the first year, suggesting that addiction is not inevitable (NSDUH, 2008). With psychostimulants (cocaine, amphetamine), people typically start with low-level sporadic use, often in social settings. For 5–10% of users, drug binges of hours or days (until supply is depleted) become frequent (NSDUH, 2008). The end of a binge is characterized by profound dysphoria, general depression of activity, and intense drug craving (Gawin, 1991).
The mesocorticolimbic system, comprising the ventral tegmental area (VTA), the nucleus accumbens (NAc), and the prefrontal cortex (PfC), is considered a primary mediator of acute drug reward (Wise and Rompre, 1989; Iremonger and Bains, 2009; Sesack and Grace, 2010). Direct actions of drugs of abuse within this neural circuit trigger molecular, physiological, and neurochemical events that produce reward and reinforce drug-seeking behavior. Repeated drug use engages additional compensatory events that serve to oppose reward and, paradoxically, reinforce drug-seeking behavior. For example, the acute stress response originates with HPA axis activation, but direct and indirect connections with elements of the mesocorticolimbic system mediate affective components of stress (Van’t Veer and Carlezon, 2013). We will focus on a restricted set of changes that occur in response to drugs of abuse and stress within the mesocorticolimbic system, as this system is known to play a role in addiction, as well as in regulation of mood states (Nestler and Carlezon, 2006; Russo and Nestler, 2013). We will discuss representative examples of excitatory transmitters, peptides, and intracellular factors, as it is impossible to be comprehensive in an article of this type. Our thesis is that the impact of repeated drug administration and stress on the brain and behavior produces similar alternating cycles (waveforms) of hedonic state such that robust aversive states emerge upon stimulus withdrawal. We will also provide evidence for and against a novel corollary – that is, these aversive states trigger similar opponent processes such that their cessation is followed by elevations in reward function.
The opponent process
Because external and internal stimuli are constantly changing, motivational states have a temporal dynamic. This concept is articulated in the opponent process theory, which posits that ‘Hedonic states are automatically opposed by central nervous system mechanisms which reduce the intensity of hedonic feelings, both pleasant and aversive’ (Solomon and Corbit, 1974). Opponent processes are conceptualized as defending hedonic equilibrium, and specific structures within the brain are responsible for regulating these processes. This model has been used as a framework to understand the transition from occasional drug use to addiction (Koob et al., 1989). In this context, the immediate rewarding effects of a drug of abuse (State ‘A’, the ‘high’) are followed by delayed aversive effects (State ‘B’, the ‘crash’) that are due to drug-dependent recruitment of opponent processes. However, this theory also applies to stimuli that are initially aversive, such as stress, fear, and anxiety (Solomon and Corbit, 1974). We will discuss evidence that the temporal dynamics of drug withdrawal and stress include negative states, but also that these states can in themselves represent aversive stimuli that trigger opponent processes leading to rebound increases in reward function (Fig. 1) and additional cycles of reverberation.
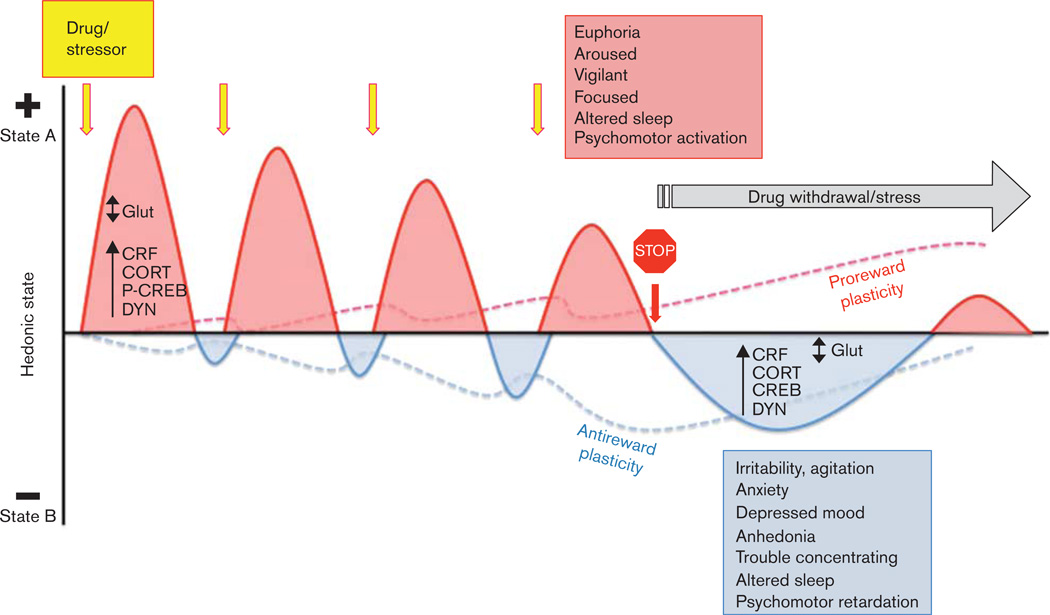
Fig. 1.
Conceptualization of drug withdrawal and stress triggering similar neuroplastic mechanisms to maintain allostasis of hedonic state. Acute exposures to drugs of abuse and stressors (yellow arrows) activate stress and reward pathways (HPA axis and mesocorticolimbic system, respectively) that are arousing (State “A”: positive deflections on hedonic axis, red curves; signs listed in red box). Each stimulus-induced increase in hedonic state is followed by a decrease in hedonic state (State “B”: negative deflections on hedonic axis, blue curves), which grows in strength with repeated presentation of the drug or stressor. Allostasis-driven neural plasticity occurs within stress and reward circuitry that oppose both the arousal/rewarding effects of repeated stimuli (red dashed line) and the depressive/anhedonic effects of stimulus offset (blue dashed line). Drug withdrawal (stop sign) eliminates the positive effects of acute drug administration on hedonic state, resulting in marked decreases in hedonic state (signs listed in blue box). This abrupt withdrawal state results in depressive-like mood states similar to those observed with chronic stress. There is evidence that withdrawal-induced increases in at least some factors (e.g. dynorphin) can themselves trigger subsequent positive deflections on the hedonic axis (red curve, far right). A gap in our knowledge is with regard to how drug withdrawal and stress impact addictive behaviors such as craving and relapse over time, as hedonic states oscillate. CORT, corticosterone; CRF, corticotropin-releasing factor; DYN, dynorphin; Glut, glutamate; HPA, hypothalamic–pituitary–adrenal; P-CREB, phosphorylated cAMP response element binding protein.
Dependent animals quickly learn that the simplest and most effective way to stop drug withdrawal-induced negative affective states is to administer the drug (Wikler and Pescor, 1967), which produces a rapid and robust elevation in reward, which is highly reinforcing (Hutcheson et al., 2001). Related to this, humans often learn that a relatively simple way to alleviate negative affective states due to stress or anxiety disorders is to self-medicate with drugs of abuse. A much more difficult approach for addicts – one that fails more often than not–would be to let withdrawal-induced negative affective states end naturally, which (as discussed below) would theoretically produce a slow and mild elevation in mood that may manifest as increased confidence in the ability to cope with the stress of abstinence. This approach is likely too gradual to be reinforcing per se and may include subtle reverberations that include periods of dysphoria, making a conscious decision to take this path challenging.
Similarities between drug withdrawal and stress
Acute versus repeated exposure
Both acute drug administration and stress elicit states of arousal and engage many of the same neural circuits (Koob, 2008; McEwen and Gianaros, 2011). Both activate the HPA axis, which consists of the paraventricular nucleus (PVN) of the hypothalamus, the anterior lobe of the pituitary gland, and the adrenal gland (Herman et al., 2005), resulting in release of stress hormones and peptides [e.g. corticotropin-releasing factor (CRF), corticosterone, glucocorticoids, endogenous opioids] (Sarnyai et al., 2001; Koob and Volkow, 2010). Release of these molecules has immediate and delayed effects on limbic circuit function, including activation of the mesocorticolimbic system (Thierry et al., 1976; Deutch et al., 1991; Marinelli and Piazza, 2002; Sheline, 2003; Pittenger and Duman, 2008). For example, corticosterone facilitates dopamine effects and potentiates drug reward (Piazza et al., 1993), and CRF injected into the VTA stimulates dopamine neurons (Kalivas et al., 1987). Rodents will self-administer glucocorticoids if the activity state of dopamine neurons is high (under conditions of novelty, during the active phase of their circadian cycle; Piazza et al., 1993). Stress also causes cross-sensitization to drugs of abuse (Kalivas and Stewart, 1991), providing a potential biological explanation for comorbidity of stress-related and addiction disorders (Sinha et al., 2006; Koob, 2008). The initial response to acute drug administration or stress is activation, which increases vigilance and goal-directed behavior. This state of arousal is protective in the short-term (Keay and Bandler, 2001) but disruptive with increasing intensity, duration, and frequency (Buydens-Branchey et al., 1990; Sapolsky, 1996; Miczek et al., 2008).
Repeated administration of drugs of abuse or stressors triggers allostasis – defined as the process of maintaining stability (i.e. homeostasis) through change – in neural circuits that control motivated behavior (Koob and Le Moal, 2008). One hypothesis is that, at a point that differs for each individual, chronic drug administration or stress ultimately leads to ‘allostatic load’ (McEwen and Gianaros, 2011), a pathological state in which homeostasis is no longer maintained. In this state, the brain and behavior are altered, perhaps irreparably.
Consequences of chronic exposure to drugs or stress
Withdrawal from most classes of abused drugs produces behavioral and emotional responses that are similar to those observed with chronic stress. Importantly, even low-intensity stressors can be perceived as highly aversive if they are unpredictable or uncontrollable (Adell et al., 1988; Foa et al., 1992). This is particularly relevant to addiction, where the knowledge that the drug will be attainable at a later time (after work, on the weekend) gives the user a sense of control that can allay the sense of panic and anxiety that, coupled with withdrawal signs, often comes with a lack of drug availability.
Drug withdrawal includes acute and protracted aversive states including anxiety, anhedonia, irritability, and depressed moods that resemble major depressive disorder (O’Brien et al., 1977; Wikler, 1980; Gawin, 1991; Barr et al., 2002; Koob and Le Moal, 2008). The neural mechanisms underlying acute versus protracted withdrawal signs are likely distinct but not mutually exclusive. For example, it is thought that drug-induced within-system adaptations might produce aversive states during early withdrawal, whereas between-system adaptations might emerge over time and contribute to protracted aversive states (Koob and Volkow, 2010). In clinical studies, most people report negative emotional states just before relapse, with anxiety and depression being the most common (Brownell et al., 1986; Markou and Koob, 1991; Sarnyai et al., 2001; Kenny and Markou, 2005; Iremonger and Bains, 2009). Repeated, prolonged, or severe stressors often precede the development of anxiety disorders, depression, and substance abuse (Kessler, 1997; Pine and Charney, 2002; Kendler et al., 2003; Volkow and Li, 2004; Fox et al., 2007). Conversely, substance abuse can also precede or exacerbate mood disorders (Kessler, 1997). Depression is characterized by anhedonia and lack of energy, whereas excessive worrying and problems concentrating are hallmark symptoms of anxiety. Many of these same negative affective states can be recapitulated in animal models that utilize chronic stress, allowing researchers to identify their underlying substrates. There are behavioral tests that quantify hedonic state, concentration, avoidance, and escape, all of which enable quantification of depressive-like and anxiety-like behaviors. Discrete stressors such as foot-shock, maternal deprivation, and restraint induce depressive-like behaviors including increased immobility in the forced swim test (Platt and Stone, 1982; Aisa et al., 2008), as well as elevations in brain reward thresholds (Zacharko and Anisman, 1991), which indicate anhedonia. Stress-related states can disrupt performance in the five-choice serial reaction time task, a procedure that quantifies attention in rodents (Van’t Veer et al., 2013). Repeated stressors such as chronic social defeat stress – an ethologically relevant stressor involving daily exposure to an aggressor – produce anhedonia (Berton et al., 2006; Golden et al., 2011; Donahue et al., 2014) and anxiogenic-like responses in subordinate mice in tests such as the elevated plus maze (Keeney and Hogg, 1999; Slattery et al., 2012), as well as decreases in social interaction with conspecifics (Avgustinovich et al., 2005; Berton et al., 2006). Although some behavioral signs of chronic social defeat stress become evident after the first exposure, they require several exposures to reach their full intensity and persist after the stress is terminated (Donahue et al., 2014), suggesting an allostatic shift in set point.
A hallmark of addiction to psychostimulants and opioids is that tolerance can develop to acute drug-induced euphoria, which is thought to be due to the strengthening of within-system and between-system opponent processes (Nestler, 2004; Koob and Le Moal, 2008). A parallel increase in the magnitude of drug offset-induced dysphoria drives negative reinforcement processes (Koob, 2008; Koob and Le Moal, 2008). If drug withdrawal and stress are similar, then tolerance should develop to the activating effects of acute stressors concomitant with an increased emergence of negative affective states upon repeated exposure to stressors. However, as the type and the timing of stressors can vary markedly, the pattern of stress-driven oscillations in affective states is not as clear-cut as with drugs of abuse. For example, habituation to the activating effects of repeated yet varied stressors may not occur, whereas tolerance typically develops to repeated and identical stressors (McEwen and Gianaros, 2011). This observation has led to the development of chronic unpredictable stress procedures (Hill et al., 2012). Traumatic or chronic uncontrollable stress that blunts the HPA axis through negative feedback can lead to depressive-like behaviors in which stressors normally coded as arousing lose their motivational properties. This occurs in part through a decrease in PfC function and an increase in extended amygdalar function [basolateral amygdala and bed nucleus of the stria terminalis (BNST)], which engenders low cognitive and behavioral control (Sinha, 2008).
In contrast to the weakening of arousal/reward and strengthening of negative affect, drugs of abuse and stressors can also produce sensitization, as reflected by amplification of their stimulant and reward-related effects (Carlezon and Nestler, 2002). A discussion of drug and stress sensitization is outside the scope of this review, but it is important to emphasize that repeated exposure to a stressor often elicits sensitization of stress-induced behaviors and may promote the transition from active to passive coping strategies (Keay and Bandler, 2001) by triggering dysphoria and anhedonia, and by the induction of active or passive coping strategies that may terminate the stress or blunt the impact of an inescapable stressor (Keay and Bandler, 2001).
Neural mechanisms common to drug withdrawal and stress
The mesocorticolimbic system has long been associated with reward states (Wise and Bozarth, 1987). However, accumulating evidence implicates these structures in both positive and negative affective states (Roitman et al., 2005; Carlezon and Thomas, 2009; McCutcheon et al., 2012; Volman et al., 2013). Aversive stimuli increase dopamine neuron population activity (Valenti et al., 2011) and dopamine release (Thierry et al., 1976; Abercrombie et al., 1989; Imperato et al., 1993; Piazza and Le Moal, 1998; Pascucci et al., 2007), which may promote or antagonize stress effects on behavior. Interestingly, aversive stimuli can also suppress dopamine release in the NAc (Maisonneuve et al., 1995; Ebner et al., 2010; McCutcheon et al., 2012). The fact that dopaminergic neurons originating in the VTA project not only to the NAc and PfC, but also to the hippocampus, amygdala, and BNST (Swanson, 1982), which play important roles in fear, anxiety, reward, and stress, supports the concept that the mesocorticolimbic system integrates and processes salient stimuli (Salamone, 1994; Pezze and Feldon, 2004; Carlezon and Thomas, 2009). Here, we describe a small set of molecular adaptations that play particularly important roles in both drug withdrawal and stress, based primarily upon our own work.
Role of glutamate systems
Overview
Glutamatergic afferents to the NAc come from brain regions known to be important for processing both positive and negative emotional stimuli, such as the basolateral amygdala (Kelley et al., 1982), and for goal-directed behavior, including the orbitofrontal cortex, insula, and cingulate cortex (Berendse et al., 1992). In addition, the NAc receives innervation from the ventral subiculum of the hippocampus (Kelley and Domesick, 1982; Groenewegen et al., 1987), which likely provides spatial and contextual information about the stimuli [for review of NAc afferents, see Brog et al. (1993); Sesack and Grace (2010)].
Drug withdrawal
Available data suggest that psychostimulant and opioid withdrawal have opposite effects on glutamatergic transmission in the NAc. Withdrawal from chronic cocaine is associated with suppression of presynaptic and postsynaptic glutamate transmission (Kalivas, 2004), whereas opiate withdrawal is associated with increased levels of extracellular glutamate in the NAc (Aghajanian et al., 1994; Desole et al., 1996; Sepulveda et al., 1998, 2004; Kalivas, 2004). Repeated cocaine use causes reductions in basal glutamate transmission in the NAc (Pierce et al., 1996; Bell et al., 2000; Hotsenpiller et al., 2001) and decreased responsiveness to ionotropic and group I metabotropic glutamate receptor (mGluR) stimulation (Swanson et al., 2001; Thomas et al., 2001). This is related, at least in part, to the reduced activity of the cystine–glutamate exchanger: restoration of this process normalizes extracellular glutamate levels (Baker et al., 2003), raising the possibility that medications targeting this process may be therapeutic. However, a cocaine injection (contingent or noncontingent) or a cue associated with repeated cocaine causes an increase in glutamate release (Pierce et al., 1996; Bell et al., 2000; Hotsenpiller et al., 2001; Miguens et al., 2008). One hypothesis is that the reduced baseline levels of gluta-mate after chronic cocaine may accentuate synaptic transmission within PfC–NAc connections following cocaine challenge (Boudreau and Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007; Iremonger and Bains, 2009). Consistent with this, virus-mediated gene transfer experiments demonstrate that elevated expression of GluR2 in the NAc enhances cocaine reward (Kelz et al., 1999). It has been proposed that this PfC–NAc synaptic enhancement is necessary for reinstatement of operant responding on levers previously paired with cocaine or heroin delivery (McFarland et al., 2003; LaLumiere and Kalivas, 2008).
In the VTA, glutamatergic transmission is enhanced for a short time period (~24 h) after chronic cocaine administration (Pierce et al., 1996; Reid et al., 1997; Kalivas and Duffy, 1998), but it returns to baseline levels by 3 weeks after cessation of cocaine (White et al., 1995; Zhang et al., 1997; Churchill et al., 1999; Ungless et al., 2001). This is partly because of alterations in the expression of AMPA and NMDA receptor subunits (Fitzgerald et al., 1996; Carlezon et al., 1997; Churchill et al., 1999; Loftis and Janowsky, 2000; Lu et al., 2002; Lane et al., 2008; Choi et al., 2011).
The acute effect of opioids occurs through activation of µ-opioid receptors (MORs), which are inhibitory Gαi-coupled G-protein-coupled receptors (Giacchino and Henriksen, 1996; Williams et al., 2001). MOR activation suppresses basal and evoked increases in extracellular glutamate in the NAc and dorsal striatum (Desole et al., 1996; Enrico et al., 1998; Sepulveda et al., 2004). Spontaneous or naloxone-precipitated withdrawal from chronic opioids leads to increases in neuronal activity and transmitter release due to the removal of inhibitory MORs (Williams et al., 2001). Glutamate release has also been shown to increase, and numerous studies have shown that systemic or intracerebroventricular administration of NMDA or AMPA receptor antagonists reduces morphine tolerance and withdrawal signs (Trujillo and Akil, 1991; Tokuyama et al., 1996; Gonzalez et al., 1997).
Extracellular glutamate levels are significantly increased in the NAc during morphine withdrawal (Aghajanian et al., 1994; Desole et al., 1996; Sepulveda et al., 1998, 2004), although excitatory synaptic transmission is not necessarily increased (Kalivas, 2009). It has been shown that presynaptic mGluR2/3 inhibitory autoreceptor function is increased during morphine withdrawal, and mGluR2/3 receptor agonists attenuate behavioral signs of morphine withdrawal (Robbe et al., 2002) and context-induced reinstatement of operant responding on levers previously paired with heroin self-administration (Bossert et al., 2006), raising the possibility that despite increases in glutamate levels, synaptic transmission may decrease. Regardless, we have demonstrated that intra-NAc microinjections of the selective AMPA receptor antagonist NBQX into morphine-dependent rats block withdrawal-induced conditioned place aversions (Sawyer et al., 2009), suggesting that activation of AMPA receptors in the NAc is necessary for withdrawal-induced negative affective states.
In the VTA, the immediate effect of opioid withdrawal is loss of MOR-mediated inhibition of glutamatergic and GABAergic afferents to dopamine neurons and an increase in glutamate and γ-aminobutyric acid (GABA) release. Subsequently, glutamate and GABA engage the more slowly acting metabotropic mGluR2/3 and GABAB receptors on glutamatergic terminals, resulting in decreased glutamatergic synaptic transmission (Manzoni and Williams, 1999). These effects lead to a strong suppression of dopamine activity (Diana et al., 1995).
These findings suggest that psychostimulant withdrawal is associated with decreased, and opioid withdrawal with increased, glutamatergic transmission in the NAc. In the VTA, psychostimulant withdrawal is associated with increased levels and availability of ionotropic glutamate receptors, whereas opioid withdrawal triggers inhibition of glutamate release. We acknowledge that this highly simplified summary does not fully address the complexities of glutamatergic transmission (Kalivas et al., 2009; Chartoff and Connery, 2014) and reflects a major gap in our understanding of the neural mechanisms of aversion. It has been proposed that aversive states are associated with activation, whereas rewarding states are associated with inhibition of NAc medium spiny neurons (Carlezon and Thomas, 2009); the development of increasingly sophisticated methods to control these systems will provide important new insights.
Stress
Similar to findings with opioid withdrawal, stress (restraint, forced swimming) increases extracellular glutamate levels in the NAc, as well as in the PfC and hippocampus (Moghaddam, 1993). Similar to findings with psychostimulant withdrawal, stress enhances synaptic strength in the NAc shell. Cold water stress causes an increase in AMPA/NMDA ratios in medium spiny neurons of the NAc shell that is glucocorticoid receptor-dependent (Campioni et al., 2009), and exogenous treatment with corticosterone is sufficient to enhance PfC-NAc synaptic strength. Further similarities to drug withdrawal include stress-induced increases in the number and function of GluR2-containing AMPA receptors in the NAc (Campioni et al., 2009). Both stress and drug withdrawal can evoke reinstatement of responding at a lever previously paired with drug administration (often interpreted as drug ‘seeking’) in animals that have undergone extinction training (Shaham et al., 2003). Further, intra-NAc infusions of AMPA can induce reinstatement (Suto et al., 2004). Taken together, synaptic plasticity of glutamatergic transmission in the NAc may be a common mechanism by which stress and withdrawal influence behavior.
As with psychostimulants and opioids, chronic restraint stress and repeated social defeat stress both increase the spontaneous and burst firing of VTA dopamine neurons (Anstrom and Woodward, 2005; Cao et al., 2010). For stress, this is thought to be due to CRF-dependent increases in glutamatergic VTA afferents. Stressors elevate CRF in the BNST (Erb and Stewart, 1999; Erb et al., 2001; Jasnow et al., 2004), a brain region that has been implicated in anxiety states (Hammack et al., 2004) and in drug withdrawal-induced negative affective states (Koob, 2008), and that sends glutamatergic projections to brain regions including the VTA (Rodaros et al., 2007). Footshock enhances BNST–VTA neuronal activity, and selective optogenetic stimulation of BNST–VTA gluta-matergic fibers produces aversion and anxiety-like behavior (Jennings et al., 2013). Through the actions of CRF, stress sensitizes VTA dopamine neurons to glutamatergic inputs through a postsynaptic mechanism that involves increasing the AMPA/NMDA ratio (Ungless et al., 2001, 2003; Saal et al., 2003). One mechanism for increased synaptic strength could be that stress, in common with drugs of abuse, increases GluR1 (AMPA) and NR1 (NMDA) receptor subunits in the VTA (Fitzgerald et al., 1996).
The idea that drugs of abuse and stress strengthen synaptic connections through enhanced glutamatergic transmission and potentiated dopamine neuron activity is consistent with observations that stress can enhance drug-taking and drug-seeking behaviors. However, it does not fit well with the idea that stress and drug withdrawal are aversive states that produce depressive-like and anxiety-like behaviors. Withdrawal from chronic regimens of psychostimulants and opioids – specifically, those expected to produce tolerance rather than sensitization (Carlezon and Nestler, 2002) – suppresses dopamine neuron activity, although this does not preclude the existence of strengthened glutamate–dopamine neuron synapses. The effects of chronic stress on dopamine neuron activity are not as well studied, but evidence suggests that, under some circumstances, mesocortico-limbic dopamine activity is increased (Miczek et al., 2008). Social defeat experiments in which an intruder rat is threatened by an aggressor reveal increases in extracellular dopamine in the NAc and PfC (Tidey and Miczek, 1996). Repeated social defeat also potentiates amphetamine-induced dopamine release in the NAc (Miczek et al., 1999), likely due to stress-induced increases in cortical–VTA glutamatergic transmission (Miczek et al., 2008). How stress-induced elevations in dopamine can be reconciled with negative affective states remains a gap in knowledge.
Implications
There have been considerable efforts to utilize drugs that alter glutamate function as treatments for addiction (Chartoff and Connery, 2014). Drugs that act at NMDA receptors (memantine) and the glutamate–cystine anti-porter (N-acetylcysteine) have generated significant enthusiasm (Bridges et al., 2012; Tomek et al., 2013). One concern with these types of treatments is that glutamate and its receptor targets are ubiquitous in the brain and that actions that are beneficial in one region may have disruptive effects in another, making the treatments unpleasant or debilitating.
Role of corticotropin-releasing factor
Overview
CRF is the principal regulator of the stress response (Vale et al., 1981). It is produced by cells in the PVN of the hypothalamus and triggers hormonal stress responses by activating the HPA axis (Herman et al., 2005). CRF and its two receptor subtypes, CRFR1 and CRFR2, have widespread and overlapping expression in the cortex, extended amygdala (including the NAc), medial septum, hypothalamus, thalamus, cerebellum, and midbrain and hindbrain nuclei (Swanson and Sawchenko, 1983; Lemos et al., 2012).
There is increasing evidence that CRF acts within the mesocorticolimbic system to influence motivated behavior. CRF-immunoreactive fibers and CRF receptors are found in the VTA (Swanson and Sawchenko, 1983; Van Pett et al., 2000) and NAc (Lemos et al., 2012). CRF is released in response to stressors in an activity-dependent manner, and its effect on neurotransmission is dependent on the type of stress and prior history of stress exposure (Lemos et al., 2012). Moderate stressors that activate both the HPA axis and the extrahypothalamic circuits are arousing and can facilitate goal-directed behavior. For example, footshock increases CRF release in the VTA, which then selectively facilitates glutamate release in rats that are drug-experienced (Wang et al., 2005). Traumatic or chronic uncontrollable stress that blunts the HPA axis through negative feedback can lead to depressive-like behaviors in which stressors normally coded as arousing lose their motivational properties. For example, intracerebroventricular infusion of relatively high CRF doses produces conditioned place aversions (Cador et al., 1992) and disrupts cognition (Van’t Veer et al., 2013).
Drug withdrawal
Acute administration of most drugs of abuse activates the HPA axis to elicit corticosterone secretion, as well as stimulates extrahypothalamic systems to release CRF (Piazza and Le Moal, 1997; Kash et al., 2008). Corticosterone and CRF can potentiate dopamine release in terminal fields and dopamine neuron excitability (Piazza et al., 1993; Piazza and Le Moal, 1997; Marinelli and Piazza, 2002; Kash et al., 2008). Acute withdrawal (following single-drug treatment) from drugs of abuse also results in activation of the HPA stress response. Although it may seem contradictory that acute drug effects (typically associated with euphoria) can have the same molecular consequences as acute drug withdrawal (typically associated with dysphoria), it is important to remember that people often report that initial drug use has aversive aspects. For example, psychostimulants can elicit anxiety, panic, and overwhelming autonomic symptoms (Yamamoto et al., 2007), whereas opioids can produce nausea and sluggishness (Coluzzi et al., 2012). For only a small percentage of users are the euphoric effects strong enough to drive continued drug use into dependence and addiction. Similarly, acute stressors are not exhilarating and euphorogenic for everyone.
In contrast, the HPA response is blunted during withdrawal from chronic drug administration due to negative feedback mechanisms (Basso et al., 1999), whereas the extrahypothalamic CRF stress system response is sensitized (Koob, 2008). Extracellular CRF is increased in the extended amygdala during drug withdrawal (Merlo Pich et al., 1995; Richter and Weiss, 1999; Weiss et al., 2001). Further, CRFR1 receptor antagonists block withdrawal-induced negative affective states, including anxiogenic effects in the elevated plus maze and defensive burying test, anhedonia in the intracranial self-stimulation (ICSS) test, and naloxone-precipitated conditioned place aversions in morphine-dependent rats (Basso et al., 1999; Knapp et al., 2004; Overstreet et al., 2004; Smith and Aston-Jones, 2008). Given that the actions of CRF in the VTA are excitatory and most likely contribute to arousal and the reinforcing aspects of stress, drug withdrawal-induced CRF release in the amygdala and BNST may play a more important role in negative affective states.
Stress
Similar to the effects of acute drug administration, stressors activate the HPA axis and increase CRF expression in the extended amygdala (Merlo Pich et al., 1995; Koob, 1999). Extrahypothalamic CRF released from the central amygdala and BNST is part of a feedforward system that activates an animal to mobilize the body for appropriate (e.g. ‘fight or flight’) behavioral responses. In the NAc of naive mice, CRF released in response to an acute, moderate stressor acts to increase dopamine release through coactivation of CRFR1 and CRFR2, providing a putative mechanism for stress-induced enhancement of drug effects. It has been recently reported that CRF acting through CRFR1 and protein kinase C enhances hyperpolarization-activated (Ih) currents to increase the firing rate of dopamine neurons (Wanat et al., 2008). Prolonged acute (10–20 min) exposure to CRF also activates CRFR2 to increase glutamate-dependent transmission in dopamine neurons (Ungless et al., 2003). However, severe stress exposure abolishes this stimulatory effect on dopamine release in the NAc, an effect that lasts up to 90 days (Lemos et al., 2012). Loss of the capacity of CRF to facilitate dopamine release in the NAc is consistent with stress-induced depressive-like states, and is accompanied by a switch in the emotional response to CRF from appetitive to aversive (Lemos et al., 2012).
As with drug withdrawal, chronic activation of the extrahypothalamic CRF system can trigger pathological states (Koob, 1999; Lemos et al., 2012). When animals are exposed to chronic, unpredictable, or more severe stressors, the effects of CRF on behavior change; for example, a dose of CRF that produces robust behavioral activation in the home cage produces behavioral suppression in a novel (i.e. stressful) environment (Sutton et al., 1982; Takahashi et al., 1989). Exogenously administered CRF has dose-dependent anxiogenic and fearful effects in the elevated plus maze (Baldwin et al., 1991), as well as the acoustic startle (Swerdlow et al., 1989), conditioned fear (Cole and Koob, 1988), and stress-induced freezing (Sherman and Kalin, 1988) tests. Finally, high doses of CRF can produce both taste and place aversions (Heinrichs et al., 1991; Cador et al., 1992).
Implications
Drug withdrawal and stress have many of the same effects on CRF. These observations have led to interest in the development of CRF antagonists to treat addiction. Thus far, the development of CRF receptor antagonists as therapeutics has been hindered by the high lipophilicity of initial drugs, although novel compounds are of continued interest (Zorrilla and Koob, 2010). More research on how CRF receptor activation affects other systems downstream may provide additional targets for new treatments.
Role of cyclic AMP response element binding protein
Overview
Cyclic AMP response element binding protein (CREB) is a transcriptional regulator expressed in the nucleus of all cells in the brain. It is constitutively expressed and bound to the promoter regions of genes that contain cAMP response elements. CREB becomes transcriptionally active when the Ser133 residue is phosphorylated in response to various stimuli, including adenylate cyclase-mediated increases in cAMP (Lonze and Ginty, 2002). As such, CREB is critical for coupling the transmission of events that occur at cell membranes into alterations in gene expression. Some examples of CREB-regulated gene-products that have been implicated in mood regulation include CRF (Itoi et al., 1996), BDNF (Finkbeiner et al., 1997), dynorphin (Cole et al., 1995), and the GluR1 AMPA receptor subunit (Borges and Dingledine, 2001). Interestingly, alterations in the expression of these target genes and the proteins they encode can, in turn, cause changes in neural activity that would be expected to further elevate CREB activity, a notable exception being the actions of dynorphin at κ-opioid receptors (KORs). There are numerous comprehensive reviews that describe the molecular mechanisms involved in CREB-mediated gene transcription (Abel and Kandel, 1998; West et al., 2001; Carlezon et al., 2005).
Drug withdrawal
Evidence suggests that CREB within the mesocorticolimbic system regulates drug reward-induced and drug withdrawal-induced aversive states. The rewarding effects of a single exposure to psychostimulants or opioids are associated with decreased P-CREB levels in the striatum, including the NAc (Turgeon et al., 1997; Tenayuca and Nazarian, 2012). In contrast, chronic morphine causes a compensatory increase in adenylate cyclase activity in MOR-expressing cells (Sharma et al., 1975a,b; Van Vliet et al., 1990). When morphine is discontinued, or withdrawal is precipitated with naloxone, cAMP levels markedly increase (Nestler and Aghajanian, 1997), which leads to intracellular adaptations including activation of cAMP-dependent protein kinase (PKA) and phosphorylation of CREB at Ser133 (Chartoff et al., 2003, 2006). Correlations between the timing of upregulated activity within cAMP pathways and signs of withdrawal support the idea that CREB contributes to aversive or dysphoric states associated with drug withdrawal.
Accumulating evidence indicates that CREB activation is part of a process that opposes the actions of drugs of abuse (Carlezon et al., 2005). Stimulation of PKA and virus-mediated elevation of CREB in the NAc both decrease cocaine reward, whereas antagonism of PKA and overexpression of a dominant-negative form of CREB in the NAc increase cocaine and morphine reward (Carlezon et al., 1998; Self et al., 1998; Pliakas et al., 2001; Barrot et al., 2002). Elevated expression of CREB in the NAc is associated with increased depressive-like behaviors, which may be qualitatively similar to depressive-like behaviors and dysphoria observed during drug withdrawal (Pliakas et al., 2001; Muschamp et al., 2011). For example, opioid withdrawal triggers PKA-dependent phosphorylation of CREB in vivo and in dissociated striatal neurons, suggesting that CREB activation results from direct morphine action in the NAc and striatum (Chartoff et al., 2003, 2006; Edwards et al., 2009). Further, mice with a targeted disruption of CREB α and Δ iso-forms (CREBαΔ) show reductions in signs of precipitated withdrawal after chronic morphine administration (Maldonado et al., 1996; Walters and Blendy, 2001). Together, these findings suggest that CREB activation in the NAc may reduce acute drug reward and lead to aversive or depressive-like motivational states that accompany drug withdrawal.
CREB activation in the VTA also regulates drug-induced affective states. The VTA has a distinct rostral to caudal neurochemical and functional topography; increased CREB function in rostral portions of the VTA increases the rewarding effects of cocaine and morphine, whereas similar changes in more caudal regions have opposite effects (Olson et al., 2005). Irrespective of rostral–caudal placement, intra-VTA injection of a dopamine D1 receptor agonist, which activates CREB, blocks morphine withdrawal-induced conditioned place aversions (Chartoff et al., 2009a), consistent with the idea that elevated CREB in the VTA increases reward-like states under some conditions.
A persistent gap in knowledge, however, is related to understanding the effect that NAc CREB-mediated aversive states has on drug-seeking and drug-taking behaviors. The negative reinforcement hypothesis would predict that CREB contributes to maintenance of drug-taking behavior and relapse because negative affective states motivate these behaviors. This view is supported by a study demonstrating that CREB over-expression in the NAc potentiates the rewarding and reinforcing aspects of cocaine self-administration (Larson et al., 2011). In contrast, a separate study showed that chronic cocaine self-administration upregulates a microRNA (miR-212) in striatal regions that enhances CREB activity, and yet prevents the escalation of cocaine self-administration when rats are allowed extended access to cocaine (Hollander et al., 2010). Even a third outcome was described by Green et al. (2010): rats raised in an enriched environment have reduced levels of P-CREB in the NAc and show an enhanced sensitivity to cocaine-induced conditioned place preferences, consistent with early virus-mediated gene transfer studies (Carlezon et al., 1998); however, reduced CREB activation in environmentally enriched rats is also associated with a downward and rightward shift in the cocaine self-administration dose–effect curve (Green et al., 2010), suggesting a decrease in the reinforcing efficacy of cocaine. There are sufficient methodological differences among these studies to account for the seemingly incongruous results, including the timing of the experiments; regardless, the ways in which NAc CREB contributes to drug dependence and addiction are not fully understood and may require resolution of CREB activation at a cellular level (i.e. discriminating CREB activation in either dopamine D1 or dopamine D2 receptor-expressing medium spiny neurons in the NAc).
Stress
The effects of stress on CREB have been most thoroughly studied in the NAc. Swim stress increases CREB activity in the NAc (Pliakas et al., 2001) and decreases the latency to immobility when rats are exposed to a second day of swim stress (Porsolt et al., 1977). Mimicking these changes by virus-mediated gene transfer of CREB in rats not previously exposed to forced swimming decreases the latency to immobility compared with that in rats treated with a control virus. In contrast, disruption of CREB function produces the opposite effect – that is, increased latency to immobility (Pliakas et al., 2001; Barrot et al., 2002) – which resembles the effects of standard anti-depressants in this assay. Footshock produces similar effects on CREB, and rats with viral vector-induced elevations in CREB show resistance to the extinction of fear conditioning (Muschamp et al., 2011), a hallmark of post-traumatic stress disorder in humans (Parsons and Ressler, 2013). Collectively, these data indicate that stress-induced elevations in CREB function within the NAc produce depressive-like signs, suggesting that stimulus-induced elevations in this region represent an allostatic adaptation that opposes the reinforcing effects of drugs and other types of rewards (Carlezon et al., 2005). Elevations in CREB function within the amygdala, a brain region often associated with aversive states, also potentiate fear responses in rats (Josselyn et al., 2001), whereas elevations in CREB function in the hippocampus are associated with beneficial effects of anti-depressants (Chen et al., 2001).
Implications
CREB regulates many genes that can contribute to addiction and stress-like states. CREB itself is unlikely to be a feasible target for therapeutic intervention because broad and unselective activation or inhibition of CREB throughout the brain would be expected to produce a mixture of beneficial and detrimental effects (Carlezon et al., 2005). However, interest remains in the development of medications that can affect systems that are regulated by CREB but have more limited brain distribution. Below, we focus on dynorphin and brain KOR systems, as efforts to develop medications that alter signaling through this system have advanced to clinical trials (see Carroll and Carlezon, 2013). However, it is important to note that BDNF systems have been implicated in addiction (Graham et al., 2007; Vassoler and Sadri-Vakili, 2014) and stress (Berton et al., 2006; Duman and Monteggia, 2006) despite the challenges associated with the current lack of pharmacological agents available to study BDNF, making it necessary to work with the peptide itself or peptide-blocking antibodies.
Role of dynorphin and κ-opioid receptor systems
Overview
The endogenous opioid dynorphin is cleaved from the precursor protein preprodynorphin and predominantly activates KORs (Chavkin et al., 1982), which are Gαi-coupled (Law et al., 2000). Dynorphin and KORs are expressed throughout limbic brain areas implicated in the pathophysiology of stress and drug addiction in humans and rodents (Fallon and Leslie, 1986; Mansour et al., 1995; Sukhov et al., 1995; Hurd, 1996; Peckys and Landwehrmeyer, 1999; Shuster et al., 2000; Alheid, 2003; Nestler and Carlezon, 2006; Koob and Le Moal, 2008). Within the NAc, dynorphin is expressed in medium spiny neurons and KORs are expressed on dopaminergic nerve terminals, as well as on nerve terminals that contain either GABA or glutamate, where they act to inhibit transmitter release (Fallon et al., 1985; Simmons and Chavkin, 1996; Rusin et al., 1997; Svingos et al., 1999; Meshul and McGinty, 2000; Hjelmstad and Fields, 2003; Margolis et al., 2006). There is also evidence for postsynaptic colocalization of KORs and dynorphin on dendritic spines in the NAc (Arvidsson et al., 1995; Svingos et al., 1999), although evidence for functional effects of these postsynaptic receptors is lacking. Dynorphin is released from dense core vesicles upon high levels of sustained neuronal activity (Weisskopf et al., 1993; Simmons et al., 1997); new evidence indicates that dynorphin is copackaged in vesicles with orexin, a peptide with opposing effects (Muschamp et al., 2014). Activity-dependent dynorphin release, especially from dendritic sites, can then serve as an effective mechanism by which neurons regulate their own activity (Drake et al., 1994; Brown and Bourque, 2004; Ludwig and Leng, 2006; Kreibich et al., 2008; Iremonger and Bains, 2009). Within the VTA, KORs are expressed on both the cell bodies and the terminals of dopamine neurons (Svingos et al., 1999, 2001), resulting in inhibition of dopamine release in areas that receive VTA input (e.g. NAc and PfC; Margolis et al., 2003; Margolis et al., 2006; Ford et al., 2007).
Drug withdrawal
Chronic exposure to drugs of abuse increases the activity of the KOR system within mesocorticolimbic circuits (Hurd and Herkenham, 1993; Spangler et al., 1993; Mathieu-Kia and Besson, 1998; Shippenberg et al., 2007; Zhou et al., 2008; Piras et al., 2010). In general, KOR activation produces aversive states that, at least in laboratory animals, have characteristics of both withdrawal and stress (Bals-Kubik et al., 1993; Newton et al., 2002; McLaughlin et al., 2003, 2006a; Bruchas et al., 2010; Knoll and Carlezon, 2010; Muschamp et al., 2011). Further, KOR activation is crucial in facilitating stress-induced reinstatement of operant responding in animals with substantial self-administration experience that have undergone extinction of operant responding for drug (Beardsley et al., 2005; Land et al., 2009; Bruchas et al., 2010; Sun et al., 2010; Schank et al., 2012; Graziane et al., 2013; Grella et al., 2014; Sedki et al., 2014) or reinstatement of drug-induced conditioned place preference (Carey et al., 2007; Redila and Chavkin, 2008; Cordery et al., 2012; Al-Hasani et al., 2013; Jackson et al., 2013; Aldrich et al., 2014). An implicit assumption in these studies is that stress-induced dynorphin release produces negative affective states that drive reinstatement (Koob, 2008). However, this has not been empirically determined because there are as yet no published studies that directly demonstrate that KOR blockade inhibits both drug withdrawal-induced negative affective states and reinstatement of drug seeking in the same animal. Further, it has been shown that KOR agonists actually decrease, and the KOR antagonist JDTic potentiates, cocaine-primed reinstatement of operant responding on a lever previously paired with cocaine delivery in animals that have undergone extinction training (Beardsley et al., 2005; Morani et al., 2009; Ruedi-Bettschen et al., 2010). These findings suggest that endogenous dynorphin decreases the effects of the cocaine prime such that it is a weaker stimulus for reinstatement.
The reinstatement paradigm, which is thought to model craving and relapse, does not specifically address how dynorphin or KORs affect the strength of withdrawal-induced stress-like states. We recently demonstrated that intracerebroventricular administration of the long-lasting KOR antagonist norBNI before initiation of an experimenter-administered binge cocaine regimen [three intraperitoneal injections of 15 mg/kg cocaine per day for 14 days, as described by Spangler et al. (1993)] blocks the development and expression of cocaine withdrawal-induced anhedonia, as measured using the ICSS test (Chartoff et al., 2012). Similarly, the KOR antagonists JDTic and norBNI attenuate the expression of nicotine withdrawal signs (Jackson et al., 2010). Both somatic withdrawal signs and hyperalgesia, as well as anxiety-related behavior and conditioned place aversion, were blocked. In contrast, several studies have shown that KOR antagonism can potentiate aversive states associated with morphine withdrawal: norBNI increased naloxone-induced conditioned place aversions and somatic withdrawal signs in morphine-dependent rats (Maldonado et al., 1992; Spanagel et al., 1994). Together, these findings raise the possibility that the role of dynorphin in drug withdrawal depends on the drug of abuse.
Stress
Just as swim stress elevates CREB function within the NAc, it also elevates prodynorphin expression (Chartoff et al., 2009b). Levels of footshock that produce fear conditioning elevate prodynorphin within the amygdala, and these increases are correlated with expression of fear (Knoll et al., 2011). Systemic administration of KOR agonists produces dysphoria in humans (Pfeiffer et al., 1986) and has aversive and stress-like effects in rodents in a variety of tests (Mague et al., 2003; McLaughlin et al., 2003, 2006b; Todtenkopf et al., 2004; Land et al., 2008; Bruchas et al., 2010). These effects are blocked by KOR antagonists and disrupted in mutant mice in which dynorphin or KORs are ablated (Mague et al., 2003; McLaughlin et al., 2003; Todtenkopf et al., 2004; Land et al., 2008; Van’t Veer et al., 2013). Activation of KORs in the NAc is sufficient to produce many of these aversive effects, although roles for other regions including VTA, PfC, amygdala, hippocampus, and raphe nucleus have been described (Bals-Kubik et al., 1989; Shirayama et al., 2004; Bruchas et al., 2011; Knoll et al., 2011; Muschamp et al., 2011). Administered on their own, KOR antagonists have combined antidepressant (Pliakas et al., 2001; Newton et al., 2002; Mague et al., 2003) and anxiolytic (Knoll et al., 2007) effects in rodents and block the acute and chronic effects of stressful stimuli (Beardsley et al., 2005; Knoll et al., 2007). Interestingly, KOR antagonists prevent the cognition-disrupting effects of CRF itself (Van’t Veer et al., 2012), consistent with other reports that the dysphoric effects of CRF involve KORs (Land et al., 2008). In the context of addiction, KOR antagonists block reinstatement of ‘cocaine-seeking’ behavior triggered by stress, but not by cocaine itself (Beardsley et al., 2005). Accumulating evidence suggests that KOR antagonists are most effective when administered before a stressor (Van’t Veer and Carlezon, 2013). To the extent that cocaine withdrawal can be considered a stressor, this concept is exemplified in studies examining the effects of cocaine withdrawal on ICSS. As described above, pretreatment with a KOR antagonist before a ‘binge’ regimen of cocaine attenuated the development of withdrawal-induced anhedonia, but administration after withdrawal effects had become detectable was ineffective (Chartoff et al., 2012). A similar prophylactic effect has been reported in rats self-administering heroin in long-access sessions, where pretreatment with KOR antagonists prevents escalation of drug intake that normally develops over time (Schlosburg et al., 2013). One interpretation of these data is that stress triggers a cascade of neuroadaptations that recruit other systems that are insensitive to alterations in KOR function.
Implications
There is considerable enthusiasm for the development of KOR antagonists for the treatment conditions ranging from addiction to depression and anxiety (Carroll and Carlezon, 2013). The broad ability to reduce the impact of stress may explain how KOR antagonists can have efficacy in such a wide variety of animal models that represent different disease states. However, the fact that KOR antagonists seem most efficacious if administered before a stressor – suggesting that they block a cascade of events that, once initiated, are difficult to reverse – represents a limitation that may narrow their utility.
Differences between drug withdrawal and stress
Stress, but not drug withdrawal, triggers opponent reward states
Stressful, painful, and aversive stimuli can be reinforcing or perceived as rewarding (Solomon and Corbit, 1974). Under some circumstances, nonhuman primates will self-administer shock (Laurence et al., 1994). In addition, mice that are exposed to an inescapable stressor (elevated stand) demonstrate a rewarding after-effect, measured as a conditioned place preference to stand exposure, which is correlated with an increase in NAc dopamine (Shen et al., 2011). In humans, termination of painful stimuli can produce pleasure (Becerra and Borsook, 2008; Leknes et al., 2011). Finally, there are numerous examples of stress (sensation)-seeking behaviors including racecar driving, skydiving, and roller coaster riding (Zuckerman, 1990). It is thought that sensation seekers requiring higher levels of arousal to feel positive emotion, alertness, and interest (Hebb, 1955; Piazza et al., 1993), although this fails to explain how more consistent effects can be observed in laboratory animals.
The idea that aversive stimuli can trigger opponent processes to subsequently produce positive affective states (Solomon and Corbit, 1974) is important in the context of stress-related disorders and addiction. It has been difficult to pinpoint neural mechanisms because it is not clear how factors such as the type, magnitude, frequency, or timing of stressors interact with baseline affective states to modulate reward function and mood. For example, chronic mild stress has been shown to reduce brain stimulation reward when rats press for electrical stimulation of the VTA (Moreau et al., 1992) but has no effect when the stimulation is in the lateral hypothalamus (Lin et al., 2002). Interestingly, ICSS thresholds actually decreased below baseline (indicating increased reward function) in the days following cessation of chronic mild stress (Lin et al., 2002), reminiscent of the proposed oscillations in affective state shown in Fig. 1. In contrast, chronic social defeat stress produces robust elevations in ICSS thresholds (indicating decreased reward function) in mice, which remain elevated several days after the defeat sessions are halted (Donahue et al., 2014).
A more reductionist approach is to isolate specific components of the stress response and track their impact on hedonic state and neural plasticity over time. We found that activation of KORs with the highly selective and potent KOR agonist salvinorin A (salvA; Roth et al., 2002) produces an immediate (0–2 h) anhedonic response and a delayed (23–24 h) hedonic response as measured with ICSS (Fig. 2a; Potter et al., 2011). KOR-mediated anhedonia has been well documented (Muschamp et al., 2011), but the delayed (and transient) increase in reward function 24 h after salvA administration is relatively novel and suggests that KOR activation triggers opponent processes within reward circuits. Consistent with this, there are reports that dynorphin and KOR agonists can be reinforcing. For example, rats can be trained to self-administer the KOR agonist RU-51599 (Marinelli et al., 1998), and dynorphin can produce conditioned place preferences and sustain self-administration behavior under some conditions (Khazan et al., 1983; Iwamoto, 1988). Humans self-administer salvA, although generally not compulsively (Zawilska and Wojcieszak, 2013), just as they use a variety of other chemicals (lysergic acid diethylamide, organic solvents) that may have aversive or hallucinogenic effects.
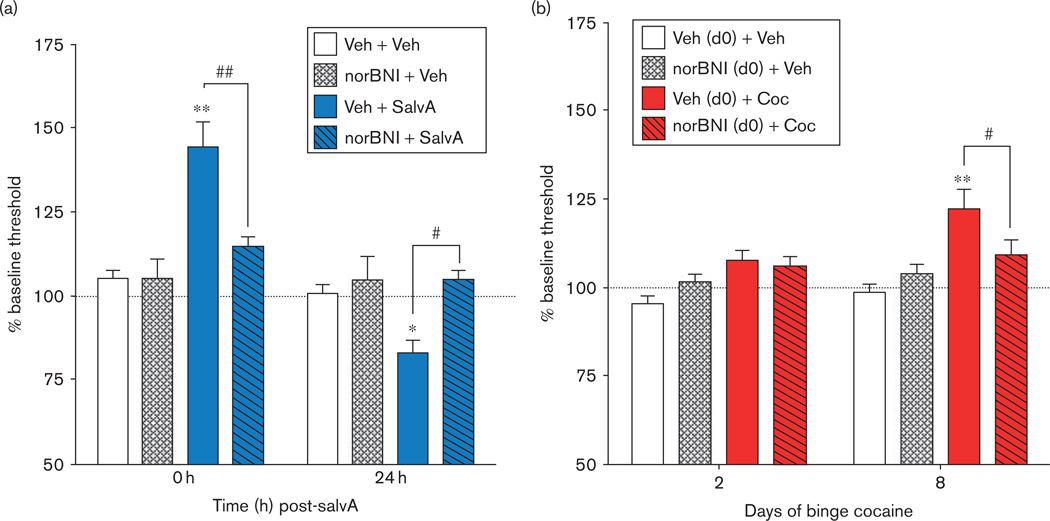
Fig. 2.
Evidence that processes that have an affective valence are followed in time by an opponent process that has the opposite valence. Hedonic states are determined by intracranial self-stimulation (ICSS). An increase in % baseline threshold [lowest frequency (Hz) of stimulation at which rats will perform operant behavior] reflects a decrease in sensitivity to brain stimulation reward (i.e. anhedonia), whereas a decrease in % baseline threshold reflects an increase in sensitivity to brain stimulation reward. (a) Rats treated with salvinorin A (salvA, 2.0 mg/kg, intraperitoneally) show an immediate (0 h post injection) increase in ICSS thresholds and a delayed (24 h post injection) decrease in ICSS thresholds compared with vehicle-treated rats (Veh, 75% dimethyl sulfoxide). Both are sensitive to blockade by the κ-opioid receptor (KOR) antagonist norBNI (10 mg/kg, intraperitoneally, given 24 h before salvA; adapted with permission from Potter et al., 2011). (b) Rats treated with cocaine (Coc) in a binge-like regimen (3× 15 mg/kg/day for 14 days, intraperitoneally) show normal ICSS thresholds 21 h after the first day of cocaine injection but develop increased ICSS thresholds compared with vehicle-treated rats (Veh, 0.9% saline), which plateau after ~8 days of binge treatment. Treatment with norBNI [20 µg, intracerebroventricularly, day 0 (d0), 24 h before the start of the cocaine binge regimen] prevents cocaine withdrawal-induced anhedonia (adapted with permission from Chartoff et al., 2012). *P < 0.05, **P <0.01 compared with Veh +Veh; #P< 0.05, ##P<0.01 comparing groups under the bars.
KOR activation, as might occur with drug withdrawal, stress, or administration of a potent agonist such as salvA, triggers opponent neuroadaptations in dopamine-mediated signaling and dopamine neurotransmission. Acute administration of salvA causes long-lasting (~2 h) decreases in dopamine levels in the extracellular NAc (Carlezon et al., 2006; Ebner et al., 2010), which likely initiates compensatory changes in dopamine signaling (Acri et al., 2001; Chefer et al., 2005). Consistent with this, repeated treatment of rats with the KOR agonist U69593 potentiates locomotor sensitization to a D2 receptor agonist (Perreault et al., 2005), and the D2 receptor number is significantly increased after withdrawal from repeated U69593 administration (Izenwasser et al., 1998). We have shown that prior, repeated salvA treatment potentiates the locomotor response to a D1 receptor agonist (Chartoff et al., 2008). Taken together, these data raise the possibility that as the KOR-related component of the aversive response to stress or drug withdrawal diminishes, an upregulated dopamine system is unmasked, allowing for higher baseline reward function and an altered hedonic response to drugs of abuse.
In contrast, there is little direct evidence that drug withdrawal-induced negative affective states trigger an opponent reward state. Rats administered a binge-like regimen of cocaine (Fig. 2b) or allowed to self-administer cocaine or heroin for an extended period each day (6 h/day) show a progressive decrease in brain stimulation reward (Ahmed and Koob, 1998; Kenny et al., 2006; Chartoff et al., 2012). When drug access is stopped, reward thresholds return to baseline over the course of a week, but do not fall below predrug baseline levels (Chartoff et al., 2012). This suggests that drug withdrawal-induced negative affective states do not result in overt increases in reward that are expressed once withdrawal signs end. Alternatively, drug withdrawal may trigger a variety of neuroadaptations that simultaneously increase and decrease reward function, as has been demonstrated with chronic drug use (Nestler and Aghajanian, 1997; Koob, 2009). Indirect support for this idea comes from findings that, with sustained cessation of drug administration, mesolimbic dopamine transmission normalizes from a depressed level (Weiss et al., 1992) and the ability of acute exposure to drugs of abuse to increase NAc dopamine levels is enhanced (Kalivas and Duffy, 1993; Heidbreder et al., 1996). The time course of this enhancement parallels the progressive increase in the locomotor-activating (i.e. sensitization) and reinforcing (i.e. incubation of craving) effects of cocaine that occur as the time after the last drug administration increases (Kalivas and Duffy, 1993; Heidbreder et al., 1996; Lu et al., 2004).
Stress, but not drug withdrawal, can act as a positive reinforcer
Animals will self-administer electrical stimulation into brain areas (i.e. dorsal mesencephalic central gray area, lateral tegmentum, reticular formation) that, when stimulated noncontingently, elicit vigorous escape responses (Cazala et al., 1985). Similarly, nonhuman primates will self-administer mild electric shocks under some conditions (Barrett et al., 1978; Malagodi et al., 1981; Laurence et al., 1994). One explanation for these behaviors is that the rewarding effects of stimulation offset are perceived as more reinforcing than the aversive effects of the stimulation itself. A more nuanced view is that the way in which the temporal dynamics of stress onset, duration, and offset coincide with operant behavior determines whether approach or avoidance behavior will be observed. This has been elegantly demonstrated in fruit flies; a shock can condition approach or avoidance to an odor depending on the relative timing of the shock and the conditioning odor (Tanimoto et al., 2004). If the conditioning odor comes after the shock, it can come to signify shock offset, and flies will learn to approach that odor.
In contrast, direct evidence that drug withdrawal per se can serve as a positive reinforcer is lacking, perhaps owing to challenges in the design of studies that would address this issue.
Inconsistencies and gaps in knowledge
Impact of stress and drug withdrawal on reinstatement of drug taking
The idea that drug withdrawal and stress elicit similar negative affective states through similar mechanisms is not novel (Kreek and Koob, 1998; Koob, 2008). An important and unresolved question is how drug withdrawal as a stressor influences the motivation to self-administer drugs of abuse, because blocking stress may be a feasible intervention (Van’t Veer and Carlezon, 2013). In the case of stress, there is evidence supporting both facilitative and suppressive effects on drug taking and relapse. Brief exposure to stressors or injections of corticosterone can sensitize the mesocorticolimbic dopamine system to the activating (Antelman et al., 1980; Kalivas and Stewart, 1991; Rouge-Pont et al., 1995; Prasad et al., 1998) and rewarding (Lett, 1989; Piazza et al., 1990) effects of cocaine and other addictive drugs. Likewise, stress can trigger craving in drug-dependent humans (Sinha et al., 1999) and facilitate the acquisition and reinstatement of drug self-administration (Piazza et al., 1990; Goeders and Guerin, 1996; Shaham et al., 2003). Stress-induced reinstatement of operant responding on a lever previously paired with cocaine delivery requires CRF release into the VTA, which activates glutamatergic inputs to dopamine neurons (Wang et al., 2005). Although exposure to a stressor itself may be aversive, there is considerable evidence that the net result is an increased sensitivity to the reinforcing effects of the drug (McLaughlin et al., 2006a; Bruchas et al., 2010). For instance, people who are more sensitive to stress (Piazza and Le Moal, 1997) or who do not feel that they have adequate control over stress may be more likely to abuse drugs. In contrast, there is evidence that prolonged or uncontrollable stress suppresses the mesocorticolimbic dopamine system, reward function, and drug intake (Willner et al., 1992; Miczek et al., 2008; Miczek et al., 2011). The complexity of this issue stems mainly from the infinite stress permutations that can be invoked.
The case for drug withdrawal motivating continued drug taking and relapse appears to be more complex. The central tenet of negative reinforcement as it applies to addiction is that drug withdrawal-induced negative affective states motivate behaviors that terminate the negative affect. This has been difficult to demonstrate unequivocally in animal models. It has been shown that rats allowed extended access to self-administration of drugs of abuse progressively escalate drug intake in parallel with an escalation of anhedonia as measured with ICSS (Koob, 2009), suggesting that withdrawal-induced negative affective states drive drug intake, which is perfectly consistent with the opponent process theory. Although powerful because both drug intake and affect were measured in the same animals, these findings are correlative. Early studies in morphine-dependent rodents and nonhuman primates trained to self-administer morphine reported that µ-opioid receptor antagonist treatment precipitated withdrawal and increased rates of morphine self-administration (Schuster and Thompson, 1969). A more recent study that controlled for dependence, withdrawal state, dose of self-administered opioid (remifentanil), and reinforcement history showed that morphine-withdrawn rats initiated remifentanil self-administration at much lower doses and at much earlier time points than nondependent rats (Cooper et al., 2008). These findings support the idea that drug withdrawal-induced negative affective states serve as negative reinforcers.
However, the studies described above did not directly address whether withdrawal could precipitate relapse. Rats trained to self-administer heroin that underwent extinction training could be motivated to reinstate lever pressing when administered a morphine prime but not when administered the opioid antagonist naltrexone (Stewart and Wise, 1992), suggesting that proponent, rather than opponent, processes drive reinstatement. A subsequent study demonstrated that rats required previous experience with heroin self-administration during withdrawal in order for drug ‘seeking’ to be enhanced during subsequent bouts of withdrawal (Hutcheson et al., 2001). The implications of these findings on drug addiction are important because they suggest that self-medication is a learned rather than an inherent process. If true, then it may be possible to take advantage of this learning process for treatment. For example, the memory that heroin is more rewarding during withdrawal could potentially be disrupted during reconsolidation processes. Alternatively, treatments that alleviate withdrawal states could be purposefully paired with precipitated withdrawal to help stamp in the memory that therapeutic alleviation of withdrawal can serve as a reinforcer to remain abstinent.
Another major gap in current knowledge is related to understanding how aversive state-mediated increases in reward function might contribute to addictive behavior (Fig. 1). For example, KOR activation has been shown to modulate aspects of dopamine signaling in the NAc. Repeated administration of KOR agonists decreases dopamine uptake in the NAc (Thompson et al., 2000), which subsequently increases basal and cocaine-potentiated levels of extracellular dopamine (Heidbreder et al., 1998). In addition, postsynaptic dopamine D2 receptor levels are increased in the NAc after repeated KOR activation (Izenwasser et al., 1998), an effect that could be considered proreward. We have shown that repeated administration of salvA increases the locomotor stimulant effects of cocaine (Potter et al., 2011), but effects on reward-related or reinforcement-related effects of cocaine have not been tested.
Conclusion
Preventing disease tends to be easier than reversing it (Van’t Veer and Carlezon, 2013). The idea of preventing addiction is appealing but provocative. Considering that stress can play a role in causing new cases of addiction and comorbid psychiatric illnesses, and can exacerbate existing cases, one strategy may be to mitigate the effects of stress. As described above, CRF antagonists and KOR antagonists have attracted interest as medications that reduce illness by targeting stress. There have been practical limitations in the development of CRF antagonists (Zorrilla and Koob, 2010), and KOR antagonists seem most efficacious if administered before a stressor (Van’t Veer and Carlezon, 2013). Importantly, some of the most traumatic and costly forms of stress (e.g. combat, responding to a disaster) can be predicted in advance; hence, intervention with these types of agents may be effective in preventing addiction or psychiatric conditions that are highly comorbid with addiction (e.g. depressive and anxiety-related illnesses; Kessler et al., 2008). These agents may also be effective for treating individuals who have been in recovery for long periods of time, by preventing stress-induced relapse of drug-seeking behavior.
A more effective strategy for faster intervention would be to find treatments that can cause lateral or vertical shifts in the affective oscillations triggered by repeated drug use; shortening or flattening the negative dips in the hedonic state (Fig. 1). Conceptualized in this way, some of the most highly effective (but still controversial) treatments for addiction (such as methadone, nicotine patch) work through this mechanism. The most tenable strategy may be to find treatments that reverse drug-induced neuroadapations, both antireward and proreward, to cure the disease rather than to treat its symptoms; however, this requires a more thorough understanding of the neurobiology of addiction.
Acknowledgements
Dr. Chartoff’s contributions to this article were supported by the National Institute on Drug Abuse (Grant Numbers DA026552 and DA023606). Dr. Carlezon’s contributions to this article were supported by the National Institute of Mental Health (Grant Numbers MH063266 and MH097860).
Dr. Carlezon has a US patent covering the use of kappaopioid receptor antagonists in the treatment of depressive disorders (Assignee: McLean Hospital). In the last 3 years, Dr. Carlezon has received compensation for professional services from The American College of Neuropsychopharmacology and Concert Pharmaceuticals.
Footnotes
Conflicts of interest
Dr. Chartoff reports no conflicts of interest.
References
- Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Brain Res Rev. 1998;26:360–378. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Acri JB, Thompson AC, Shippenberg T. Modulation of pre- and post-synaptic dopamine D2 receptor function by the selective kappa-opioid receptor agonist U69593. Synapse. 2001;39:343–350. doi: 10.1002/1098-2396(20010315)39:4<343::AID-SYN1018>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Adell A, Garcia-Marquez C, Armario A, Gelpi E. Chronic stress increases serotonin and noradrenaline in rat brain and sensitizes their responses to a further acute stress. J Neurochem. 1988;50:1678–1681. doi: 10.1111/j.1471-4159.1988.tb02462.x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Kogan JH, Moghaddam B. Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res. 1994;636:126–130. doi: 10.1016/0006-8993(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: Change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Aldrich JV, Senadheera SN, Ross NC, Reilley KA, Ganno ML, Eans SE, et al. Alanine analogs of [D-Trp]CJ-15,208: Novel opioid activity profiles and prevention of drug- and stress-induced reinstatement of cocaine-seeking behavior. Br J Pharmacol. 2014;171:3212–3222. doi: 10.1111/bph.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Bruchas MR. Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Front Pharmacol. 2013;4:96. doi: 10.3389/fphar.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, et al. The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci USA. 1995;92:5062–5066. doi: 10.1073/pnas.92.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgustinovich DF, Kovalenko IL, Kudryavtseva NN. A model of anxious depression: Persistence of behavioral pathology. Neurosci Behav Physiol. 2005;35:917–924. doi: 10.1007/s11055-005-0146-6. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. Crf antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 1989;98:203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23:475–482. doi: 10.1016/s0165-6147(02)02086-2. [DOI] [PubMed] [Google Scholar]
- Barrett JE, Stanley JA, Weinberg ES. Schedule-induced water and ethanol consumption as a function of interreinforcement interval in the squirrel monkey. Physiol Behav. 1978;21:453–455. doi: 10.1016/0031-9384(78)90108-7. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, et al. Creb activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs. cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain. 2008;12:866–869. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Borges K, Dingledine R. Functional organization of the GluR1 glutamate receptor promoter. J Biol Chem. 2001;276:25929–25938. doi: 10.1074/jbc.M009105200. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Natale NR, Patel SA. System xc(−) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165:20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. Am Psychol. 1986;41:765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, et al. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buydens-Branchey L, Noumair D, Branchey M. Duration and intensity of combat exposure and posttraumatic stress disorder in Vietnam veterans. J Nerv Ment Dis. 1990;178:582–587. doi: 10.1097/00005053-199009000-00005. [DOI] [PubMed] [Google Scholar]
- Cador M, Ahmed SH, Koob GF, Le Moal M, Stinus L. Corticotropin-releasing factor induces a place aversion independent of its neuroendocrine role. Brain Res. 1992;597:304–309. doi: 10.1016/0006-8993(92)91487-y. [DOI] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao JL, Covington HE, 3rd, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC, et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277:812–814. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, Dinieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Carlezon WA., Jr Development of kappa opioid receptor antagonists. J Med Chem. 2013;56:2178–2195. doi: 10.1021/jm301783x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazala P, Bendani T, Zielinski A. Self-stimulation of an ‘aversive’ brain structure: the mesencephalic central gray area. Brain Res. 1985;327:53–60. doi: 10.1016/0006-8993(85)91498-2. [DOI] [PubMed] [Google Scholar]
- Chartoff E, Connery HS. It’s more exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol. 2014;5:116. doi: 10.3389/fphar.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, Konradi C, Carlezon WA., Jr Dopamine-dependent increases in phosphorylation of cAMP response element binding protein (CREB) during precipitated morphine withdrawal in primary cultures of rat striatum. J Neurochem. 2003;87:107–118. doi: 10.1046/j.1471-4159.2003.01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Mague SD, Barhight MF, Smith AM, Carlezon WA., Jr Behavioral and molecular effects of dopamine D1 receptor stimulation during naloxone-precipitated morphine withdrawal. J Neurosci. 2006;26:6450–6457. doi: 10.1523/JNEUROSCI.0491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Barhight MF, Mague SD, Sawyer AM, Carlezon WA., Jr Anatomically dissociable effects of dopamine D1 receptor agonists on reward and relief of withdrawal in morphine-dependent rats. Psychopharmacology (Berl) 2009a;204:227–239. doi: 10.1007/s00213-008-1454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, Carlezon WA., Jr Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAC tissue. Mol Pharmacol. 2009b;75:704–712. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001;49:753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- Choi KH, Edwards S, Graham DL, Larson EB, Whisler KN, Simmons D, et al. Reinforcement-related regulation of AMPA glutamate receptor subunits in the ventral tegmental area enhances motivation for cocaine. J Neurosci. 2011;31:7927–7937. doi: 10.1523/JNEUROSCI.6014-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Koob GF. Propranolol antagonizes the enhanced conditioned fear produced by corticotropin releasing factor. J Pharmacol Exp Ther. 1988;247:902–910. [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluzzi F, Rocco A, Mandatori I, Mattia C. Non-analgesic effects of opioids: opioid-induced nausea and vomiting: mechanisms and strategies for their limitation. Curr Pharm Des. 2012;18:6043–6052. doi: 10.2174/138161212803582540. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Truong YN, Shi YG, Woods JH. Morphine deprivation increases self-administration of the fast- and short-acting muopioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther. 2008;326:920–929. doi: 10.1124/jpet.108.139196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordery SF, Taverner A, Ridzwan IE, Guy RH, Delgado-Charro MB, Husbands SM, Bailey CP. A non-rewarding, non-aversive buprenorphine/naltrexone combination attenuates drug-primed reinstatement to cocaine and morphine in rats in a conditioned place preference paradigm. Addict Biol. 2014;19:575–586. doi: 10.1111/adb.12020. [DOI] [PubMed] [Google Scholar]
- Coyne JC, Aldwin C, Lazarus RS. Depression and coping in stressful episodes. J Abnorm Psychol. 1981;90:439–447. doi: 10.1037//0021-843x.90.5.439. [DOI] [PubMed] [Google Scholar]
- Desole MS, Esposito G, Fresu L, Migheli R, Enrico P, Mura MA, et al. Effects of morphine treatment and withdrawal on striatal and limbic monoaminergic activity and ascorbic acid oxidation in the rat. Brain Res. 1996;723:154–161. doi: 10.1016/0006-8993(96)00235-1. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ. Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cereb Cortex. 1991;1:273–292. doi: 10.1093/cercor/1.4.273. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni A, Gessa G. Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. J Pharmacol Exp Ther. 1995;272:781–785. [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.12.014. pii: S0006-3223:00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, Chavkin C. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J Neurosci. 1994;14:3736–3750. doi: 10.1523/JNEUROSCI.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Whisler KN, Self DW. Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse. 2009;63:224–235. doi: 10.1002/syn.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrico P, Mura MA, Esposito G, Serra P, Migheli R, De Natale G, et al. Effect of naloxone on morphine-induced changes in striatal dopamine metabolism and glutamate, ascorbic acid and uric acid release in freely moving rats. Brain Res. 1998;797:94–102. doi: 10.1016/s0006-8993(98)00371-0. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress-induced relapse to drug seeking in the rat: Role of the bed nucleus of the stria terminalis and amygdala. Stress. 2001;4:289–303. doi: 10.3109/10253890109014753. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Leslie FM, Cone RI. Dynorphin-containing pathways in the substantia nigra and ventral tegmentum: a double labeling study using combined immunofluorescence and retrograde tracing. Neuropeptides. 1985;5:457–460. doi: 10.1016/0143-4179(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: Common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Ford CP, Beckstead MJ, Williams JT. Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J Neurophysiol. 2007;97:883–891. doi: 10.1152/jn.00963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Axelrod SR, Paliwal P, Sleeper J, Sinha R. Difficulties in emotion regulation and impulse control during cocaine abstinence. Drug Alcohol Depend. 2007;89:298–301. doi: 10.1016/j.drugalcdep.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Giacchino JL, Henriksen SJ. Systemic morphine and local opioid effects on neuronal activity in the medial prefrontal cortex. Neuroscience. 1996;70:941–949. doi: 10.1016/0306-4522(95)00409-2. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF. Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996;64:337–348. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez P, Cabello P, Germany A, Norris B, Contreras E. Decrease of tolerance to, and physical dependence on morphine by, glutamate receptor antagonists. Eur J Pharmacol. 1997;332:257–262. doi: 10.1016/s0014-2999(97)01099-6. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–954. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, et al. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry. 2010;67:28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella SL, Funk D, Coen K, Li Z, Le AD. Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. Behav Brain Res. 2014;265:188–197. doi: 10.1016/j.bbr.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hebb DO. Drives and the C.N.S. (conceptual nervous system) Psychol Rev. 1955;62:243–254. doi: 10.1037/h0041823. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1996;278:490–502. [PubMed] [Google Scholar]
- Heidbreder CA, Schenk S, Partridge B, Shippenberg TS. Increased responsiveness of mesolimbic and mesostriatal dopamine neurons to cocaine following repeated administration of a selective kappa-opioid receptor agonist. Synapse. 1998;30:255–262. doi: 10.1002/(SICI)1098-2396(199811)30:3<255::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Britton KT, Koob GF. Both conditioned taste preference and aversion induced by corticotropin-releasing factor. Pharmacol Biochem Behav. 1991;40:717–721. doi: 10.1016/0091-3057(91)90075-d. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo–pituitary–adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Im H-I, Amelio AL, Kocerha J, Bali P, Lu Q, et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Hurd YL. Differential messenger RNA expression of prodynorphin and proenkephalin in the human brain. Neuroscience. 1996;72:767–783. doi: 10.1016/0306-4522(96)00002-4. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4:943–947. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Imperato A, Cabib S, Puglisi-Allegra S. Repeated stressful experiences differently affect the time-dependent responses of the mesolimbic dopamine system to the stressor. Brain Res. 1993;601:333–336. doi: 10.1016/0006-8993(93)91732-8. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29:7349–7358. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoi K, Horiba N, Tozawa F, Sakai Y, Sakai K, Abe K, et al. Major role of 3’,5’-cyclic adenosine monophosphate-dependent protein kinase a pathway in corticotropin-releasing factor gene expression in the rat hypothalamus in vivo. Endocrinology. 1996;137:2389–2396. doi: 10.1210/endo.137.6.8641191. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET. Dynorphin a [1–17] induces “reward” in rats in the place conditioning paradigm. Life Sci. 1988;43:503–508. doi: 10.1016/0024-3205(88)90151-8. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Acri JB, Kunko PM, Shippenberg T. Repeated treatment with the selective kappa opioid agonist U-69593 produces a marked depletion of dopamine D2 receptors. Synapse. 1998;30:275–283. doi: 10.1002/(SICI)1098-2396(199811)30:3<275::AID-SYN5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Jackson K, Carroll FI, Negus S, Damaj M. Effect of the selective kappaopioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl) 2010;210:285–294. doi: 10.1007/s00213-010-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McLaughlin JP, Carroll FI, Damaj MI. Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2013;226:763–768. doi: 10.1007/s00213-012-2716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Shi C, Carlezon WA, Jr, Neve RL, Nestler EJ, Davis M. Long-term memory is facilitated by cAMP response element-binding protein over-expression in the amygdala. J Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Latimer LG. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J Pharmacol Exp Ther. 1987;242:757–763. [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28:13856–13865. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Keeney AJ, Hogg S. Behavioural consequences of repeated social defeat in the mouse: preliminary evaluation of a potential animal model of depression. Behav Pharmacol. 1999;10:753–764. doi: 10.1097/00008877-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat-an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, et al. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci. 2005;25:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the national comorbidity survey follow-up. Psychol Med. 2008;38:365–374. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazan N, Young GA, Calligaro D. Self-administration of dynorphin-[1–13] and D-ala2-dynorphin-[1–11] (kappa opioid agonists) in morphine (mu opioid agonist)-dependent rats. Life Sci. 1983;33(Suppl 1):559–562. doi: 10.1016/0024-3205(83)90564-7. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. Sb242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ, Valentino RJ. Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci. 2008;28:6516–6525. doi: 10.1523/JNEUROSCI.0390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DA, Lessard AA, Chan J, Colago EE, Zhou Y, Schlussman SD, et al. Region-specific changes in the subcellular distribution of AMPA receptor GluR1 subunit in the rat ventral tegmental area after acute or chronic morphine administration. J Neurosci. 2008;28:9670–9681. doi: 10.1523/JNEUROSCI.2151-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Graham DL, Arzaga RR, Buzin N, Webb J, Green TA, et al. Overexpression of CREB in the nucleus accumbens shell increases cocaine reinforcement in self-administering rats. J Neurosci. 2011;31:16447–16457. doi: 10.1523/JNEUROSCI.3070-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence MT, Hineline PN, Bersh PJ. The puzzle of responding maintained by response-contingent shock. J Exp Anal Behav. 1994;61:135–153. doi: 10.1901/jeab.1994.61-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: hedonic and neural responses to safety from pain. PloS one. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, Van Bockstaele EJ, et al. Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature. 2012;490:402–406. doi: 10.1038/nature11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Lin D, Bruijnzeel AW, Schmidt P, Markou A. Exposure to chronic mild stress alters thresholds for lateral hypothalamic stimulation reward and subsequent responsiveness to amphetamine. Neuroscience. 2002;114:925–933. doi: 10.1016/s0306-4522(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Regulation of NMDA receptor subunits and nitric oxide synthase expression during cocaine withdrawal. J Neurochem. 2000;75:2040–2050. doi: 10.1046/j.1471-4159.2000.0752040.x. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lu W, Monteggia LM, Wolf ME. Repeated administration of amphetamine or cocaine does not alter AMPA receptor subunit expression in the rat midbrain. Neuropsychopharmacology. 2002;26:1–13. doi: 10.1016/S0893-133X(01)00272-X. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Ho A, Kreek MJ. Chronic administration of a cocaine “binge” alters basal extracellular dopamine levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1995;272:652–657. [PubMed] [Google Scholar]
- Malagodi EF, Gardner ML, Ward SE, Magyar RL. Responding maintained under intermittent schedules of electric-shock presentation: “Safety” or schedule effects? J Exp Anal Behav. 1981;36:171–190. doi: 10.1901/jeab.1981.36-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Negus S, Koob GF. Precipitation of morphine withdrawal syndrome in rats by administration of mu-, delta- and kappa-selective opioid antagonists. Neuropharmacology. 1992;31:1231–1241. doi: 10.1016/0028-3908(92)90051-p. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Blendy JA, Tzavara E, Gass P, Roques BP, Hanoune J, Schutz G. Reduction of morphine abstinence in mice with a mutation in the gene encoding CREB. Science. 1996;273:657–659. doi: 10.1126/science.273.5275.657. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Le Moal M, Piazza PV. Sensitization to the motor effects of contingent infusions of heroin but not of kappa agonist ru 51599. Psychopharmacology (Berl) 1998;139:281–285. doi: 10.1007/s002130050717. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Mathieu-Kia AM, Besson MJ. Repeated administration of cocaine, nicotine and ethanol: effects on preprodynorphin, preprotachykinin A and pre-proenkephalin mRNA expression in the dorsal and the ventral striatum of the rat. Brain Res Mol Brain Res. 1998;54:141–151. doi: 10.1016/s0169-328x(97)00338-0. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci. 2012;6:137. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006a;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006b;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca , Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, McGinty JF. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate-putamen is primarily associated with synaptic vesicles in axons. Neuroscience. 2000;96:91–99. doi: 10.1016/s0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH, van Erp AM, Blank AD, McInerney SC. D-amphetamine “cue” generalizes to social defeat stress: behavioral sensitization and attenuated accumbens dopamine. Psychopharmacology (Berl) 1999;147:190–199. doi: 10.1007/s002130051160. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE3rd. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE3rd. Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 2008;196:303–313. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Morani AS, Kivell B, Prisinzano TE, Schenk S. Effect of kappa-opioid receptor agonists U69593, U50488h, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol Biochem Behav. 2009;94:244–249. doi: 10.1016/j.pbb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur Neuropsychopharmacol. 1992;2:43–49. doi: 10.1016/0924-977x(92)90035-7. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Van’t Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, et al. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci. 2011;31:3095–3103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, et al. Hypocretin (orexin) facilitates reward by attenuating the antire-ward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci USA. 2014;111:E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, et al. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. The NSDUH Report – substance use and dependence following initiation of alcohol or illicit drug use. Rockville, MD: NSDUH; 2008. [Google Scholar]
- O’Brien CP, Testa T, O’Brien TJ, Brady JP, Wells B. Conditioned narcotic withdrawal in humans. Science. 1977;195:1000–1002. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, et al. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci. 2013;16:146–153. doi: 10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, Latagliata EC, Cabib S, Puglisi-Allegra S. The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine. Cereb Cortex. 2007;17:2796–2804. doi: 10.1093/cercor/bhm008. [DOI] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Graham D, Bisnaire L, Simms J, Hayton S, Szechtman H. Kappa-opioid agonist U69593 potentiates locomotor sensitization to the D2/D3 agonist quinpirole: pre- and postsynaptic mechanisms. Neuropsychopharmacology. 2005;31:1967–1981. doi: 10.1038/sj.npp.1300938. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev. 1997;25:359–372. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: Implications for sensation-seeking behaviors. Proc Natl Acad Sci USA. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine D, Charney D. Children, stress, and sensitization: an integration of basic and clinical research on emotion? Biol Psychiatry. 2002;52:773. doi: 10.1016/s0006-3223(02)01569-x. [DOI] [PubMed] [Google Scholar]
- Piras AP, Zhou Y, Schlussman SD, Ho A, Kreek MJ. Acute withdrawal from chronic escalating-dose binge cocaine administration alters kappa opioid receptor stimulation of [35S] guanosine 5′-O-[gamma-thio]triphosphate acid binding in the rat ventral tegmental area. Neuroscience. 2010;169:751–757. doi: 10.1016/j.neuroscience.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Platt JE, Stone EA. Chronic restraint stress elicits a positive anti-depressant response on the forced swim test. Eur J Pharmacol. 1982;82:179–181. doi: 10.1016/0014-2999(82)90508-8. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Potter DN, Damez-Werno D, Carlezon WA, Jr, Cohen BM, Chartoff EH. Repeated exposure to the kappa-opioid receptor agonist salvinorin a modulates extracellular signal-regulated kinase and reward sensitivity. Biol Psychiatry. 2011;70:744–753. doi: 10.1016/j.biopsych.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BM, Ulibarri C, Sorg BA. Stress-induced cross-sensitization to cocaine: effect of adrenalectomy and corticosterone after short- and long-term withdrawal. Psychopharmacology (Berl) 1998;136:24–33. doi: 10.1007/s002130050535. [DOI] [PubMed] [Google Scholar]
- Redila V, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Hsu K, Jr, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Richter RM, Weiss F. in vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Robbe D, Bockaert J, Manzoni OJ. Metabotropic glutamate receptor 2-/3-dependent long-term depression in the nucleus accumbens is blocked in morphine withdrawn mice. Eur J Neurosci. 2002;16:2231–2235. doi: 10.1046/j.1460-9568.2002.02273.x. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, et al. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci USA. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouge-Pont F, Marinelli M, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. II. Sensitization of the increase in extracellular dopamine induced by cocaine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7189–7195. doi: 10.1523/JNEUROSCI.15-11-07189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedi-Bettschen D, Rowlett JK, Spealman RD, Platt DM. Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys: kappa opioid and serotonergic mechanisms. Psychopharmacology (Berl) 2010;210:169–177. doi: 10.1007/s00213-009-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusin KI, Giovannucci DR, Stuenkel EL, Moises HC. Kappa-opioid receptor activation modulates Ca2+ currents and secretion in isolated neuroendocrine nerve terminals. J Neurosci. 1997;17:6565–6574. doi: 10.1523/JNEUROSCI.17-17-06565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res. 1994;61:117–133. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Sawyer A, Carlezon WA, Jr, Chartoff EH. Nucleus accumbens AMPA glutamate receptors contribute to precipitated morphine withdrawal signs in rats. Chicago: Society for Neuroscience; 2009. [Google Scholar]
- Schank JR, Goldstein AL, Rowe KE, King CE, Marusich JA, Wiley JL, et al. The kappa opioid receptor antagonist JDTic attenuates alcohol seeking and withdrawal anxiety. Addict Biol. 2012;17:634–647. doi: 10.1111/j.1369-1600.2012.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Whitfield TW, Jr, Park PE, Crawford EF, George O, Vendruscolo LF, Koob GF. Long-term antagonism of kappa opioid receptors prevents escalation of and increased motivation for heroin intake. J Neurosci. 2013;33:19384–19392. doi: 10.1523/JNEUROSCI.1979-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Sedki F, Eigenmann K, Gelinas J, Schouela N, Courchesne S, Shalev U. A role for kappa-, but not mu-opioid, receptor activation in acute food deprivation-induced reinstatement of heroin seeking in rats. Addict Biol[Epub ahead of print] 2014 doi: 10.1111/adb.12133. [DOI] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Sepulveda MJ, Hernandez L, Rada P, Tucci S, Contreras E. Effect of precipitated withdrawal on extracellular glutamate and aspartate in the nucleus accumbens of chronically morphine-treated rats: an in vivo microdialysis study. Pharmacol Biochem Behav. 1998;60:255–262. doi: 10.1016/s0091-3057(97)00550-9. [DOI] [PubMed] [Google Scholar]
- Sepulveda J, Oliva P, Contreras E. Neurochemical changes of the extracellular concentrations of glutamate and aspartate in the nucleus accumbens of rats after chronic administration of morphine. Eur J Pharmacol. 2004;483:249–258. doi: 10.1016/j.ejphar.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: micro-circuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci USA. 1975a;72:3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Nirenberg M, Klee WA. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci USA. 1975b;72:590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Shen YL, Chen JC, Liao RM. Place conditioning and neurochemical responses elicited by the aftereffect of acute stressor exposure involving an elevated stand. Neurosci Lett. 2011;504:156–159. doi: 10.1016/j.neulet.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Sherman JE, Kalin NH. ICV-CRH alters stress-induced freezing behavior without affecting pain sensitivity. Pharmacol Biochem Behav. 1988;30:801–807. doi: 10.1016/0091-3057(88)90103-7. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. The kappa opioid receptor and dynorphin co-localize in vasopressin magnocellular neurosecretory neurons in guinea-pig hypothalamus. Neuroscience. 2000;96:373–383. doi: 10.1016/s0306-4522(99)00472-8. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. K-opioid receptor activation of a dendrotoxin-sensitive potassium channel mediates presynaptic inhibition of mossy fiber neurotransmitter release. Mol Pharmacol. 1996;50:80–85. [PubMed] [Google Scholar]
- Simmons ML, Terman GW, Chavkin C. Spontaneous excitatory currents and kappa-opioid receptor inhibition in dentate gyrus are increased in the rat pilocarpine model of temporal lobe epilepsy. J Neurophysiol. 1997;78:1860–1868. doi: 10.1152/jn.1997.78.4.1860. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Uschold N, Magoni M, Bar J, Popoli M, Neumann ID, Reber SO. Behavioural consequences of two chronic psychosocial stress paradigms: anxiety without depression. Psychoneuroendocrinology. 2012;37:702–714. doi: 10.1016/j.psyneuen.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Almeida OF, Bartl C, Shippenberg TS. Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berl) 1994;115:121–127. doi: 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- Spangler R, Unterwald EM, Kreek MJ. ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res Mol Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- Stewart J, Wise RA. Reinstatement of heroin self-administration habits: morphine prompts and naltrexone discourages renewed responding after extinction. Psychopharmacologia. 1992;108:79–84. doi: 10.1007/BF02245289. [DOI] [PubMed] [Google Scholar]
- Sukhov RR, Walker LC, Rance NE, Price DL, Young WS., 3rd Opioid precursor gene expression in the human hypothalamus. J Comp Neurol. 1995;353:604–622. doi: 10.1002/cne.903530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Xue Y, Huang Z, Steketee JD. Regulation of cocaine-reinstated drug-seeking behavior by kappa-opioid receptors in the ventral tegmental area of rats. Psychopharmacology (Berl) 2010;210:179–188. doi: 10.1007/s00213-010-1812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Garzon M, Colago EE, Pickel VM. Mu-opioid receptors in the ventral tegmental area are targeted to presynaptically and directly modulate mesocortical projection neurons. Synapse. 2001;41:221–229. doi: 10.1002/syn.1079. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Britton KT, Koob GF. Potentiation of acoustic startle by corticotropin-releasing factor (CRF) and by fear are both reversed by alpha-helical CRF (9–41) Neuropsychopharmacology. 1989;2:285–292. doi: 10.1016/0893-133x(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav Neurosci. 1989;103:648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- Tanimoto H, Heisenberg M, Gerber B. Experimental psychology: event timing turns punishment to reward. Nature. 2004;430:983. doi: 10.1038/430983a. [DOI] [PubMed] [Google Scholar]
- Tenayuca JM, Nazarian A. Hydrocodone and morphine possess similar rewarding effects and reduce ERK and CREB phosphorylation in the nucleus accumbens. Synapse. 2012;66:918–922. doi: 10.1002/syn.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tokuyama S, Wakabayashi H, Ho IK. Direct evidence for a role of glutamate in the expression of the opioid withdrawal syndrome. Eur J Pharmacol. 1996;295:123–129. doi: 10.1016/0014-2999(95)00645-1. [DOI] [PubMed] [Google Scholar]
- Tomek SE, Lacrosse AL, Nemirovsky NE, Olive MF. NMDA receptor modulators in the treatment of drug addiction. Pharmaceuticals (Basel) 2013;6:251–268. doi: 10.3390/ph6020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Pollack AE, Fink JS. Enhanced CREB phosphorylation and changes in c-Fos and FRA expression in striatum accompany amphetamine sensitization. Brain Res. 1997;749:120–126. doi: 10.1016/s0006-8993(96)01316-9. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31:4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Van Vliet BJ, Mulder AH, Schoffelmeer AN. Mu-opioid receptors mediate the inhibitory effect of opioids on dopamine-sensitive adenylate cyclase in primary cultures of rat neostriatal neurons. J Neurochem. 1990;55:1274–1280. doi: 10.1111/j.1471-4159.1990.tb03135.x. [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Yano JM, Carroll FI, Cohen BM, Carlezon WA., Jr Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the kappa-opioid receptor antagonist JDTic. Neuropsychopharmacology. 2012;37:2809–2816. doi: 10.1038/npp.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Bechtholt AJ, Onvani S, Potter D, Wang Y, Liu-Chen LY, et al. Ablation of kappa-opioid receptors from brain dopamine neurons has anxiolytic-like effects and enhances cocaine-induced plasticity. Neuropsychopharmacology. 2013;38:1585–1597. doi: 10.1038/npp.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Sadri-Vakili G. Mechanisms of transgenerational inheritance of addictive-like behaviors. Neuroscience. 2014;264:198–206. doi: 10.1016/j.neuroscience.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drug addiction: The neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33:17569–17576. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Blendy JA. Different requirements for cAMP response element binding protein in positive and negative reinforcing properties of drugs of abuse. J Neurosci. 2001;21:9438–9444. doi: 10.1523/JNEUROSCI.21-23-09438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, et al. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Zalutsky RA, Nicoll RA. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature. 1993;365:188. doi: 10.1038/365188a0. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- Wikler A. A theory of opioid dependence. NIDA Res Monogr. 1980;30:174–178. [PubMed] [Google Scholar]
- Wikler A, Pescor FT. Classical conditioning of a morphine abstinence phenomenon, reinforcement of opioid-drinking behavior and “relapse” in morphine-addicted rats. Psychopharmacologia. 1967;10:255–284. doi: 10.1007/BF00401386. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Yamamoto RT, Karlsgodt KH, Rott D, Lukas SE, Elman I. Effects of perceived cocaine availability on subjective and objective responses to the drug. Subst Abuse Treat Prev Policy. 2007;2:30. doi: 10.1186/1747-597X-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharko RM, Anisman H. Stressor-induced anhedonia in the mesocorticolimbic system. Neurosci Biobehav Rev. 1991;15:391–405. doi: 10.1016/s0149-7634(05)80032-6. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Wojcieszak J. Salvia divinorum: from mazatec medicinal and hallucinogenic plant to emerging recreational drug. Hum Psychopharmacol. 2013;28:403–412. doi: 10.1002/hup.2304. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and pre-prodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague–Dawley rats. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. The psychophysiology of sensation seeking. J Pers. 1990;58:313–345. doi: 10.1111/j.1467-6494.1990.tb00918.x. [DOI] [PubMed] [Google Scholar]