Abstract
Although maternal stress and depression have been linked to adverse birth outcomes (ABOs), few studies have investigated preventive interventions targeting maternal mental health as a means of reducing ABOs. This randomized controlled study examines the impact of Family Foundations (FF)—a transition to parenthood program for couples focused on promoting coparenting quality, with previously documented impact on maternal stress and depression—on ABOs. We also examine whether intervention buffers birth outcomes from the negative effect of elevated salivary cortisol levels. We use intent-to-treat analyses to assess the main effects of the FF intervention on ABOs (prematurity, birth weight, pregnancy complications, Cesarean section, and days in hospital for mothers and infants) among 148 expectant mothers. We also test the interaction of cortisol with intervention condition status in predicting ABOs. FF participation was associated with reduced risk of C-section (OR .357, p < 0.05, 95 % CI 0.149, 0.862), but did not have main effects on other ABOs. FF significantly buffered (p < 0.05) the negative impact of maternal cortisol on birth weight, gestational age, and days in hospital for infants; that is, among women with relatively higher levels of prenatal cortisol, the intervention reduced ABOs. These results demonstrate that a psycho-educational program for couples reduces incidence of ABOs among higher risk women. Future work should test whether reduced maternal stress and depression mediate these intervention effects.
Keywords: Prenatal cortisol, Birth weight, Prematurity, Gestational age, Caesarian section, Length of newborn hospital stay, Coparenting, Psychosocial intervention, Family Foundations
Introduction
The morbidity associated with adverse birth outcomes (ABOs)—including preterm birth, low birth weight, and neonatal health problems—is considerable both in the US (relative to other industrialized countries) and globally [1–4]. Children born preterm or at low birth weight are at increased risk for mortality, medical conditions such as diabetes and hypertension, poor developmental outcomes, and behavioral problems [5–8]. ABOs are associated with increased risk for long-term problems such as chronic health issues, low-educational attainment, and psychological disorders well into adulthood [8–10]. ABOs result in substantially increased health burdens and subsequent economic consequences. Health care costs double for births at 32–36 weeks and increase exponentially at earlier gestation lengths [2, 11].
Most approaches for improving birth outcomes focus on access to prenatal health care, maternal health-promoting behaviors, and avoidance of exposure of the fetus to harmful factors such as maternal smoking and drug use [12, 13]. However, current research links prenatal maternal stress and depression with ABOs, presenting a new malleable prevention target: pregnant women’s mental and emotional health [14–19]. The research linking mother’s prenatal depression and anxiety with ABOs is robust; however, this research has been largely observational and correlational in nature [18, 19]. Experimental evidence can provide greater confidence in causal relations between prenatal maternal mental health and birth outcomes.
Recent intervention studies found that reducing high-risk pregnant women’s stress and depression via yoga, massage therapy, and cognitive behavioral therapy reduces risk for low birth weight and preterm birth [20–23]. However, despite research indicating that partner support is a key influence on maternal mental health [24–27], no intervention studies have tested whether reducing maternal psychological distress via a focus on support in the interparental (couple) relationship could have similar effects. This is important as it might be possible to incorporate prevention material concerning relationship issues into existing universal programs, such as prenatal preparation and education. Moreover, reducing ABOs via strengthening support in a pregnant woman’s primary relationship may have collateral and long-term benefits. Coparenting relations are newly formed during the transition to parenthood, often amidst some degree of stress for each parent and the couple relationship [28]. Early dynamics in the new coparenting relationship influence long-term coparenting relationship patterns. Thus early positive intervention impact on coparenting may be carried forward as more positive long-term family relationship dynamics. Such persisting positive effects would provide ongoing benefits for each parent’s mental health, the relationship quality, and—as coparenting has been shown to impact parenting quality and child outcomes—the child’s emotional/behavioral problems and competencies [29–31]. In contrast, the benefits from yoga or massage therapy may be more circumscribed in both duration and generalization to other spheres of health and family functioning.
To our knowledge, no randomized trial has shown that a universal program providing psychosocial support and education, delivered prenatally, can prevent ABOs. In this report, we test the effect on ABOs of a group-format program designed to prepare couples to enter parenthood together in a supportive manner. Family Foundations (FF)—consisting of a series of classes for first-time, expectant couples—has been shown to reduce maternal stress and depression measured at 6 months after birth [29]. We reason that, as half of the eight FF classes took place before birth (the remaining classes took place 3–6 months after birth), the program’s impact on maternal stress and depression may have begun during pregnancy. Thus, we hypothesize that the program’s impact in reducing maternal negative emotional states reduces risk of ABOs.
We also assess evidence of risk moderation. In this case, we hypothesize that FF offers relatively greater beneficial impact for women at higher risk for ABOs due to elevated cortisol during pregnancy. The hypothalamic–pituitary–adrenal (HPA) axis is linked to stress, depression, and physical health problems [32]. Cortisol levels in saliva—an indicator of HPA activity–rise to a plateau during pregnancy; near the end of pregnancy [33, 34], cortisol levels rise and likely play a role in triggering labor [35]. Thus, women with higher levels of cortisol are more likely to enter labor at shorter gestational ages and have low birth weight babies [36–39].
We test the impact of FF as a protective factor, buffering the negative impact of high cortisol levels. Because we did not measure women’s stress and depression before childbirth (apart from the pre-intervention assessment), we are not able to test whether it is in fact the program’s impact on maternal psychological well-being that is responsible for detectable improvement in birth outcomes. However, evidence of buffering ABOs would be consistent with our expectation that the program’s impact on stress and depression began during the prenatal portion of the program. Findings of program impact in this study would support further development and testing of interventions that promote maternal mental health—especially interventions that enhance the supportiveness and overall quality of primary relationships, particularly the coparenting relationship.
Patients and Methods
Study Design
This randomized controlled study assessed couples at pretest when mothers gestational ages averaged 22.4 weeks (SD = 5.3), as well as at three periods after birth: 6 months, 1 years, and 3 years. At pretest, respondents were interviewed in their homes by trained research assistants, who also collected cortisol samples from the expectant parents. At posttest, parents separately mailed in questionnaires. Control group families were mailed literature on selecting quality childcare and developmental stages.
After pretest, couples were randomized to condition. The FF intervention consisted of nine classes, with four weekly classes conducted during the 2nd or 3rd trimester of pregnancy and four weekly classes conducted within the first 6 months post-partum. Classes focused on emotional self-management, conflict management, problem solving, communication, and mutual support strategies that foster positive joint parenting of an infant. Average attendance was 5.5 classes for mothers and 5.4 classes for fathers, with only 3 % of mothers and 5 % of fathers attending no sessions. Approximately 80 % of couples attended at least 3 of the prenatal classes, and 60 % of couples attended at least 3 postnatal classes. A male–female facilitator team led each class; the female was a childbirth educator in all cases, and males came from various backgrounds but were experienced working with families and leading groups. Respondent engagement and participation were assessed through participant feedback and report of homework completion, and facilitator’s ratings of participant engagement. Observer coding of videotaped classes indicated high levels of fidelity. Further details of the study are available elsewhere [29, 31]. The study was approved by the Penn State University Institutional Review Board.
Participants
The FF intervention was tested on a sample of 169 heterosexual couples residing in two cities in central Pennsylvania with surrounding rural areas, and predominately white populations. Both areas were comprised largely of working-class and middle-class populations. Eligibility requirements stipulated that couples were age 18 and above, living together, and expecting a first child at recruitment. These couples were primarily recruited through childbirth education programs, media advertisements, fliers, and word of mouth. The analytic sample consisted of 147 mothers (71 from control, and 76 from the intervention group) who completed interviews when children were 6 months old (wave 2), interviewed from 2004 to 2006. At pretest, mothers had a mean age of 28.4 years (SD = 4.9), mean education of 15.2 years (SD = 1.8), and mean household income of $68,900 (SD = $34,629). Compared to the local populations, the FF sample had higher education levels and income [40]. Of these respondents, 92.1 % were non-Hispanic white, and 85 % were married and 15 % cohabiting. We excluded five families (two intervention and three control) because of severe parent and infant non-birth related medical problems (e.g., severe congenital defect, death of mother prior to interview) or multiple births. Excluding these five families, participation rates among the 164 eligible respondents and 132 respondents with cortisol data are, respectively, 89.6 and 93.1 %. Although the sample size is modest, analyses would provide sufficient power (0.85) to detect a medium effect in terms of standardized group mean differences [41].
To assess randomization, we performed attrition analysis and baseline equivalence testing by intervention condition. Results showed baseline equivalence across a wide array of pretest variables including self-reported measures of physical health (hypertension, BMI, overall health), mental health (depression, anxiety), and alcohol, tobacco, and substance use [29]. We also did not find evidence of differential attrition at the 6-month follow-up.
Measures
During in-home interviews, respondents completed questionnaires that included basic demographic information on the mother, including age, education (highest grade complete), marital status (cohabitating versus married), health (ranging from 1 to 4, with ‘1’ being poor and ‘4’ being excellent), height and pre-pregnancy weight. To control for the influence on substance use on ABO risk, we utilize maternal reports for tobacco usage (frequency, prior 30 days), history of alcohol related problems (number of occurrences), and illicit drug usage (frequency, prior 5 years). Salivary cortisol levels of mothers were also taken at pretest. We note that no measures were collected on paternal outcomes at childbirth, so measures provided by the father were not available for this study.
At the 6-month follow-up, data on pregnancy and delivery outcomes were collected via surveys taken by the mother (the date birth outcomes were first available). These variables included child sex and birth weight, gestational age at delivery, delivery mode, post-birth mother and newborn length of hospital stay, and complications of pregnancy and delivery. Complications were reported by mothers in an open-ended question; responses were reviewed and categorized by the study’s pediatrician co-author (I.P.); complications included failure to progress (n = 2), fetal size or position (n = 6), miscellaneous maternal complications (n = 1), maternal complications related to labor/delivery (n = 4), fetal distress (n = 16). Delivery mode is an indicator for childbirth via Caesarian section (0 = vaginal birth, 1 = Cesarean birth). Mothers reported on number of days of infant and mother length of hospital stays after birth. In order to reduce influence of outliers, we truncated extreme scores identified by box plots. The number of cases and truncation values are as follows: 1 low gestational age: four cases to 31 weeks; 2 high newborn length of hospital stay: seven cases at 16 days; 3 high maternal length of hospital stay: one case at 6 days.
Salivary cortisol samples were collected during a home visit by a trained research assistant, during which expectant mothers and fathers completed separate surveys and were videotaped during couple support and conflict discussions. A subset of 123 mothers (61 from control, and 62 from intervention) had cortisol levels measured at pretest. In a comparison of means on key sample characteristics, mothers providing cortisol data did not significantly vary from the full sample at wave 2. Due to the typical circadian rhythm, cortisol levels vary throughout the day (declining generally from peak levels after awakening); we attempted to interview couples in the afternoon or early evening to limit variability based on time of day. As such, roughly 80 % of the sample were interviewed after 4 pm (mean 24-h time = 17.9, SD = 2.3 h).
During the course of the home visit at pretest, three salivary samples were obtained from participants and later assayed for cortisol. A baseline sample (t0) was collected shortly after the interviewer explained the procedures and obtained consent. Two subsequent samples were taken during the course of the interview to assess cortisol reactivity and recovery after exposure to stressful couple dynamics. We use the baseline measure in the current analyses given that it best represents the characteristic biological state of the mother in this period. More complete details on the three cortisol measures are available elsewhere [42].
Because cortisol levels may be affected by recent meals [43], subjects were asked to avoid eating 1 h before interviews; if the subject had reported eating during that period, the cortisol-related tasks were delayed. Within our sample, there was no correlation between time since last meal and mother’s cortisol levels, suggesting these procedures addressed this potential confound.
After collection, saliva samples were kept on ice, frozen within 8 h, and stored at −20 °C until transported to Salimetrics laboratories (State College, PA, USA) where they were stored at −80 °C until the day of assay (samples were processed within 2 weeks after arriving at the lab). At that time, samples were thawed and then centrifuged at 3,000 rpm for 15 min to remove mucins. Samples were assayed using a commercially available enzyme immunoassay for salivary cortisol without modification to the manufacturers recommended protocol (Salimetrics LLC). The test used 25 µL of saliva, had a lower limit of sensitivity of .007 µg/dL, range of sensitivity from 0.007 to 3.0 µg/dL, and average intra-and inter-assay coefficients of variation of < 5 and 10 %. All samples were tested in duplicate, and the average of the duplicate tests was used in the analyses. Cortisol units reported here are expressed as micrograms per deciliter (µg/dL).
Other variables that could influence birth outcomes were included as controls. These included child gender and preintervention maternal characteristics: marital status, years of education, age, pre-pregnancy BMI, self-rated health, and substance use. For the latter, we used self-report measures for illicit drug use (number of times within prior 5 years), tobacco use (number of times within prior 30 days), and lifetime history of alcohol problems (number of distinct problems).
As expected, mothers’ baseline cortisol levels were significantly predicted by gestation period and the time of day [37]. To remove the influence of these measurement artifacts, we generated residualized cortisol scores using regression models with these two predictors. These residualized scores are used in all statistical models. To reduce the impact of outliers, five values were truncated (i.e., set to threshold levels based on distributional box plots). Residualized scores were also multiplied by a factor of ten to generate comparable variation across variables. The residualized scores had a mean of zero and range between −1.4 and 2.1.
Analysis
We used regression models to examine (a) main effects of the intervention on ABO’s for the full sample; and (b) whether the intervention moderated associations between mother cortisol and pregnancy outcomes. In all models, we also tested for non-linear moderation using a quadratic term, reporting statistically significant models (p < 0.05). (We focused on quadratic associations after finding that higher-order interactions did not improve model fit.) We also tested, but do not report results for child-gender moderation of main and moderated effects; there were no significant child-gender moderation results. We note that the term moderation here is analogous to statistical interaction or effect modification; moderation refers to systematic differences in the effect of a predictor on an outcome across levels of the moderator variable.
Standard linear regression models were used to analyze child birth weight and mother’s gestation length; logistic regression models were used to analyze pregnancy complications and Cesarean section; negative binomial regression (addressing issues of skewed count data) was used to model the number of days spent in the hospital post birth. To address heteroskedasticity among predictor variables, Huber–White standard errors were estimated for all regression models.
Below, we report supplemental analyses examining if delivery mode mediates an association between condition status and experiencing pregnancy complications. This analysis uses standard mediation models in our analysis, as outlined by MacKinnon and Dwyer [44], to see if the reduction in C-sections may have indirectly resulted in few birth complications experienced by the mother.
Results
Descriptive statistics, variable constructs, and unit-ofmeasurement (where appropriate) are reported in Table 1 for birth outcomes, baseline cortisol levels, and control variables used in analysis. The descriptive statistics are presented by condition status, and show that mothers in the treatment and control group have similar age, socioeconomic, health, and alcohol/substance usage profiles.
Table 1.
Means for intervention and control conditions
| Control N = 71 |
Intervention N = 76 |
|||
|---|---|---|---|---|
| Mean | (SD)/% | Mean | (SD)/% | |
| Pre-intervention variables | ||||
| Marital status (0 = non-married; 1 = married) | 0.86 | (0.35) | 0.84 | (0.37) |
| Mother education (highest grade completed) | 15.18 | (1.92) | 14.96 | (1.77) |
| Maternal age, pretest (years) | 27.91 | (5.29) | 28.60 | (4.65) |
| Child sex (0 = female; 1 = male) | 0.58 | (0.50) | 0.56 | (0.50) |
| Mother’s pre-pregnancy body mass index (kg/m2) | 24.70 | (5.41) | 25.01 | (5.12) |
| Mother’s self-rated health (scale: 1 = poor; 4 = excellent) | 3.31 | (0.59) | 3.46 | (0.50) |
| Mother’s tobacco use (# of times, prior 30 days) | 0.50 | (2.02) | 0.45 | (2.06) |
| Mother’s drug use (# of times, prior 5 years) | 0.99 | (2.70) | 0.89 | (2.54) |
| Mother’s lifetime history of alcohol problems (# of problems) | 1.46 | (1.80) | 1.81 | (1.28) |
| Maternal cortisol measures at pre-intervention | ||||
| Raw cortisol (µg/dL) | 0.16 | (0.10) | 0.16 | (0.11) |
| Residualized cortisol (see text) | −0.02 | (0.72) | −0.07 | (0.70) |
| Time of day of cortisol collection (24 h) | 18.04 | (2.19) | 17.85 | (2.43) |
| Number of weeks gestation at collection | 22.75 | (5.31) | 22.22 | (5.46) |
| Pregnancy-related outcomes | ||||
| Birth weight (kg s) | 3.35 | (0.64) | 3.19 | (0.66) |
| Gestational age (weeks) | 39.04 | (2.06) | 39.07 | (2.75) |
| Cesarean section (1 = yes)a | 0.40 | 40 % | 0.21 | 21 % |
| Pregnancy complications (1 = one or more complications) | 0.36 | 36 % | 0.25 | 25 % |
| Maternity length of stay (days) | 3.36 | (2.50) | 3.11 | (2.09) |
| Newborn length of stay (days) | 2.89 | (1.17) | 2.67 | (1.04) |
Significant group difference based on t test (p < 0.05)
As Table 1 also indicates, the mean, standard deviation, and ranges for both raw and residualized cortisol are similar by condition status. The ranges for raw cortisol are [0.042, 0.669] for the treatment condition and [0.042, 0.679] for the control. Although reference ranges for salivary cortisol are not yet well-established in current research, our sample mean, standard deviation and range of raw cortisol values are comparable to recent work by Giesbrecht et al. [45] for salivary cortisol samples taken late in the day during the 2nd and 3rd trimester of pregnancy. Our raw cortisol values are also roughly comparable to those described in other studies [37, 46, 47]. T tests were conducted on all pre-test variables involved in analyses in order to assess potential baseline condition differences. We found no condition differences on model predictors from wave 1.
Results of tests of main intervention effects and statistical interactions of condition X maternal cortisol level are provided in Table 2 and are presented by birth outcome.
Table 2.
Regression coefficients (standard errors) for separate models testing main and interaction effects of intervention condition on birth outcomes
| Dependent Variable | Cond. | Cortisol | Cond. × Cort. | Cort.2 | Cond. × Cort.2 |
|---|---|---|---|---|---|
| Birth weight (kg) | |||||
| Model 1: Main effects | −0.15 (0.10) | ||||
| Model 2: With interactions | −0.26 (0.14) | −0.20 (0.13) | 0.23 (0.21) | −0.28 (0.08) | 0.32* (0.13) |
| Gestational Age (weeks) | |||||
| Model 1: Main effects | 0.13 (0.37) | ||||
| Model 2: With interactions | −0.26 (0.52) | −0.72 (0.54) | −0.60 (0.82) | −0.63+ (0.38) | 1.08* (0.46) |
| Cesarean section (logistic regression) | |||||
| Model 1: Main effects | −1.03* (0.45) | ||||
| Model 2: With interactions | −1.23* (0.50) | −0.23 (0.46) | 0.16 (0.68) | ||
| Pregnancy complications (logistic regression) | |||||
| Model 1: Main effects | −0.60 (0.44) | ||||
| Model 2: With interactions | −0.72 (0.49) | 0.39 (0.41) | 0.13 (0.60) | ||
| Newborn length of stay (negative binomial regression) | |||||
| Model 1: Main effects | −0.13 (0.14) | ||||
| Model 2: With interactions | 0.05 (0.16) | 0.22 (0.19) | −0.17 (0.26) | 0.21* (0.10) | −0.27* (0.12) |
| Maternal length of stay (negative binomial regression) | |||||
| Model 1: Main effects | −0.06 (0.16) | ||||
| Model2: With interactions | −0.05 (0.07) | 0.03 (0.10) | −0.04 (0.12) | 0.21* (0.10) | −0.27* (0.12) |
Cond. = intervention condition status; Cort. = mother’s residualized baseline cortisol levels; (Cort.)2 = Residualized cortisol-squared. Model 1 includes control variables and condition; Model 2 adds mother’s cortisol and term for the interaction of mother’s cortisol and condition status, and, if significant, quadratic cortisol term and its interaction with condition. To evaluate direction of results, we present raw coefficients for logistic and negative binomial regression
p < 0.10;
p < 0.05;
p < 0.01;
p < 0.001
Birth Weight
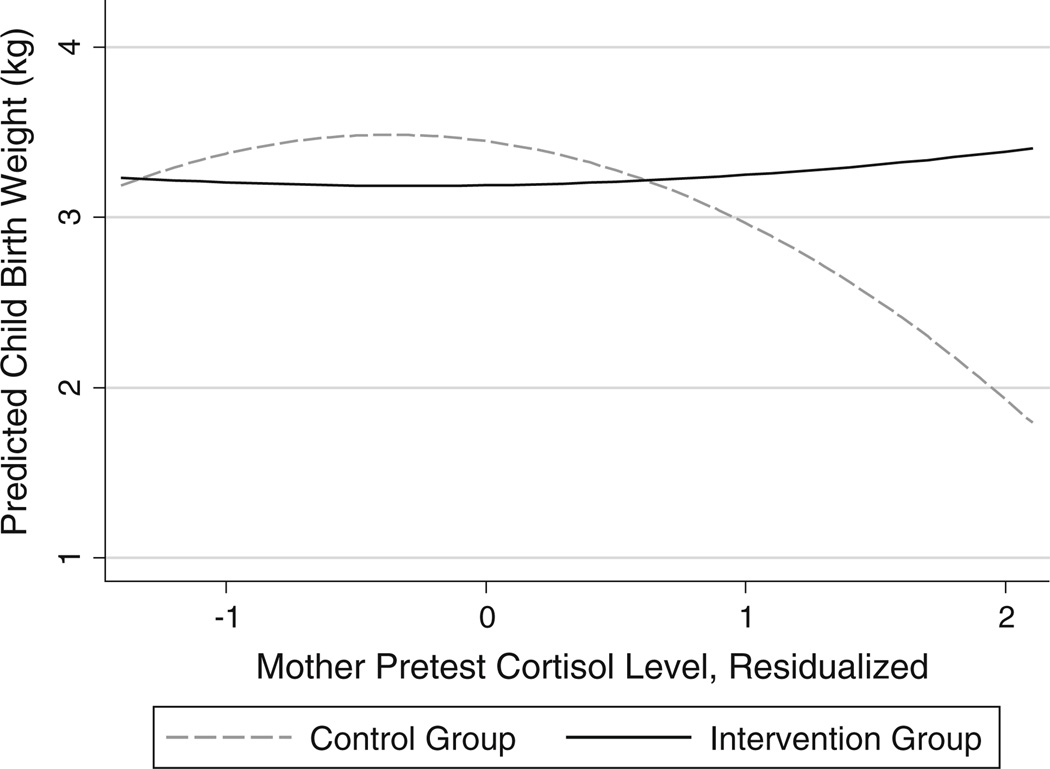
For models of infant birth weight, we found a significant non-linear moderating effect between cortisol and intervention condition (p < 0.05). As shown in Fig. 1, birth weight was relatively stable among children born to intervention group mothers across all levels of cortisol; in contrast, birth weight was negatively associated with cortisol for control mothers with moderate to high levels of cortisol. To assess whether the effect on birth weight might be attributable to premature birth (see below), we ran an additional model including a control for the number of weeks the child was born premature. Results showed that cortisol moderation was non-significant when controlling for weeks of prematurity.
Fig. 1.
Interaction of intervention condition and maternal pretest cortisol predicting child birth weight
Gestational Age at Birth
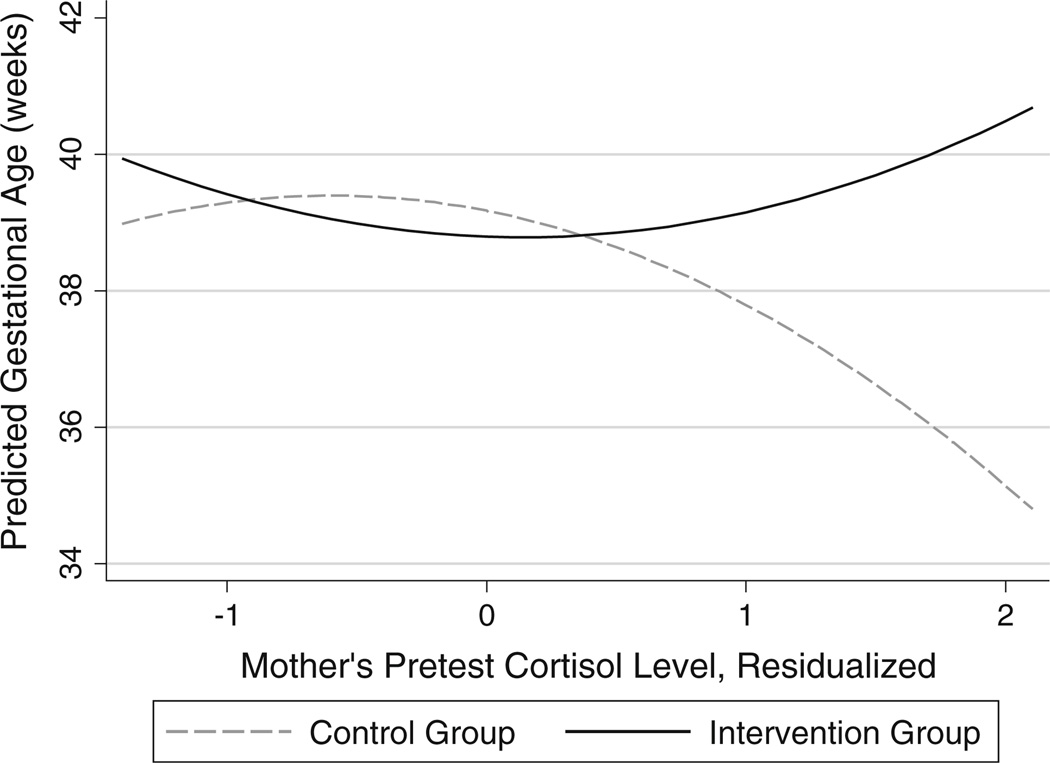
The model for gestational age also indicated a significant non-linear cortisol × condition effect (p < 0.05). As shown in Fig. 2 participants in the intervention group maintained a relatively stable gestation length regardless of cortisol level. In contrast, gestation length among control group mothers declined substantially to 35 weeks among those with high cortisol levels.
Fig. 2.
Interaction of intervention condition and maternal pretest cortisol predicting gestational age (weeks)
Delivery Mode
We found a main effect of the intervention condition indicating a lower likelihood for C-section (OR = 0.357, p < 0.05), which was not moderated by maternal cortisol level.
Pregnancy Complications
Although the percentage of those indicating pregnancy complications was lower for the intervention group (Table 1), we found no significant main or moderating effects for this outcome in statistical models.
We noted that pregnancy complications and delivery mode were significantly associated (r = 0.34). However, in supplemental analyses, we found that the relation between condition status and delivery mode (reported above) was not mediated by pregnancy complications.
Newborn Length of Stay
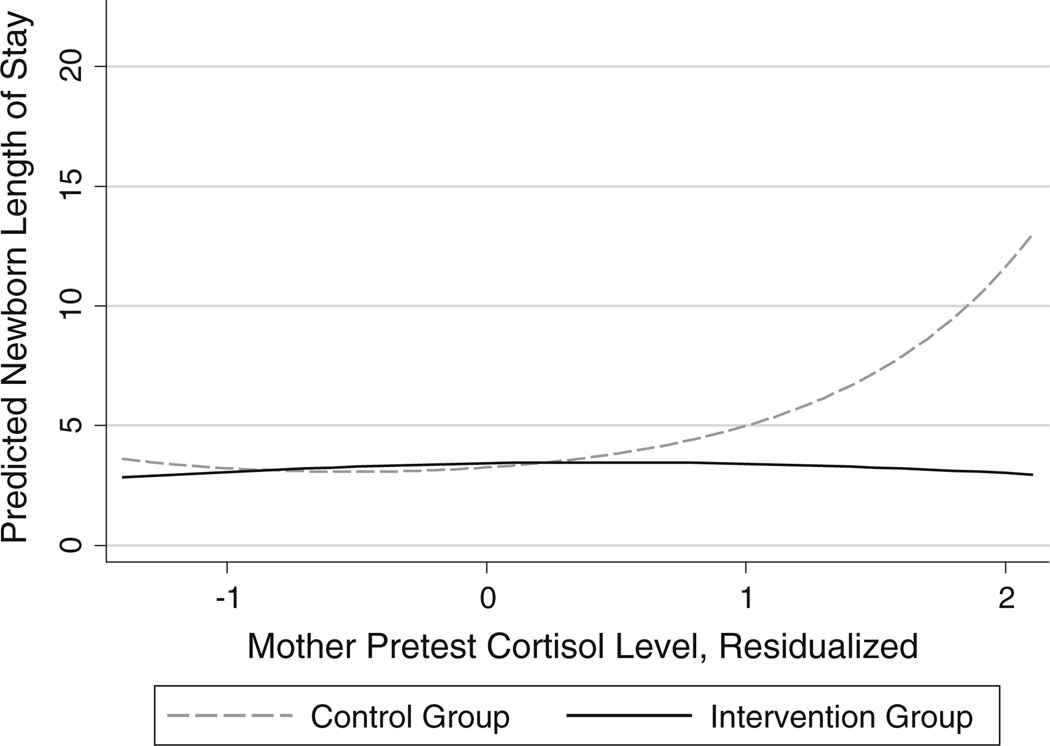
The results for newborn length of stay also revealed a significant non-linear moderated cortisol × intervention condition effect (p < 0.05). A plot of this interaction (Fig. 3) shows the number of days in hospital remained relatively constant across maternal cortisol levels for intervention group participants. In contrast, among the control group, the newborn length of stay increased from ~3 days at low levels of prenatal maternal cortisol to ~14 days at high cortisol levels.
Fig. 3.
Interaction of intervention condition an maternal pretest cortisol predicting newborn length of stay
Discussion
We found evidence in intent-to-treat analyses that assignment to the FF program was associated with reduced frequency of C-sections for the whole sample, and reduced several ABOs for women with relatively higher levels of cortisol. Given that we only found a main effect of the program for one of six outcomes, this evidence is not conclusive. Follow-up analyses (not described) indicated that the impact on C-sections was a result of reduced birth complications arising from fetal position (e.g., breech birth) and labor progression. Further research is in order with a larger sample to assess whether in fact the program is effective in reducing the rate of C-sections and, if so, what are the underlying causal mechanisms.
We found more consistent evidence that the program has a positive impact on ABOs for women with relatively high levels of cortisol during pregnancy. High cortisol levels, reflecting a high level of HPA-axis activation, may represent a physiological response to acute stress that increases risk for prematurity and low birth weight. Exposure to the FF program appears to have had a positive impact on birth outcomes among women demonstrating elevated cortisol. Unfortunately, we did not collect data on changes in maternal depression and stress until 6 months postpartum, and thus we do not know the extent to which reductions in maternal stress and depression occurred during the prenatal period as a result of the prenatal portion of the curriculum. It is reasonable to hypothesize that program effects on stress and depression were evident during pregnancy as higher attendance was obtained for the prenatal versus postnatal portion of the curriculum [29]. Thus, we propose that the program’s documented effect in reducing postpartum maternal stress and depression occurred during pregnancy and was responsible for improvements in birth outcomes. However, future work should measure maternal mental health during pregnancy and thus facilitate meditational analyses to document the pathway of program influence on birth outcomes.
Prior work has shown that yoga, massage, and cognitive-behavioral therapy also reduce risk for preterm birth and low birth weight [20–23]. FF, in contrast, targets couples rather than only mothers. Implementation of a couples-oriented program is more difficult logistically than intervening only with the mother. However, research shows that support from a partner is the strongest predictor of maternal depression—apart from a prior history of depression. [24, 26, 27, 48]. Research on “fragile families”—families consisting of unmarried, lower income, and frequently young parents—shows that even among high-risk and economically vulnerable populations, positive mother-father relations are crucial for maternal well-being and positive parenting, father involvement, and child competence and adjustment [49–51]. By focusing on enhancing the coparenting relationship, FF has demonstrated an impact on a range of important child and family outcomes, including enhanced couple relations, parent well-being, parenting quality, and child emotional/behavioral problems through at least age 6.
Practitioners and policy makers may be concerned about the feasibility of deploying couple-focused programs, especially for high-risk families where biological fathers may be difficult to engage. However, research on high-risk families indicates that most fathers and mothers are committed to one another and their child at time of birth [52, 53]. Fathers also do in fact have substantial contact with children, especially in the months after birth. For example, among families in the database of a regional home visiting database for Every Child Succeeds, located in the Cincinnati region, 86 % of fathers in high-risk families see their 6-month-old children at least weekly and 67 % see them every day. However, over time, parenting stress, role overload, and coparenting conflict play a substantial role in relationship breakup and disengagement of fathers from children [54]. Couples-oriented prevention may enhance father’s long-term engagement in parenting and support of the mother—even if the romantic relationship dissolves.
Among the strengths of this study is that we utilized intent-to-treat analyses. Thus, the impact of the program reported here is for all couples assigned to the program condition, regardless of attendance. However, some limitations of this study should be noted. The data come from samples taken in central Pennsylvania mid-sized cities, so results may not generalize to certain areas of the country. Recruitment of participants relied on their consent to attend classes, although this represents how program participation would occur in dissemination. We note, however, that randomization to intervention groups was executed properly and thus tests of group differences are valid. Differential attrition was not found to be an issue for outcomes measured within 6 months of the pre-natal classes. In analyses, cortisol was represented by one measure taken during a pre-test home visit. It is possible that a more involved assessment of cortisol (e.g., based on multiple samples) would provide a more reliable measure. We do not currently have reference ranges that would indicate salivary cortisol levels that would be considered elevated or high-risk in an absolute sense; this is an important area for future study. Thus, cortisol elevation in this study is based on internal, relative comparisons within the sample.
Future research could help us further understand how a program such as FF leads to improved birth outcomes, especially if more data are available to assess maternal physical and mental health status (e.g., stress, anxiety, depression symptoms) at several times during pregnancy. Further, examining the biological pathways by which maternal stress affects fetal development and birth outcomes is an important direction for future research, as is the development of refined models of what types of stressors in interaction with what internal coping and external supports are detrimental. Although study findings require replication and extension, results here suggest that implementing a couples-focused prevention strategy holds promise for improving birth outcomes among higher risk mothers.
Acknowledgments
This study was funded by grants from the National Institute of Child Health and Development (K23 HD042575) and the National Institute of Mental Health (R21 MH064125-01), Mark E. Feinberg, principal investigator.
Contributor Information
Mark E. Feinberg, Email: mef11@psu.edu, Prevention Research Center, Penn State University, 314 Biobehavioral Health, University Park, PA 16802, USA.
Michael E. Roettger, Prevention Research Center, Penn State University, 314 Biobehavioral Health, University Park, PA 16802, USA
Damon E. Jones, Prevention Research Center, Penn State University, 314 Biobehavioral Health, University Park, PA 16802, USA
Ian M. Paul, Department of Pediatrics, Penn State College of Medicine, Hershey, PA, USA
Marni L. Kan, RTI International, Research Triangle Park, NC, USA
References
- 1.Blencowe H, Cousens S, Oestergaard M, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Clements K, Barfield W, Ayadi M, et al. Preterm birth-associated cost of early intervention services: An analysis by gestational age. Pediatrics. 2007;119(4):866–874. doi: 10.1542/peds.2006-1729. [DOI] [PubMed] [Google Scholar]
- 3.Lawn J, Gravett M, Nunes T, et al. Global report on preterm birth and stillbirth (1 of 7): Definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10(Supp 1):S1. doi: 10.1186/1471-2393-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St John E, Nelson K, Cliver S, et al. Cost of neonatal care according to gestational age at birth and survival status. American Journal of Obstetrics and Gynecology. 2000;182(pt1):170–175. doi: 10.1016/s0002-9378(00)70509-6. [DOI] [PubMed] [Google Scholar]
- 5.Bhutta AT, Cleves MA, Casey PH, et al. Cognitive and behavioral outcomes of school-aged children who were born preterm. JAMA. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 6.Curhan GC, Willett WC, Rimm EB, et al. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 7.Hack M, Klein NK, Taylor HG. Long-term developmental outcomes of low birth weight infants. The Future of Children. 1995;5:176–196. [PubMed] [Google Scholar]
- 8.Hack M, Flannery DJ, Schluchter M, et al. Outcomes in young adulthood for very-low-birth-weight infants. New England Journal of Medicine. 2002;346(3):149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 9.Conley D, Strully KW, Bennett NG. The starting gate: Birth weight and life chances. Oakland, CA: University of California Press; 2003. [Google Scholar]
- 10.Nosarti C, Reichenberg A, Murray RM, et al. Preterm birth and psychiatric disorders in young adult life preterm birth and psychiatric disorders. Archives of General Psychiatry. 2012;69(6):610–617. doi: 10.1001/archgenpsychiatry.2011.1374. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert W, Nesbitt T, Danielsen B. The cost of prematurity: Quantification by gestational age and birth weight. Obstet and Gynecol. 2003;102(3):488–492. doi: 10.1016/s0029-7844(03)00617-3. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong M, Gonzales OV, Lieberman L, et al. Perinatal substance abuse intervention in obstetric clinics decreases adverse neonatal outcomes. Journal of Perinatology. 2003;1:3–9. doi: 10.1038/sj.jp.7210847. [DOI] [PubMed] [Google Scholar]
- 13.Barros FC, Bhutta ZA, Batra M, et al. Global report on preterm birth and stillbirth (3 of 7): Evidence for effectiveness of interventions. BMC Pregnancy and Childbirth. 2010;10(1):S3. doi: 10.1186/1471-2393-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buss C, Davis E, Shahbaba B, et al. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy Science. 2012;109(20):E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buss C, Entringer S, Swanson J, et al. The role of stress in brain development: The gestational environment’s long-term effects on the brain. Cerebrum. 2012 http://dana.org/Cerebrum/2012/The_Role_of_Stress_in_Brain_Development__The_Gestational_Environment%E2%80%99s_Long-Term_Effects_on_the_Brain/ [PMC free article] [PubMed] [Google Scholar]
- 16.Beijers R, Jansen J, Riksen-Walraven M, et al. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126(2):e401–e409. doi: 10.1542/peds.2009-3226. [DOI] [PubMed] [Google Scholar]
- 17.Conde A, Figueiredo B, Tendais I, et al. Mother’s anxiety and depression and associated risk factors during early pregnancy: Effects on fetal growth and activity at 20–22 weeks of gestation. Journal of Psychosomatic Obstetrics and Gynecology. 2010;31(2):70–82. doi: 10.3109/01674821003681464. [DOI] [PubMed] [Google Scholar]
- 18.Grote N, Bridge J, Gavin A, et al. A Meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Archives of General Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schetter CD, Tanner L. Anxiety, depression and stress in pregnancy: Implications for mothers, children, research, and practice. Current Opinion in Psychiatry. 2012;25(2):141–148. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Mohandes AA, Kiely M, Gantz MG, et al. Very preterm birth is reduced in women receiving an integrated behavioral intervention: A randomized controlled trial. Maternal and Child Health Journal. 2011;15(1):19–28. doi: 10.1007/s10995-009-0557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field T, Diego M, Hernandez-Reif M. Prenatal depression effects and interventions: A review. Infant Behavior and Development. 2010;33(4):409–418. doi: 10.1016/j.infbeh.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Field T, Diego M, Hernandez-Reif M, et al. Yoga and massage therapy reduce prenatal depression and prematurity. Journal of Bodywork and Movement Therapies. 2012;16(2):204–209. doi: 10.1016/j.jbmt.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narendran S, Nagarathna R, Narendran V, et al. Efficacy of yoga on pregnancy outcome. Journal of Alternative and Complementary Medicine. 2005;11(2):237–244. doi: 10.1089/acm.2005.11.237. [DOI] [PubMed] [Google Scholar]
- 24.Crnic K, Greenberg MT. Maternal stress, social support, and coping: Influences on the early mother-infant relationship. In: Boukydis CFZ, editor. Research on support for parents and infants in the postnatal period. Norwood, NJ: Ablex; 1987. pp. 25–40. [Google Scholar]
- 25.Dunn J. Relations among relationships. New York: Wiley; 1988. [Google Scholar]
- 26.Gottlieb NH, Brink SG, Gingiss PL. Correlates of coalition effectiveness: The smoke free class of 2000 program. Health Education Research. 1993;8(3):375–384. doi: 10.1093/her/8.3.375. [DOI] [PubMed] [Google Scholar]
- 27.Harmon DK, Perry AR. Fathers’ unaccounted contributions: Paternal involvement and maternal stress. Families in Society. 2011;92(2):176–182. [Google Scholar]
- 28.Feinberg ME. Coparenting and the transition to parenthood: A framework for prevention. Clinical Child and Family Psychology Review. 2002;5:173–195. doi: 10.1023/a:1019695015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feinberg ME, Kan ML. Establishing Family Foundations: Intervention effects on coparenting, parent/infant well-being, and parent-child relations. Journal of Family Psychology. 2008;22(2):253–263. doi: 10.1037/0893-3200.22.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feinberg ME, Kan ML, Goslin M. Enhancing coparenting, parenting, and child self-regulation: Effects of Family Foundations 1 year after birth. Prevention Science. 2009;10:276–285. doi: 10.1007/s11121-009-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinberg ME, Jones DE, Kan ML, et al. Effects of Family Foundations on parents and children: 3.5 years after baseline. Journal of Family Psychology. 2010;24(5):532–542. doi: 10.1037/a0020837. [DOI] [PubMed] [Google Scholar]
- 32.Hellhammer DH, Hellhammer J. Stress: The brainbody connection. Basel: Kerger; 2008. [Google Scholar]
- 33.Hillhouse EW, Grammatopoulos DK. Role of stress peptides during human pregnancy and labour. Reproduction. 2002;124:323–329. doi: 10.1530/rep.0.1240323. [DOI] [PubMed] [Google Scholar]
- 34.Wadhwa P, Entringer S, Buss C, et al. The contribution of maternal stress to preterm birth: Issues and considerations. Clinics in Perinatology. 2011;38(3):351–384. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snegovskikh V, Park JS, Norwitz ER. Endocrinology of parturition. Endocrinology and Metabolism Clinics of North America. 2006;35(1):173–192. doi: 10.1016/j.ecl.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Baibazarova E, van de Beek C, Cohen-Kettenis P, et al. Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychoneuroendocrinology. 2013;38(6):907–915. doi: 10.1016/j.psyneuen.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Entringer S, Buss C, Shirtcliff E, et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010;3:258–268. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hobel C, Dunkel-Schetter C, Roesch S, et al. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. American Journal of Obstetrics and Gynecology. 1999;180(1 pt 3):S257–S263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 39.Wadhwa P. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 40.United States Census Bureau. State and metropolitan area data book, 2009. Washington, DC: United States Government Printing Office; 2009. [Google Scholar]
- 41.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. London: Lawrence Erlbaum; 1988. [Google Scholar]
- 42.Feinberg ME, Jones DE, Bontempo D, et al. Relation of intimate partner violence to salivary cortisol among couples expecting a first child. Aggressive Behavior. 2011;37:1–11. doi: 10.1002/ab.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibson EL, Checkley S, Papadopoulos A, et al. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosomatic Medicine. 1999;61:214–224. doi: 10.1097/00006842-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 44.MacKinnon D, Dwyer J. Estimating mediated effects in prevention studies. Evaluation Review. 1993;17:144–158. [Google Scholar]
- 45.Giesbrecht G, Campbell T, Letourneau N, et al. Advancing gestation does not attenuate biobehavioural coherence between psychological distress and cortisol. Biological Psychology. 2013;93(1):45–51. doi: 10.1016/j.biopsycho.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 46.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81(1):131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis E, Sandman C. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37(8):1224–1233. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn J. Relations among relationships. In: Duck SW, editor. Handbook of personal relationships. England: John Wiley & Sons; 1988. pp. 193–209. [Google Scholar]
- 49.Choi JK, Jackson AP. Fathers’ involvement and child behavior problems in poor African American single-mother families. Children and youth services review. 2010;33(5):698–704. [Google Scholar]
- 50.Jia R, Schoppe-Sullivan SJ. Relations between coparenting and father involvement in families with preschoolage children. Developmental Psychology. 2011;47(1):106–118. doi: 10.1037/a0020802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gee CB, McNerney CM, Reiter MJ, et al. Adolescent and young adult mothers’ relationship quality during the transition to parenthood: Associations with father involvement in fragile families. Journal of Youth and Adolescence. 2007;36(2):213–224. [Google Scholar]
- 52.Cabrera N, Ryan RM, Mitchell SJ, et al. Lowincome, nonresident father involvement with their toddlers: Variation by father’s race and ethnicity. Journal of Family Psychology. 2008;22(4):647–649. doi: 10.1037/0893-3200.22.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garfinkel I, McLanahan S. Unwed parents in the US: Myths, realities, and policy making. Social Policy and Society. 2003;2(02):143–150. [Google Scholar]
- 54.Ryan RM, Tolani N, Brooks-Gunn J. Relationship trajectories, parenting stress, and unwed mothers’ transition to a new baby. Parenting Science and Practice. 2009;9(1–2):160–177. doi: 10.1080/15295190802656844. [DOI] [PMC free article] [PubMed] [Google Scholar]