Abstract
Objectives
Biofilm acids contribute to secondary caries, which is a main reason for dental restoration failures. The objectives of this study were to: (1) develop a protein-repellent and antibacterial composite, and (2) investigate the effects of combining 2-methacryloyloxyethyl phosphorylcholine (MPC) with quaternary ammonium dimethylaminohexadecyl methacrylate (DMAHDM) on composite mechanical properties and biofilm response for the first time.
Methods
MPC, DMAHDM and glass particles were mixed into a dental resin composite. Mechanical properties were measured in three-point flexure. Protein adsorption onto the composites was measured by a micro bicinchoninic acid method. A human saliva microcosm model was used to grow biofilms on composites. Colony-forming unit (CFU) counts, live/dead assay, metabolic activity, and lactic acid production of biofilms were determined.
Results
Incorporation of 3% MPC and 1.5% DMAHDM into composite achieved protein-repellent and antibacterial capabilities without compromising the mechanical properties. Composite with 3% MPC + 1.5% DMAHDM had protein adsorption that was 1/10 that of a commercial composite (p < 0.05). The composite with 3% MPC + 1.5% DMAHDM had much greater reduction in biofilm growth than using MPC or DMAHDM alone (p < 0.05). Biofilm CFU counts on composite with 3% MPC + 1.5% DMAHDM were more than three orders of magnitude lower than that of commercial control.
Conclusions
Dental composite with a combination of strong protein-repellent and antibacterial capabilities was developed for the first time. Composite with MPC and DMAHDM greatly reduced biofilm activity and is promising to inhibit secondary caries. The dual agents of MPC plus DMAHDM may have wide applicability to other dental materials.
Keywords: Resin composite, protein repellent, antibacterial property, mechanical property, human saliva microcosm biofilm, caries inhibition
1. Introduction
Dental caries remains the most common and widespread biofilm-dependent oral disease.1,2 Because of their esthetics and direct-filling capability, resin composites are widely used to restore tooth cavities.3,4 Extensive efforts have improved the resin compositions and cure conditions, and reduced the polymerization shrinkage.5-12 Nonetheless, secondary caries still limits the lifetime of composite restorations.13,14 More than half of the restorations placed annually are replacements of failed restorations,15 and the annual cost for tooth cavity restorations was approximately $46 billion in 2005 in the United States.16 Dental composites generally do not inhibit bacterial adhesion and biofilm formation. On the contrary, previous studies have shown that composites tend to accumulate more biofilms and plaques in vivo than other restorative materials.17,18
Efforts have been made to incorporate antibacterial agents into composites. One class of such composites involved the use of quaternary ammonium methacrylates (QAMs).19-23 Composites containing 12-methacryloyloxydodecylpyridinium bromide (MDPB) were effective in reducing bacterial viability.19,20 Other antibacterial composites used agents including methacryloxylethyl cetyl dimethyl ammonium chloride and cetylpyridinium chloride.21-23 Recently, a quaternary ammonium dimethacrylate (QADM) was synthesized and incorporated into composite, achieving strong antibacterial effects.24-27 The antibacterial potency of quaternary ammonium compounds was shown to increase with increasing the alkyl chain length (CL) of the ammonium groups.28 A series of new QAMs with CL varying from 3 to 18 were synthesized and incorporated into composites and bonding agents.27,29 The results showed that a new dimethylaminohexadecyl methacrylate (DMAHDM) with CL of 16 had the strongest antibacterial activity.29
Other efforts were made to develop surfaces with bacteria-repellent capability by coating the surface with layers of highly hydrophilic material.30 Hydrophilic material surfaces can repel protein adsorption and bacterial adhesion.31,32 2-methacryloyloxyethyl phosphorylcholine (MPC) is a methacrylate with a phospholipid polar group in the side chain, and is one of the most common biocompatible and hydrophilic biomedical polymers.33 Highly hydrophilic surface coatings using MPC polymers are well known to reduce protein adsorption and bacterial adhesion.34-37 However, there has been no report on the development of protein-repellent dental composite. Furthermore, there has been no report on dental composite that incorporates both MPC and DMAHDM to possess double benefits of protein-repellent and antibacterial capabilities.
One drawback of QAM-containing composites is that the adsorption of salivary proteins on composite surfaces could decrease the efficacy of “contact-inhibition”, thereby reducing the antibacterial potency.21,22 Therefore, a composite containing both MPC and QAM may protect the antibacterial potency of the composite by repelling protein adsorption, thereby increasing the composite surface-bacteria contact and hence the contact-killing efficacy. Hence, it would be highly desirable to combine MPC with DMAHDM to achieve double benefits of protein-repellent and antibacterial activities for dental composites.
Accordingly, the objectives of this study were to: (1) develop a novel protein-repellent and antibacterial composite, and (2) investigate the combined effects of MPC and DMAHDM on protein adsorption, dental plaque microcosm biofilm response, and mechanical properties of the composite for the first time. It was hypothesized that: (1) The composite containing MPC and DMAHDM would have good mechanical properties matching those with 0% MPC and 0% DMAHDM, and those of a commercial control composite; (2) composite containing MPC and DMAHDM would have much less protein adsorption than the controls; (3) incorporating MPC or DMAHDM individually into composite would yield substantial decreases in biofilm growth on composite; and (4) incorporating both MPC and DMAHDM into composite would achieve much greater biofilm-inhibition than using MPC or DMAHDM alone.
2. Materials and methods
2.1. Preparation of composites containing MPC and DMAHDM
MPC was obtained commercially (Sigma-Aldrich, St. Louis, MO) which was synthesized via a method reported by Ishihara et al.33 BisGMA (bisphenol glycidyl dimethacrylate) and TEGDMA (triethylene glycol dimethacrylate) (Esstech, Essington, PA) were mixed at a mass ratio = 1:1, and rendered light-curable with 0.2% camphorquinone and 0.8% ethyl 4-N,Ndimethylaminobenzoate (mass fractions). The MPC powder was mixed with the photo-activated BisGMA-TEGDMA resin (referred to as BT) at a MPC/(BT + MPC) mass fraction of 10%. Preliminary study on a series of mass fractions indicated that this mass fraction yielded a strong protein-repellent property without compromising mechanical properties of the resin.
DMAHDM with an alkyl chain length of 16 was synthesized using a modified Menschutkin reaction where a tertiary amine group was reacted with an organo-halide.29,38 A benefit of this reaction is that the reaction products are generated at virtually quantitative amounts and require minimal purification. Briefly, 10 mmol of 2-(dimethylamino) ethyl methacrylate (DMAEMA, Sigma-Aldrich, St. Louis MO) and 10 mmol of 1-bromohexadecane (BHD, TCI America, Portland, OR) were combined with 3 g of ethanol in a 20 mL scintillation vial. The vial was stirred at 70 °C for 24 h. The solvent was then removed via evaporation, yielding DMAHDM as a clear, colorless, and viscous liquid.27,29 DMAHDM was incorporated into the BisGMA-TEGDMA resin at DMAHDM/(BT + DMAHDM) mass fractions of 0%, 5%, 7.5%, and 10%. The 10% DMAHDM was used following previous studies.27,29 The 5% and 7.5% DMAHDM were used because 10% DMAHDM appeared to lower the composite strength when combined with MPC.
Each resin was filled with glass particles (barium boroaluminosilicate, mean size = 1.4 μm, Caulk/ Dentsply, Milford, DE) silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine.40 A filler mass fraction of 70% was used to yield a cohesive paste. Since the resin mass fraction in the composite was 30%, the MPC mass fraction in the final composite was 3%. The DMAHDM mass fractions in the composite were 0%, 1.5%, 2.25%, and 3%, respectively. The composite with 0% MPC and 0% DMAHDM served as a control. In addition, a commercial composite (Heliomolar, Ivoclar, Ontario, Canada) also served as a control. The fillers were silica and ytterbium-trifluoride with particle sizes of 40-200 nm at a filler level of 66.7%. Heliomolar is indicated for Class I and Class II restorations in the posterior region and Classes III-V restorations.
2.2. Mechanical properties
Nine composites were tested for mechanical properties:
-
(1)
Commercial control (Heliomolar);
-
(2)
Experimental control: 70% glass + 30% BT (termed “0% MPC + 0% DMAHDM”);
-
(3)
70% glass + 27% BT + 3% MPC (termed “3% MPC”);
-
(4)
70% glass + 28.5% BT + 1.5% DMAHDM (“1.5% DMAHDM”);
-
(5)
70% glass + 27.75% BT + 2.25% DMAHDM (“2.25% DMAHDM”);
-
(6)
70% glass + 27% BT + 3% DMAHDM (“3% DMAHDM”);
-
(7)
70% glass + 25.5% BT + 3% MPC + 1.5% DMAHDM (“3% MPC + 1.5% DMAHDM”);
-
(8)
70% glass + 24.75% BT + 3% MPC + 2.25% DMAHDM (“3% MPC + 2.25% DMAHDM”);
-
(9)
70% glass + 24% BT + 3% MPC + 3% DMAHDM (“3% MPC + 3% DMAHDM”).
Each composite paste was placed into rectangular molds of 2 mm × 2 mm × 25 mm. The specimens were photo-cured (Triad 2000, Dentsply, York, PA) for 1 min on each open side.25,26 Six specimens were made for each composite. The specimens were immersed in distilled water at 37 °C for 24 h.25,26 The specimens were then fractured in three-point flexure with a 10-mm span at a crosshead-speed of 1 mm/min on a computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC). Flexural strength (S) was calculated as: S = 3PmaxL/(2bh2), where P is the fracture load, L is span, b is specimen width and h is thickness. Elastic modulus (E) was calculated as: E = (P/d)(L3/[4bh3]), where load P divided by displacement d is the slope in the linear elastic region. The specimens were wet and not dried, and were fractured within a few minutes after being taken out of the water.25,26
2.3. Characterization of protein adsorption
The mechanical testing results showed that composites with 3% MPC plus 2.25% or 3% DMAHDM had lower composite strength. Therefore, only the 1.5% DMAHDM was used. Hence, five composites were tested for protein adsorption and biofilm experiments:
-
(1)
Commercial control (Heliomolar);
-
(2)
Experimental control: 70% glass + 30% BT (termed “0% MPC + 0% DMAHDM”);
-
(3)
70% glass + 27% BT + 3% MPC (termed “3% MPC”);
-
(4)
70% glass + 28.5% BT + 1.5% DMAHDM (“1.5% DMAHDM”);
-
(5)
70% glass + 25.5% BT + 3% MPC + 1.5% DMAHDM (“3% MPC + 1.5% DMAHDM”).
For protein adsorption and biofilm experiments, each composite paste was placed into disk molds of 9 mm in diameter and 2 mm in thickness and light-curd as described above. The specimens were immersed in distilled water at 37 °C for 24 h. The amount of protein adsorbed on composite disks was determined by the micro bicinchoninic acid (BCA) method.41,42 Each disk was immersed in phosphate buffered saline (PBS) for 2 h. The disks then were immersed in bovine serum albumin (BSA) (Sigma-Aldrich) solutions at 37 °C for 2 h. The protein solutions contained BSA at a concentration of 4.5 g/L following previous studies.41,42 The disks then were rinsed with fresh PBS by stirring at a speed of 300 rpm for 5 min (Bellco Glass, Vineland, NJ), immersed in sodium dodecyl sulfate (SDS) 1 wt % in PBS, and sonication at room temperature for 20 minutes to completely detach the BSA adsorbed onto the surface of disk.41,42 A protein analysis kit (micro BCA protein assay kit, Fisher Scientific, Pittsburgh, PA) was used to determine the BSA concentration in the SDS solution.41,42 Briefly, 25μL of the SDS solution was mixed with 200 μL of the BCA working reagent in a 96-well plate, which was incubated at 60 °C for 30 minutes.41,42 Then the 96-well plate was cooled down to room temperature and the absorbance at 562 nm was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA). Standard curves were prepared using the BSA standard. From the concentration of protein, the amount of protein adsorbed on the composite disk surface was calculated.41,42
2.4. Saliva collection for biofilm inoculum
Oral biofilm growth and viability on the composite disks were investigated using a dental plaque microcosm model following previous studies.25,26 Saliva is ideal for growing dental plaque microcosm biofilms in vitro, with the advantage of maintaining much of the complexity and heterogeneity of the dental plaque in vivo.43 The saliva for biofilm inoculums was collected from ten healthy adult donors having natural dentition without active caries or periopathology, and without the use of antibiotics within the last 3 months, following previous studies.25,26 The donors did not brush teeth for 24 h and abstained from food and drink intake for 2 h prior to donating saliva. Stimulated saliva was collected during parafilm chewing and was kept on ice. An equal volume of saliva from each of the ten donors was combined to form the saliva sample. The saliva was diluted in sterile glycerol to a concentration of 70%, and stored at −80 °C.44
2.5. Dental plaque microcosm biofilm formation and live/dead assay
The saliva-glycerol stock was added, with 1:50 final dilution, into the growth medium as inoculum.25,26 The growth medium contained mucin (type II, porcine, gastric) at a concentration of 2.5 g/L; bacteriological peptone, 2.0 g/L; tryptone, 2.0 g/L; yeast extract, 1.0 g/L; NaCl, 0.35 g/L, KCl, 0.2 g/L; CaCl2, 0.2 g/L; cysteine hydrochloride, 0.1 g/L; hemin, 0.001 g/L; vitamin K1, 0.0002 g/L, at pH 7.45 The composite disks were sterilized in ethylene oxide (Anprolene AN 74i, Andersen, Haw River, NC). 1.5 mL of inoculum was added to each well of 24-well plates with a disk, and incubated at 37 °C in 5% CO2 for 8 h. Then, the composite disks were transferred to new 24-well plates filled with fresh medium and incubated. After 16 h, the composite disks were transferred to new 24-well plates with fresh medium and incubated for 24 h. This totaled 48 h of incubation, which was adequate to form plaque microcosm biofilms as shown in a previous study.44
For live/dead bacterial staining assay, composite disks with 2-day biofilms were washed with PBS and stained using the BacLight live/dead kit (Molecular Probes, Eugene, OR).25,26 Live bacteria were stained with Syto 9 to produce a green fluorescence. Bacteria with compromised membranes were stained with propidium iodide to produce a red fluorescence. The stained disks were examined using an inverted epifluorescence microscope (Eclipse TE2000-S, Nikon, Melville, NY). The area of green staining (live bacteria) was computed with NIS Elements imaging software (Nikon). The area fraction of live bacteria = green staining area/total area of the image.26 Six specimens were evaluated for each composite. Three randomly chosen fields of view were photographed from each disk, yielding a total of 18 images for each composite.
2.6. MTT metabolic assay
Composite disks with 2-day biofilms were transferred to a new 24-well plate for the MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay.25,26 MTT is a colorimetric assay that measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan. A total of 1 mL of MTT was added to each well and incubated for 1 h. Disks were transferred to a new 24-well plate, and 1 mL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals. The plate was incubated for 20 min with gentle mixing at room temperature in the dark. Then, 200 μL of the DMSO solution from each well was collected, and its absorbance at 540 nm was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnvale, CA). A higher absorbance is related to a higher formazan concentration, which indicates a higher metabolic activity in the biofilm on the disk.25,26
2.7. Lactic acid production
Composite disks with 2-day biofilms were rinsed with cysteine peptone water (CPW) to remove loose bacteria.25,26 The disks were transferred to 24-well plates and 1.5 mL of buffered-peptone water (BPW) supplemented with 0.2% sucrose was added. The disks were incubated at 37 °C in 5% CO2 for 3 h to allow the biofilms to produce acid.25,26 The BPW solutions were then stored for lactate analysis. Lactate concentrations in the BPW solutions were determined using an enzymatic (lactate dehydrogenase) method, following previous studies.25,26 The microplate reader was used to measure the absorbance at 340 nm (optical density OD340) for the collected BPW solutions. Standard curves were prepared using a lactic acid standard (Supelco, Bellefonte, PA).25,26
2.8. Colony-forming unit (CFU) counts
Composite disks with 2-day biofilms were transferred into tubes with 2 mL CPW, and the biofilms were harvested by sonication and vortexing (Fisher, Pittsburgh, PA).25,26 Three types of agar plates were used to measure the CFU counts to assess the microorganism viability. First, tryptic soy blood agar culture plates were used to determine total microorganisms.45 Second, mitis salivarius agar (MSA) culture plates containing 15% sucrose were used to determine total streptococci.46 This is because MSA contains selective agents including crystal violet, potassium tellurite and trypan blue, which inhibit most Gram-negative bacilli and most Gram-positive bacteria except streptococci, thus enabling the streptococci to grow.46 Third, cariogenic mutans streptococci is known to be resistant to bacitracin, and this property is used to isolate mutans streptococci from the highly heterogeneous oral microflora.45 Therefore, MSA agar culture plates plus 0.2 units of bacitracin per mL was used to determine mutans streptococci.45 The bacterial suspensions were serially diluted, spread onto agar plates and incubated at 37 °C in 5% CO2 for 24 h.25,26 The number of colonies that grew was counted and used, along with the dilution factor, to calculate the CFU counts on each composite disk.25,26
2.9. Statistical analysis
One-way and two-way analyses of variance (ANOVA) were performed to detect the significant effects of the variables. Tukey's multiple comparison test was used to compare the data at a p value of 0.05.
3. Results
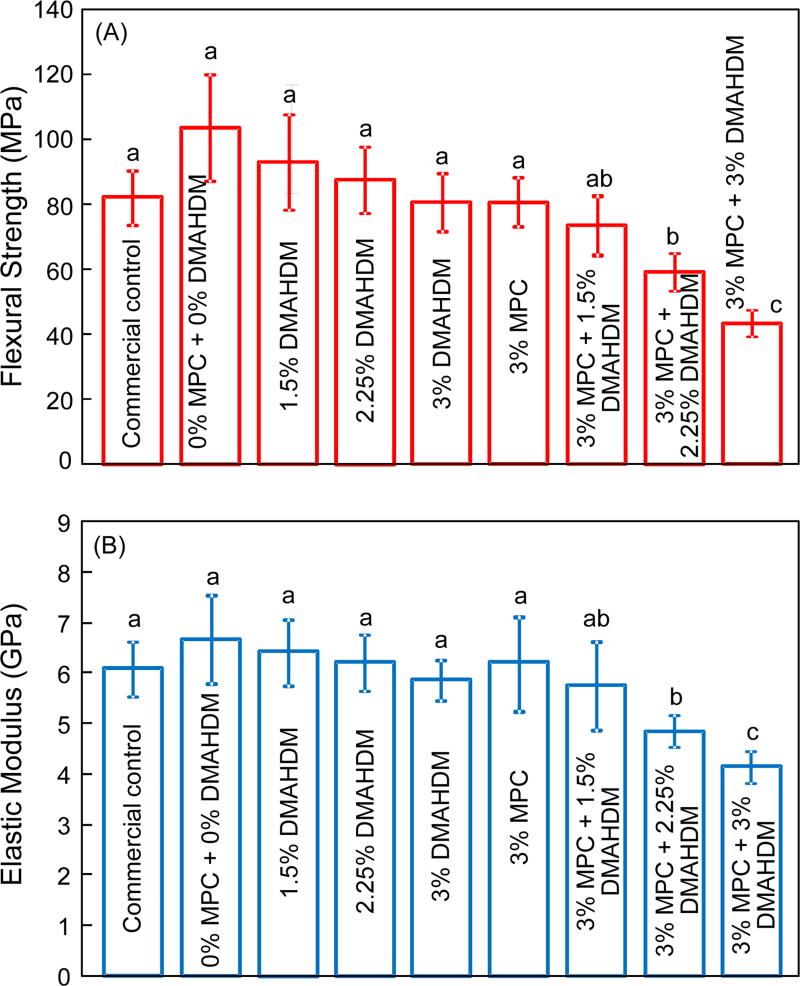
Fig. 1 plots of the mechanical properties of composites: (A) Flexural strength, and (B) elastic modulus (mean ± sd; n = 6). The first seven composites had flexural strength and elastic modulus that were not significantly different from each other (p > 0.1). The last two composites with 3% MPC + 2.25% DMAHDM and 3% MPC + 3% DMAHDM had significantly lower mechanical properties than the controls (p < 0.05). The composite containing 3% MPC + 1.5% DMAHDM had strength and elastic modulus similar to those of the commercial control (p > 0.1).
Figure 1.
Mechanical properties of composites: (A) Flexural strength, and (B) elastic modulus (mean ± sd; n = 6). The composite with 3% MPC + 1.5% DMAHDM had strength and elastic modulus similar to those of a commercial control (p > 0.1). Bars with dissimilar letters indicate values that are significantly different from each other (p < 0.05).
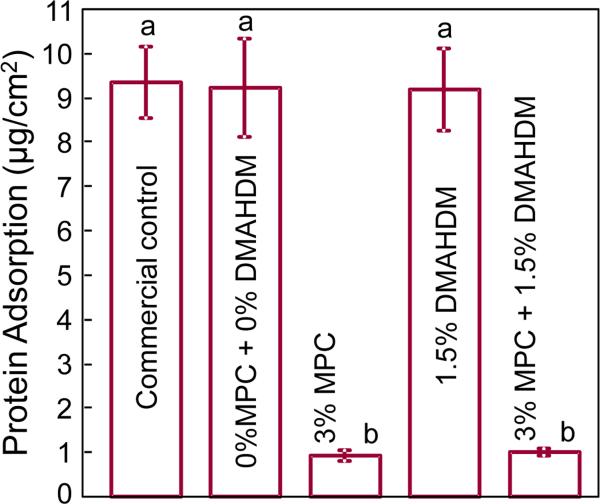
Protein adsorption onto composite surfaces is plotted in Fig. 2 (mean ± sd; n = 6). Adding 3% of MPC into the composite greatly reduced the protein adsorption, compared to that with 0% MPC and the commercial control (p < 0.05). Adding 1.5% DMAHDM had no effect on protein adsorption (p > 0.1). The composite with 3% MPC + 1.5% DMAHDM had the same protein adsorption as that containing 3% MPC without DMAHDM (p > 0.1). The composite with 3% MPC + 1.5% DMAHDM had protein adsorption about an order of magnitude less than that of control (p < 0.05).
Figure 2.
Protein adsorption onto composite surfaces (mean ± sd; n = 6). The composite with 3% MPC, and the composite with 3% MPC + 1.5% DMAHDM, both had much less protein adsorption, which was about 1/10 that of commercial control composite (p < 0.05). Bars with dissimilar letters indicate values that are significantly different from each other (p < 0.05).
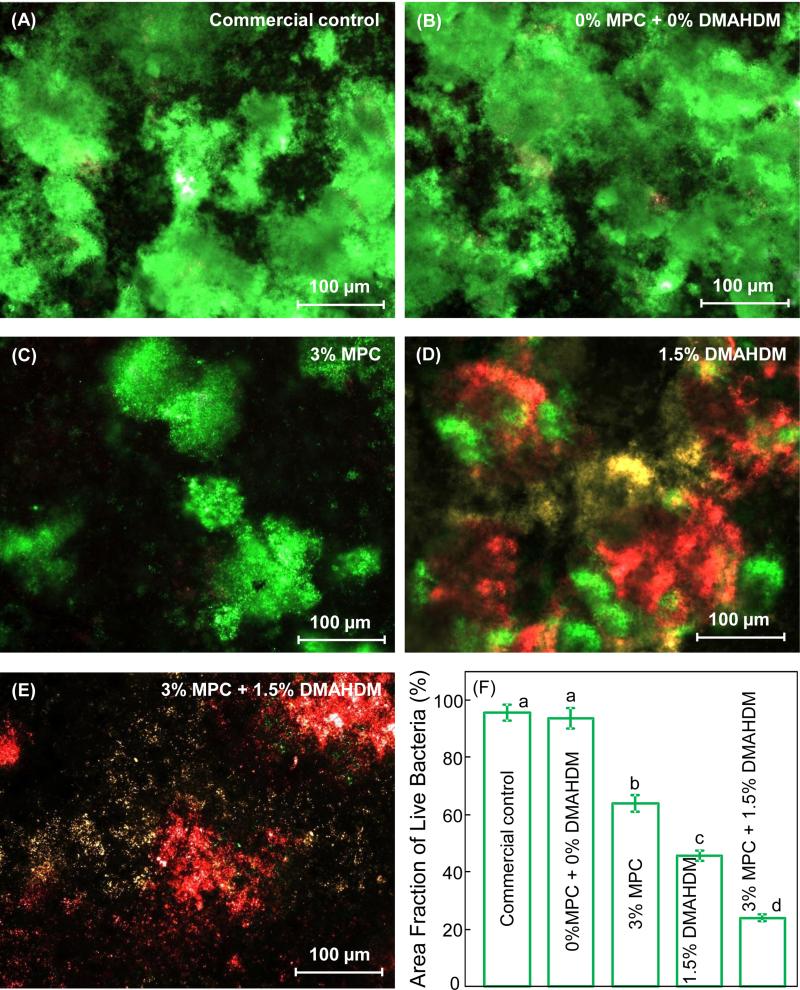
Typical live/dead staining images of 2-day biofilms grown on composite disks are shown in Fig. 3(A-E), and the area fraction of composite surface covered by live bacteria is plotted in (F) (mean ± sd; n = 6). The two control composites were fully covered by live bacteria (A and B). In contrast in (C), composite with 3% MPC had much less bacterial adhesion, although the bacteria were mostly alive (green staining). On the other hand, composite with 1.5% DMAHDM had a strong antibacterial activity yielding substantial amounts of dead bacteria (red staining). The yellowish staining was likely caused by live and dead bacteria being close together or on the top of each other. Finally, the combined use of 3% MPC + 1.5% DMAHDM had much less bacterial adhesion, and the bacteria were mostly dead. In (F) for quantification of live bacteria coverage, values with dissimilar letters are significantly different from each other.
Figure 3.
Representative live/dead staining images of biofilms adherent on composite disks cultured for 2 days: (A) Commercial control composite, (B) control composite with 0% MPC + 0% DMAHDM, (C) composite with 3% MPC, (D) composite with 1.5% DMAHDM, (E) composite with 3% MPC + 1.5% DMAHDM. (F) area fraction of green staining of live bacteria coverage on composite surface (mean ± sd; n = 6). The live bacteria were stained green, and the dead bacteria were stained red. When live and dead bacteria were in close proximity or on the top of each other, the staining had yellow or orange colors. The composite with 3% MPC + 1.5% DMAHDM had greatly decreased bacterial adhesion, and the biofilms consisted of primarily dead bacteria. Dissimilar letters in (E) indicate values that are significantly different from each other (p < 0.05).
Quantitative viability of the 2-day biofilms on composites is shown in Fig. 4: (A) Metabolic activity, and (B) lactic acid production (mean ± sd; n = 6). Incorporation of MPC or DMAHDM alone greatly decreased the metabolic activity and lactic acid production of the biofilms, compared to the controls (p < 0.05). The composite containing 3% MPC + 1.5% DMAHDM had the least metabolic activity and lactic acid production.
Figure 4.
Biofilm viability on composite disks cultured for 2 days: (A) metabolic activity, and (B) lactic acid production (mean ± sd; n = 6). Biofilms on the composite with 3% MPC + 1.5% DMAHDM had metabolic activity that was about 5% that on commercial control (p < 0.05). Lactic acid production by the biofilms on the composite with 3% MPC + 1.5% DMAHDM was about 7% that on the commercial control (p < 0.05). In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
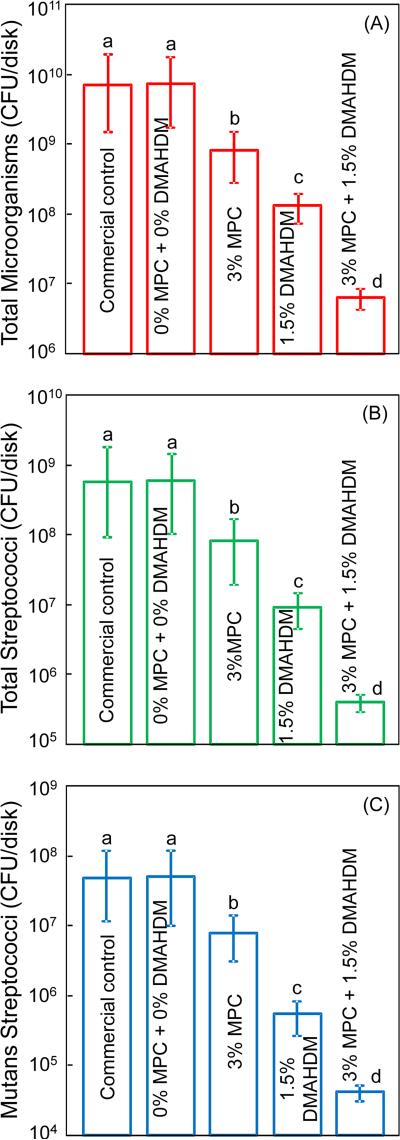
Fig. 5 plots the CFU of the 2-day biofilms grown on the composite disks: (A) Total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). Incorporating MPC or DMAHDM alone into the composite decreased the biofilm CFU, compared to the two controls (p < 0.05). The composite with double agents, 3% MPC + 1.5% DMAHDM, had much less biofilm CFU than using either MPC or DMAHDM alone (p < 0.05). All three CFU counts on the composite with 3% MPC + 1.5% DMAHDM were more than 3 orders of magnitude lower than those of the two control composites.
Figure 5.
Biofilm CFU counts on composite disks cultured for 2 days: (A) total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). All three CFU counts on the composite with 3% MPC + 1.5% DMAHDM were more than 3 orders of magnitude lower than those on commercial control. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
4. Discussion
To date there has been no report on protein-repellent dental composite, or composite with a combination of protein-repellent and antibacterial properties. In the present study, novel composite was developed with MPC for protein-repellent ability and DMANDM for antibacterial property for the first time. The composite with 3% MPC + 1.5% DMAHDM greatly reduced protein adsorption, bacteria attachment and biofilm growth, metabolic activity, CFU counts, and lactic acid production. These benefits were achieved without compromising mechanical properties. Therefore, the composite with 3% MPC + 1.5% DMAHDM is promising for dental restorations to inhibit biofilm acids and secondary caries, and the approach of using dual agents with protein-repellent and antibacterial capabilities may have applicability to other dental materials.
Resin composites are the principal materials for tooth cavity restorations.3,47 However, composites not only had no antibacterial function, but also accumulated more biofilms in vivo than other restorative materials.17,18 Biofilms contribute to secondary caries, which is a main reason for restoration failures.13,14 It was suggested that protein adsorption from physiological fluids, such as saliva-derived protein films, is an initial step in bacteria attachment and biofilm formation.48 Previous studies reported that most proteins were found to adsorb preferentially to hydrophobic surfaces.31,32 MPC is a methacrylate with a phospholipid polar group in the side chain.33 Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers.49 The structure of the phospholipid molecule generally consists of a hydrophilic head (attracted to water) and hydrophobic tails (repelled by water).49 When placed in water, phospholipids will orient themselves into a bilayer in which the non-polar tail region faces the inner area of the bilayer. The polar head region faces outward and interacts with the water. Hence, the MPC polymers are highly hydrophilic.33 Highly hydrophilic surface coatings using MPC are well known to reduce both protein adsorption and bacterial attachment.35-37 Regarding the protein-repellent mechanism, it was reported that there is an abundance of free water but no bound water in the hydrated MPC polymer. The presence of bound water would cause protein adsorption.34,50,51 On the other hand, the large amount of free water around the phosphorylcholine group is considered to detach proteins effectively, thereby repelling protein adsorption.34,50 The results of protein adsorption assay confirmed that incorporation of MPC into the composite significantly decreased protein adsorption. This was confirmed via the composite containing 3% MPC, which had protein adsorption 1/10 that of control composite. The present study also confirmed that the MPC composite with protein-repellent capability indeed had much less bacteria attachment and biofilm CFU.
QAMs were shown to be promising for dental applications including use in composite, primer and adhesive.19-29 In the present study, DMAHDM and MPC were combined for use in the composite. The results showed that DMAHDM indeed imparted a strong antibacterial function to the composite. Furthermore, the present study showed that the antibacterial potency of DMAHDM-containing composite can be further increased by the incorporation of MPC. The combined use of MPC and DMAHDM in the composite was supported by two benefits: (1) the use of dual agents in the composite achieved much greater reduction in biofilm activity, compared to DMAHDM or MPC alone; (2) the use of dual agents in the composite did not adversely affect the mechanical properties. All three CFU counts of biofilm on the composite with 1.5% DMAHDM was nearly two orders of magnitude lower than the control. However, the composite with 3% MPC + 1.5% DMAHDM reduced the biofilm CFU by more than three orders of magnitude. The reason may be that, the mode of antibacterial action of DMAHDM-containing composite is contact-inhibition.21,22 Previous studies suggested that when the negatively-charged bacterial cell contacts the positively-charged sites of QAM, the electric balance of the cell membrane could be disturbed, and the bacterium could explode under its own osmotic pressure.21,22,52 This contact-killing mechanism would indicate that, when a salivary protein pellicle separates the antibacterial resin surface from the overlaying biofilm, the antibacterial effect of the resin could be decreased.21,22 Indeed, several studies demonstrated that a saliva-derived protein film on the cationic antibacterial surfaces reduced the original bactericidal effect.30,53,54 Because MPC can greatly reduce the protein adsorption, it would enhance the antibacterial effectiveness of DMAHDM. This factor likely contributed to the further reduction in biofilm CFU by more than an order of magnitude over that with DMAHDM alone.
Regarding the long-term durability of protein-repellent and antibacterial properties, the advantage of QAM composite is that the antibacterial agent is copolymerized with the resin by forming a covalent bonding with the polymer network.19,20 Therefore the QAM is immobilized in the composite and not released or lost over time.19,20 This method imparts a durable antibacterial capability to the composite. Several publications have demonstrated the long-term durability of QAM composites.26,55-58 For example, a study on MDPB monomer, a bromide monomethacrylate, showed that the antibacterial effect was maintained after the composite was immersed in water for 3 months.58 Regarding the MPC, previous studies showed that the MPC-modified surface layer formed by photo-induced graft polymerization was resistant to mechanical stresses.59,60 In previous studies, MPC was grafted onto the surface through covalent bonding, and the strong C-C bonding provided durable resistance to protein adsorption.59,60 Another study reported that the MPC-modified layer provided high lubricity for the surface.61 This lubrication may result in durability against the mechanical stress caused by brushing, thus offering sufficient durability for clinical application.61 In the present study, MPC was mixed and copolymerized with the BisGMA-TEGDMA resin, leading to MPC immobilization in the composite. Therefore, the MPC was incorporated throughout the composite volume, and not limited to the surface only; as a result, the MPC effect will not be lost in wear and chewing actions. Therefore, the protein-repellent activity of the composite is expected to be durable. Further study is needed to test the long-term properties of the novel protein-repellent and antibacterial composite.
Regarding potential clinical applications, the composite containing MPC and DMAHDM may be especially useful in patients who are prone to developing caries. Furthermore, the dual agents method of MPC plus DMAHDM may have a wide applicability to other types of dental materials. This includes bonding agents, cements, sealants and various types of composites such as flowable composites for root caries restorations. Studies are needed to incorporate MPC and DMAHDM into various dental materials to gain protein-repellent and antibacterial benefits without adversely affecting other desirable properties. Further studies are also needed to test the novel protein-repellent and antibacterial composite containing MPC and DMAHDM under in vivo conditions.
5. Conclusions
The present study reported the first dental composite with a combination of protein-repellent and antibacterial capabilities to combat biofilms and caries. The effects of MPC and DMAHDM incorporation into composite on mechanical properties, protein adsorption, and dental plaque microcosm biofilm response were determined for the first time. The composite with 3% MPC showed a strong protein-repellent capability and substantially reduced bacteria attachment. Furthermore, the use of dual agents, 3% MPC + 1.5% DMAHDM, in the composite achieved the greatest reduction in biofilm growth and lactic acid production. The composite with 3% MPC + 1.5% DMAHDM had strength and elastic modulus matching those of a commercial composite without protein-repellent and antibacterial properties. Therefore, the novel composite with MPC plus DMAHDM is promising to reduce biofilm formation and plaque buildup, and inhibit secondary caries. The method of dual agents MPC plus DMAHDM may have wide applicability to other bonding systems, composites, sealants and cements.
Acknowledgments
We thank Dr. Junling Wu and Chen Chen for discussions and experimental help. This study was supported by the School of Stomatology at the Capital Medical University in China (NZ), NIH R01 DE17974 (HX), and a Seed Grant (HX) from the University of Maryland School of Dentistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–9. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 2.Hu DY, Hong X, Li X. Oral health in China – trends and challenges. International Journal of Oral Science. 2011;3:7–12. doi: 10.4248/IJOS11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. Journal of Dental Research. 2008;87:710–9. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferracane JL. Resin composite – State of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dental Materials. 2002;18:436–44. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 6.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dental Materials. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 7.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. Journal of Dental Research. 2005;84:822–6. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Ling L, Wang R, Burgess JO. Formation and characterization of a novel fluoride-releasing dental composite. Dental Materials. 2006;22:1014–23. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Wan Q, Sheffield J, McCool J, Baran GR. Light curable dental composites designed with colloidal crystal reinforcement. Dental Materials. 2008;24:1694–1701. doi: 10.1016/j.dental.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. State-of-the-art techniques in operative dentistry: contemporary teaching of posterior composites in UK and Irish dental schools. British Dental Journal. 2010;209:129–36. doi: 10.1038/sj.bdj.2010.674. [DOI] [PubMed] [Google Scholar]
- 11.Milward PJ, Adusei GO, Lynch CD. Improving some selected properties of dental polyacid-modified composite resins. Dental Materials. 2011;27:997–1002. doi: 10.1016/j.dental.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. Journal of the American Dental Association. 2011;142:612–20. doi: 10.14219/jada.archive.2011.0243. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dental Materials. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dental Materials. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Primary Dental Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 16.Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. Economic impact of regulating the use of amalgam restorations. Public Health Report. 2007;122:657–63. doi: 10.1177/003335490712200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. Accumulation of Streptococcus mutans on light-cured composites and amalgam: an in vitro study. Journal of Esthetic Dentistry. 1998;10:187–90. doi: 10.1111/j.1708-8240.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 18.Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. Journal of Dentistry. 2007;35:201–6. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Imazato S. Review: antibacterial properties of resin composites and dentin bonding systems. Dental Materials. 2003;19:449–57. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 20.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dental Materials Journal. 2009;28:11–9. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 21.Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Namba N, Yoshida Y, Nagaoka N, Takashima S, Matsuura-Yoshimoto K, Maeda H, Van Meerbeek B, Suzuki K, Takashiba S. Antibacterial effect of bactericide immobilized in resin matrix. Dental Materials. 2009;25:424–30. doi: 10.1016/j.dental.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. Journal of Dentistry. 2009;37:289–96. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, Lin-Gibson S, Zhou X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dental Materials. 2012;28:561–72. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng L, Weir MD, Zhang K, Wu EJ, Xu SM, Zhou X, Xu HH. Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dental Materials. 2012;28:853–62. doi: 10.1016/j.dental.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng L, Weir MD, Zhang K, Xu SM, Chen Q, Zhou X, Xu HH. Antibacterial nanocomposite with calcium phosphate and quaternary ammonium. Journal of Dental Research. 2012;91:460–6. doi: 10.1177/0022034512440579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Weir MD, Zhang K, Deng D, Cheng L, Xu HH. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dental Materials. 2013;29:859–70. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J, Soderling E, Osterblad M, Vallittu PK, Lassila LVJ. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules. 2011;16:9755–63. doi: 10.3390/molecules16119755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Li F, Weir MD, Xu HH. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. Journal of Dentistry. 2013;41:1122–31. doi: 10.1016/j.jdent.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, Imazato S, Ruhl S, Schmalz G. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30:4921–9. doi: 10.1016/j.biomaterials.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 31.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. Journal of Biomedical Materials Research. 1998;43:338–48. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 32.Katsikogianni M, Missirlis YF. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria material interactions. European Cells and Materials. 2004;8:37–5. doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- 33.Ishihara K, Ueda T, Nakabayashi N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polymer Journal. 1990;22:355–60. [Google Scholar]
- 34.Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipid polymers reduce protein adsorption? Journal of Biomedical Materials Research. 1998;39:323–30. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 35.Kuiper KK, Nordrehaug JE. Early mobilization after protamine reversal of heparin following implantation of phosphorylcholine-coated stents in totally occluded coronary arteries. American Journal of Cardiology. 2000;85:698–702. doi: 10.1016/s0002-9149(99)00843-7. [DOI] [PubMed] [Google Scholar]
- 36.Lewis AL, Tolhurst LA, Stratford PW. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre-and post-implantation. Biomaterials. 2002;23:1697–706. doi: 10.1016/s0142-9612(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 37.Moro T, Kawaguchi H, Ishihara K, Kyomoto M, Karita T, Ito H. Wear resistance of artificial hip joints with poly( 2-methacryloyloxyethyl phosphorylcholine) grafted polyethylene: comparisons with the effect of polyethylene cross-linking and ceramic femoral heads. Biomaterials. 2009;30:2995–3001. doi: 10.1016/j.biomaterials.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–28. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HH. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41:345–55. doi: 10.1016/j.jdent.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dental Materials. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishihara K, Ziats NP, Tierney BP, Nakabayashi N, Anderson JM. Protein adsorption from human plasma is reduced on phospholipid polymers. Journal of Biomedical Materials Research. 1991;25:1397–407. doi: 10.1002/jbm.820251107. [DOI] [PubMed] [Google Scholar]
- 42.Sibarani J, Takai M, Ishihara K. Surface modification on microfluidic devices with 2-methacryloyloxyethyl phosphorylcholine polymers for reducing unfavorable protein adsorption. Colloids and Surfaces B: Biointerfaces. 2007;54:88–93. doi: 10.1016/j.colsurfb.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 43.McBain AJ. In vitro biofilm models: an overview. Advances in Applied Microbiology. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]
- 44.Cheng L, Exterkate RA, Zhou X, Li J, ten Cate JM. Effect of Galla chinensis on growth and metabolism of microcosm biofilms. Caries Research. 2011;45:87–92. doi: 10.1159/000324084. [DOI] [PubMed] [Google Scholar]
- 45.McBain AJ, Sissons C, Ledder RG, Sreenivasan PK, De Vizio W, Gilbert P. Development and characterization of a simple perfused oral microcosm. Journal of Applied Microbiology. 2005;98:624–34. doi: 10.1111/j.1365-2672.2004.02483.x. [DOI] [PubMed] [Google Scholar]
- 46.Lima JP, Sampaio de Melo MA, Borges FM, Teixeira AH, Steiner-Oliveira C, Nobre Dos Santos M, Rodrigues LK, Zanin IC. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. European Journal of Oral Sciences. 2009;117:568–74. doi: 10.1111/j.1600-0722.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 47.Lynch CD, Opdam NJ, Hickel R, Brunton PA, Gurgan S, Kakaboura A, Shearer AC, Vanherle G, Wilson NH. Guidance on posterior resin composites: Academy of Operative Dentistry - European Section. Journal of Dentistry. 2014;42:377–83. doi: 10.1016/j.jdent.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clinical Microbiology Reviews. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mashaghi S, Jadidi T, Koenderink G, Mashaghi A. Lipid Nanotechnology. International Journal of Molecular Sciences. 2013;14:4242–82. doi: 10.3390/ijms14024242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamasaki A, Imamura Y, Kurita K, Iwasaki Y, Nakabayashi N, Ishihara K. Surface mobility of polymers having phosphorylcholine groups connected with various bridging units and their protein adsorption-resistance properties. Colloids and Surfaces B: Biointerfaces. 2003;28:53–62. [Google Scholar]
- 51.Goda T, Konno T, Takai M, Ishihara K. Photoinduced phospholipid polymer grafting on Parylene film: Advanced lubrication and antibiofouling properties. Colloids and Surfaces B: Biointerfaces. 2007;54:67–73. doi: 10.1016/j.colsurfb.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. Permanent, non-leaching antibacterial surfaces-2: how high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28:4870–9. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 53.Li F, Weir MD, Fouad AF, Xu HH. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dental Materials. 2014;30:182–91. doi: 10.1016/j.dental.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imazato S, Ebi N, Takahashi Y, Kaneko T, Ebisu S, Russell RR. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials. 2003;24:3605–9. doi: 10.1016/s0142-9612(03)00217-5. [DOI] [PubMed] [Google Scholar]
- 55.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dental Materials. 2007;23:170–6. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 57.Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing antibacterial monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41:504–13. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. Journal of Dental Research. 1994;73:1437–43. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 59.Kyomoto M, Moro T, Miyaji F, Hashimoto M, Kawaguchi H, Takatori Y, Nakamura K, Ishihara K. Effects of mobility/immobility of surface modification by 2-methacryloyloxyethyl phosphorylcholine polymer on the durability of polyethylene for artificial joints. Journal of Biomedical Materials Research. Part A. 2009;90:362–71. doi: 10.1002/jbm.a.32092. [DOI] [PubMed] [Google Scholar]
- 60.Tateishi T, Kyomoto M, Kakinoki S, Yamaoka T, Ishihara K. Reduced platelets and bacteria adhesion on poly(ether ether ketone) by photoinduced and self-initiated graft polymerization of 2-methacryloyloxyethyl phosphorylcholine. Journal of Biomedical Materials Research. Part A. 2014;102:1342–9. doi: 10.1002/jbm.a.34809. [DOI] [PubMed] [Google Scholar]
- 61.Kyomoto M, Ishihara K. Self-initiated surface graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on poly(ether ether ketone) by photoirradiation. ACS Applied Materials & Interfaces. 2009;1:537–42. doi: 10.1021/am800260t. [DOI] [PubMed] [Google Scholar]