Abstract
Background
Aging is characterized by a chronic low-grade inflammation that has been found to be related to mortality risk in older persons.
Objectives
The aim of the present study was to investigate whether interleukin-6 (IL-6), C-reactive protein (CRP) and Tumor Necrosis Factor-alpha (TNF-α) protein levels predict all-cause mortality in a sample of older persons living in the community.
Design and Setting
Data are from the Aging and Longevity Study in the Sirente Geographic Area (ilSIRENTE Study), a prospective cohort study that collected information on individuals aged 80 years and older living in an Italian mountain community (n=362). The main outcome was the hazard ratio of death after four years of follow-up.
Participants and measurements
Participants were classified according to the median value of the 3 inflammatory markers (IL-6: 2.08 pg/mL; TNF-α: 1.43 pg/mL and CRP: 3.08 mg/L). In addition, a composite summary score of inflammation was created.
Results
A total of 150 deaths occurred during a 4-year follow-up. In the unadjusted model, high levels of each of the 3 markers were associated with increased mortality. After adjusting for potential confounders, high levels of IL-6 and CRP were associated with a significantly increased risk of death (HR, 2.18; 95% CI 1.29–3.69 and 2.58; 95% CI 1.52–4.40, respectively); whereas the association between TNF-α protein levels and mortality lost significance (1.26; 95% CI: 0.74 to 2.15). The composite summary score of inflammation was strongly associated with mortality, with the highest risk estimated for individuals with all three inflammatory markers above the median.
Conclusions
Low levels of inflammatory markers are associated with better survival in elderly, independently of age and other clinical and functional variables.
Keywords: Interleukin-6, C-Reactive Protein, TNF-alpha, Mortality, Frail Elderly
INTRODUCTION
Aging is characterized by a chronic low-grade inflammatory status which is involved in the pathogenesis and course of several age-related disorders such as Alzheimer’s disease , Parkinson’s disease, atherosclerosis, type 2 diabetes , sarcopenia, osteoporosis, cognitive decline and frailty (1, 2, 3). This peculiar inflammatory activity, leading to long-term tissue damage, is detrimental for longevity and has been found to be related to all-cause mortality (4, 5, 6).
IL-6 is the major factor involved in the acute phase response, inducing the synthesis of CRP, as well as other acute phase reactants (7). Given the role of IL-6 in CRP regulation, the combined use of IL-6 and CRP levels as indicators of inflammation may provide a better prediction of risk associated with inflammation than the use of either indicator alone. TNF-α determines strength, effectiveness and duration of inflammatory reactions by opposing IL-10 role (8). Interindividual differences in TNF-α regulation may be critical with respect to the final outcome of an inflammatory response. Moreover, plasma levels of TNF-α are reported to be linearly related with IL-6 and CRP in centenarians, indicating an interrelated activation of the entire inflammatory cascade in the oldest old (9). In previous studies, IL-6 and CRP have been found to predict cardiovascular disease and mortality(10, 11, 12), all-cause mortality (4, 5, 6), disability (13) and loss of muscle strength (14). TNF-alpha resulted as an independent prognostic marker of mortality in persons aged 100 years(15).
The present study focuses on the prognostic role of three inflammatory markers, interleukin-6 (IL-6), C-reactive protein (CRP) and tumor necrosis factor- alpha (TNF-α) in long-lived people and it is aimed at investigating whether IL-6, CRP and TNF-α protein levels predict all-cause mortality in a sample of frail octogenarians and nonagenarians living in community, enrolled in the “Invecchiamento e Longevità nel Sirente” (Aging and longevity in the Sirente geographic area, ilSIRENTE Study) study. We hypothesized that IL-6, CRP and TNF-α protein levels may be associated with mortality and that the combined elevation of these three markers would be associated with the greatest and most consistent increment in mortality.
METHODS
The ilSIRENTE study is a prospective cohort study performed in the mountain community living in the Sirente geographic area (L’Aquila, Abruzzo) in the Central Italy. This study was designed by the Department of Gerontology, Geriatrics, and Physiatric Medicine of the Catholic University of Sacred Heart (Rome, Italy) and developed by the teaching nursing home, Opera Santa Maria della Pace (Fontecchio, L’Aquila, Italy) in a partnership with local administrators and primary care physicians of Sirente Mountain Community Municipalities. The Catholic University of Sacred Heart ethical committee ratified the entire study protocol. All the participants signed an informed consent at the baseline visit. Details of the ilSIRENTE study protocol are described in details elsewhere (16).
Study population
A preliminary list of all persons living in this well defined area was obtained at the end of October 2003 from the Registry Offices of the 13 municipalities involved in the study. Everyone living in the area has to register at birth or after relocation to receive primary health care. This mandatory registration ensured a complete coverage of the population living in the area, including persons in nursing homes. From this preliminary list, potential study participants were identified by selecting all persons living in the Sirente area who were born before January 1st, 1924. Of the initial 514 individuals screened, 32 men and 53 women died or moved away from the area before the baseline assessment. Among those eligible (n=429), the prevalence of refusal was very low (16%), without significant differences across age or gender groups. As a result, the overall sample population enrolled in the ilSIRENTE study consisted of 364 individuals. Two patients were excluded because the blood sample for the haematological assessment was insufficient. Finally, the study sample included 362 individuals.
Data collection
Baseline participants’ assessment began in December 2003 and was completed in September 2004. Assessors were trained on how to perform each component of the ilSIRENTE study protocol (16). The Minimum Data Set for Home Care (MDS-HC) form was administered to all study participants following the guidelines published in the MDS-HC manual (17, 18). The MDS-HC contains over 350 data elements including socio-demographics, physical and cognitive status variables, as well as major clinical diagnoses (19). Moreover, the MDS-HC includes information about an extensive array of signs, symptoms, syndromes, and treatments (17, 18). The MDS items have shown an excellent inter-rater and test-retest reliability when completed by nurses performing usual assessment duties (average weighted Kappa = 0.8) (18, 20). Additional information about family history, lifestyle, physical activity and behavioral factors were collected using specific questionnaires shared with the “Invecchiare in Chianti Study”(21).
Blood measurements
Venous blood samples were drawn in the morning after an overnight fast. The samples were immediately centrifuged and stored at −80 C until final analysis. Standard determinations of serum albumin, cholesterol (total and HDL), and triglycerides were performed by commercially available kits (Olympus, Italy) suitable on Olympus 2700 instrumentation.
IL-6, PCR and TNF-α measurements
Plasma interleukin-6 levels and Tumor Necrosis Factor -α were measures by enzyme-linked immunosorbent assay (ELISA; High Sensitivity Quantikine KitR&D Systems, Minneapolis, MN). Values were measured in duplicate and the average value was reported for all the assays. C-reactive protein was performed by commercially available kits (Olympus, Italy) suitable on Olympus 2700 instrumentation.
Covariates
Basic Activities of Daily Living were assessed by the assessor using the MDS-HC instrument (17, 18). The ADL scale is based on seven levels of self-performance including dressing, eating, toilet use, bathing, mobility in bed, locomotion, transfer. The IADL scale is based on eight domains of function including ability to use phone, shopping, food preparation, house keeping, laundering, mode of transportation, responsibility for own medications, ability to handle finances. Cognitive performance was assessed using a six-items, seven-category scale (Cognitive Performance Scale - CPS). The CPS was scored on a 7-point ordinal scale in which higher scores were associated with worse cognitive performance. The CPS considers two cognitive items (short-term memory, skills for daily decision making), one item describing communication ability (understood by others), one ADL measure (self performance in eating), and whether in comatose status.
Medical diagnoses and drugs were directly collected by general practitioners. Medical diagnoses were defined as conditions that have a relationship to the patient’s functional, cognitive, and behavioral status, medical treatment, and risk of death. The diagnoses were listed on the MDS-HC form in a check-box section containing 27 specific diagnostic categories. General practitioner collected information on up to 18 different drugs received by each patient in the 7 days preceding the assessment. Body weight was measured with light clothes using a calibrated bathroom scale. Body height was measured using a standard stadiometer. Body mass index (BMI) was defined as weight (kilograms) divided by the square of height (meters). Alcohol consumption was assessed asking the participants about the number of glasses of wine drunk during a standard day. Alcohol abuse was defined as a consumption of more than half of liter of wine per day.
Survival status
Vital status was obtained from general practitioners and confirmed by the National Death Registry. Time to death was calculated from the date of baseline assessment to the date of death. All individuals were followed-up for 48 months.
Statistical analysis
For the present study, we excluded 2 participants from the initial sample of 364 participants, because the three markers were not determined. This selection resulted in a final sample size of 362 participants. Differences in proportions and means of covariates between deceased individuals and survivors were assessed using Mann-Whitney U test and t test statistics, respectively. A p <0.05 level was chosen for statistical significance. To test the hypothesis that joint elevation of IL-6, CRP and TNF-α protein levels would confer the greatest risk of death, participants were classified according to the median value of the 3 inflammatory markers (IL-6: 2.08 pg/mL; TNF-α: 1.43 pg/mL and CRP: 3.08 mg/L). Composite summary indicators of inflammation have been adopted in previous longitudinal studies investigating the effect of inflammation on mortality (10, 22). According to such methodology, we constructed a composite summary score of inflammation. In this variable, the first category consists of (i) participants whose values were below the median in all of the three markers. The other categories – (ii) above the median in any one of the three markers, (iii) above the median in any two of the three markers, (iv) above the median in all of the three markers – were each contrasted to category (i). The three inflammatory markers showed an independent effect on mortality when sequentially included in Cox proportional models adjusted for age and gender.
Cox proportional hazards models were used to estimate crude and adjusted hazard ratios and 95% confidence intervals of death by IL-6, PCR and TNF-α levels. Time to death was calculated from the date of baseline assessment to the date of death. We examined all events which occurred during the 4 year follow up. Analyses were adjusted for age, gender, living alone, functional impairment (ADL scale score), cognitive impairment (CPS scale score), number of diseases, hearing impairment, albumin levels, total cholesterol levels, BMI.
In the adjusted model, age was treated as a continuous variable. The impact of IL-6, CRP and TNF-α levels on survival was also tested comparing the survival curves obtained with the Kaplan-Meier method. Moreover, to test the hypothesis that a joint elevation of IL-6, CRP and TNF-α protein levels would confer the greatest risk of mortality, we evaluated the impact of the composite summary indicator of inflammation on survival. Differences between curves were evaluated using the log-rank test. Statistical analysis was performed using the SPSS 10.0 package (Chicago, Illinois).
RESULTS
The sample consisted of 362 individuals, 244 (67%) were women and mean age was 85.8 ± 4.8 years. Characteristics of the study population according to the vital status are summarized in Table 1. Individuals showing functional and cognitive impairment had a higher risk to die during 4-years follow-up relative to those functionally and cognitively not impaired. Similarly, comorbidity, and low body mass index were more frequently reported for deceased individuals.. In particular, hypertension, congestive heart failure, diabetes and cardiovascular diseases were more prevalent among the participants who died furing the follow-up relative to those who survived.
Table 1.
Characteristics of Study Population according to Death (4-years Follow-up).
| Total sample (n=362) | |||
|---|---|---|---|
| Characteristic | Deceased (n=150) n (%) |
Survivors (n=212) n (%) |
p |
| Age, years (mean ± SD) | 87.5 ± 5.1 | 84.6 ± 4.2 | <0.001 |
| Female | 95 (63) | 149 (70) | 0.2 |
| Marital status | |||
| Married | 34 (23) | 26 (25) | |
| Widowed | 98 (65) | 127 (59) | 0.1 |
| Never married | 18 (12) | 20 (10) | |
| Living alone | 29 (20) | 74 (36) | <0.001 |
| Education, years (mean ± SD) | 4.9 ± 1.1 | 5.2 ± 1.8 | 0.09 |
| Activity of Daily Living Scale score ^ (mean ± SD) | 2.6 ± 2.8 | 0.5 ± 1.6 | <0.001 |
| Instrumental Activity of Daily Living Scale score ∞(mean ± SD) | 4.4 ± 2.5 | 2.0 ± 2.1 | <0.001 |
| Cognitive Performance Scale score + (mean ± SD) | 1.4 ± 1.8 | 0.5 ± 1.1 | <0.001 |
| Depression | 41 (27) | 51 (24) | 0.4 |
| Number of diseases (mean ± SD) | 2.4 ± 1.3 | 2.0 ± 1.2 | 0.007 |
| Specific Disease | |||
| Hypertension | 99 (66) | 164(76) | 0.01 |
| Ischemic Heart Disease | 22 (15) | 22 (10) | 0.1 |
| Congestive Heart Failure | 20 (14) | 2 (1) | <0.001 |
| Cancer | 9 (6) | 8 (4) | 0.2 |
| Chronic Obstructive Pulmonary Disease | 23 (15) | 26 (12) | 0.2 |
| Diabetes | 57 (38) | 52 (24) | 0.004 |
| Cerebrovascular Diseases | 15 (10) | 2 (1) | <0.001 |
| Osteoarthritis | 25 (17) | 46 (21) | 0.1 |
| Renal disease | 1 (1) | 2 (1) | 0.7 |
| Number of medications (mean ± SD) | 3.7 ± 2.4 | 3.0 ± 2.0 | 0.01 |
| Body Mass Index, kg/m2 (mean ± SD) | 24.5 ± 4.7 | 26.3 ± 4.2 | <0.001 |
| Alcohol abuse # | 22 (15) | 23 (11) | 0.2 |
| Smoking habit | 4 (3) | 4 (2) | 0.6 |
| Sensory impairment | |||
| Hearing | 46 (31) | 37 (17) | 0.003 |
| Vision | 41(27) | 45(21) | 0.1 |
| Hematological parameters | |||
| Albumin, g/dl (mean ± SD) | 4.1 ± 0.3 | 4.2 ± 2.2 | 0.002 |
| Total cholesterol, mg/dl (mean ± SD) | 182.5 ± 42.7 | 206.6 ± 43.6 | <0.001 |
| HDL-cholesterol, mg/dl (mean ± SD) | 42.3 ± 13.0 | 48.0 ± 13.3 | <0.001 |
| Triglycerides, mg/dl (mean ± SD) | 138.4 ± 61 | 151.5 ± 62.6 | 0.04 |
| IL-6 (pg/mL) (median; IQR) | 3.33; 1.57–5.23 | 1.69; 1.00–2.58 | <0.001 |
| TNF-α (pg/mL) (median; IQR) | 1.73; 1.07–2.67 | 1.15; 0.67–2.09 | 0.01 |
| CRP (mg/mL) (median; IQR) | 4.74; 2.37–8.02 | 2.2; 1.20–4.10 | <0.001 |
Data are given as number (percent) for the following variables: gender, marital status, living alone, depression, alcohol abuse, smoking habit, sensory impairment; for all the other variables means ± SD are reported.
Activity of Daily Living Scale ranges from 0 (no functional impairment) to 7 (severe functional impairment).
Instrumental Activity of Daily Living Scale ranges from 0 (no functional impairment) to 8 (severe functional impairment).
Cognitive Performance Scale ranges from 0 (no cognitive impairment) to 6 (severe cognitive impairment).
More than ½ liter of wine per day.
A total of 150 deaths (55 men and 95 women) occurred during the 4-year follow-up. The deceased participants had a significantly higher median level of IL-6 (3.33 IQR (sta per?) 1.57–5.23 vs 1.69 IQR 1.00–2.58 , p < 0.001), CRP (4.74 IQR 2.37–8.02 vs 2.2 IQR 1.2–4.1, p < 0.001) and TNF-α (1.73 IQR 1.07–2.67 vs 1.15 IQR 0.67–2.09, p = 0.01) relative to survivors (Table 1). In addition, there was a direct association between the level of each inflammatory marker and mortality during the follow-up (Table 2). In the unadjusted model, high levels of each of the 3 markers were associated with increased mortality. After adjusting for potential confounders, high levels of IL-6 and CRP levels were still associated with a significantly increased risk of death (HR, 2.18; 95% CI 1.29–3.69 and 2.58; 95% CI 1.52–4.40, respectively) whereas the association between TNF-α protein levels and mortality lost statistical significance (1.26; 95% CI: 0.74 to 2.15).
Table 2.
Association between IL-6.CRP, TNF-α systemic levels and All-Cause Mortality, after Adjustment for Various Confounders.
| Inflammatory Markers |
Mortality | Unadjusted | Adjusted* | |
|---|---|---|---|---|
| Deceased/Alive | HR (95 %Cl) | HR (95 %CI) | ||
| IL-6 median (pg/mL) | ||||
| ≤ 2.08 | 48/133 | - | - | |
| > 2.08 | 29190 | 3.58 (2.30–5.57) | 2.18 (1.29–3.69) | |
| TNF-α median (pg/mL) | ||||
| ≤ 1.43 | 59/122 | - | - | |
| > 1.43 | 91/90 | 2.09 (1.37–3.20) | 1.26 (0.74–2.15) | |
| CRP median (mg/mL) | ||||
| ≤ 3.08 | 51/129 | - | - | |
| > 3.08 | 99/80 | 3.13 (2.02–4.85) | 2.58 (1.52–4.40) | |
| Inflammation composite score^ | ||||
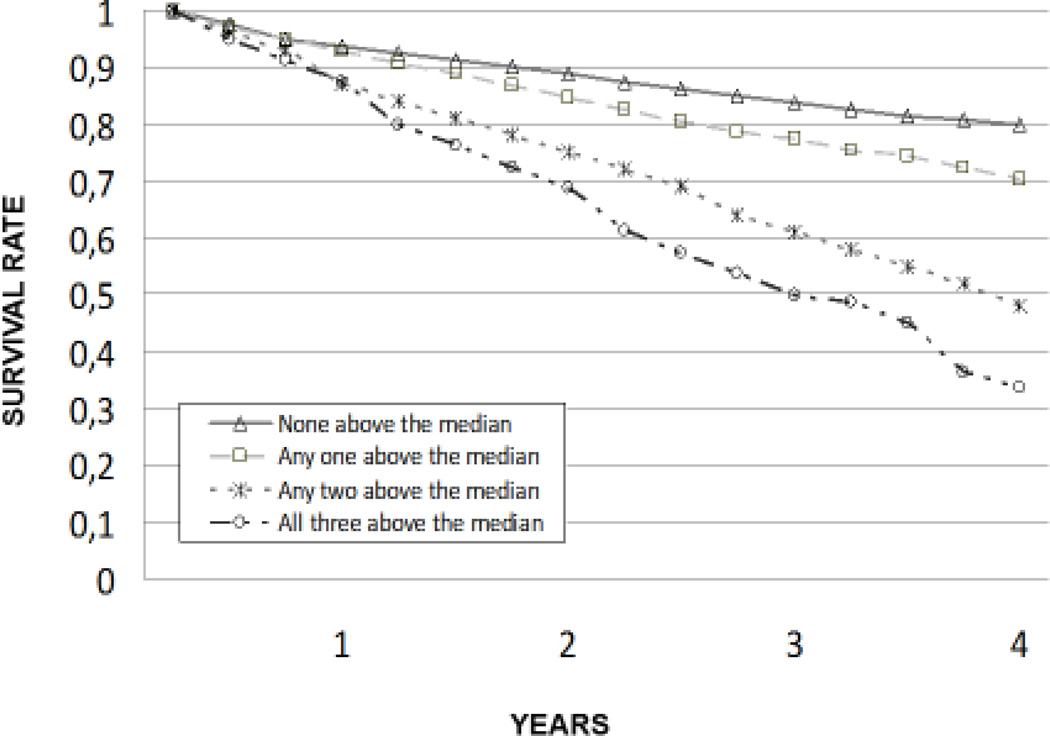
| None above the median (i) | 16/64 | - | - | |
| Any one above the median (ii) | 29/69 | 1.68 (0.84–3.38) | 1.49 (0.68–3.26) | |
| Any two above the median (iii) | 52/48 | 4.33 (2.21–8.50) | 3.03 (1.39–6.59) | |
| All Three above the median (iv) | 53/27 | 7.85 (3.83–16.09) | 3.83 (1.62–9.10) | |
Analyses are adjusted for age, gender, living alone, functional impairment (ADL scale score), cognitive impairment (CPS scale score),number of diseases, hearing impairment, albumin, total and HDL cholesterol, BMI.
In this variable, the first category consists of (i) participants whose values were below the median in all of the three markers. The other categories – (ii) above the median in any one of the three markers, (iii) above the median in any two of the three markers, (iv) above the median in all of the three markers – were each contrasted to category (i).
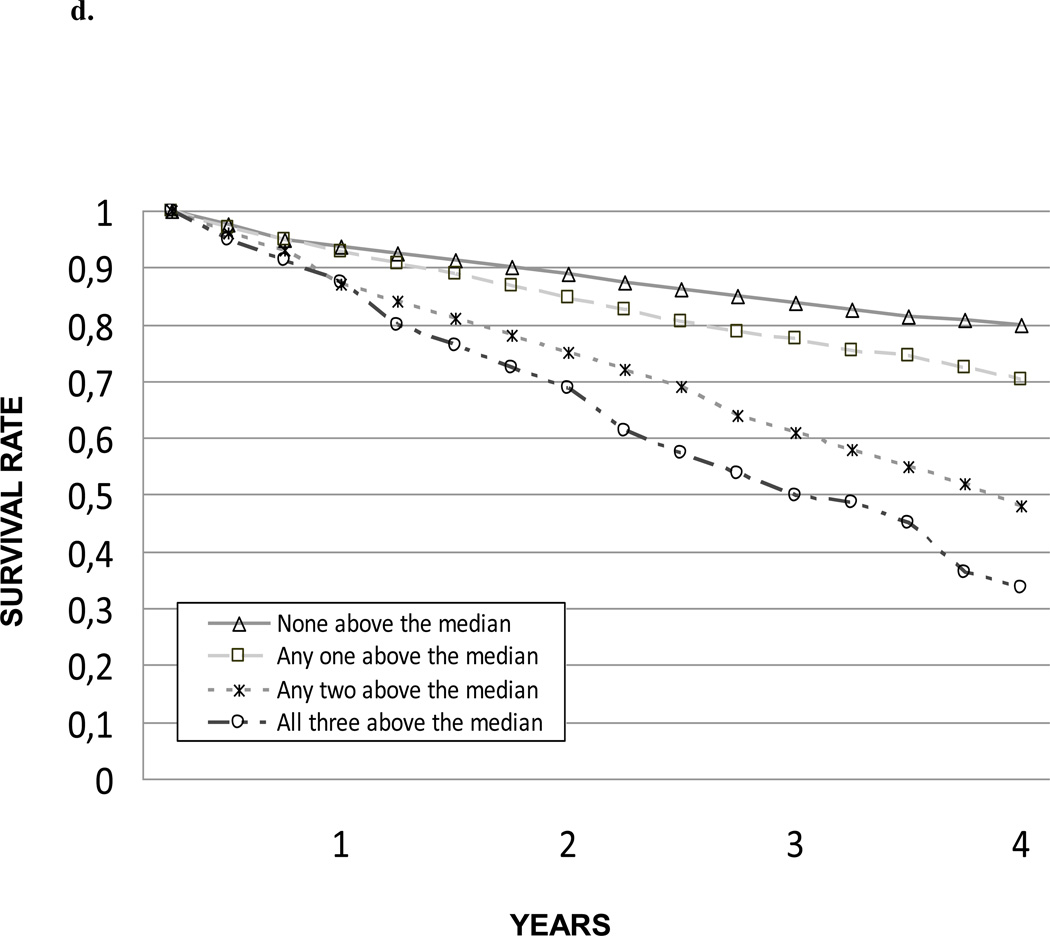
In the unadjusted model, the risk of death significantly and progressively increased as the summary composite score of inflammation increased. This association and trend were confirmed after adjusting for potential confounders.
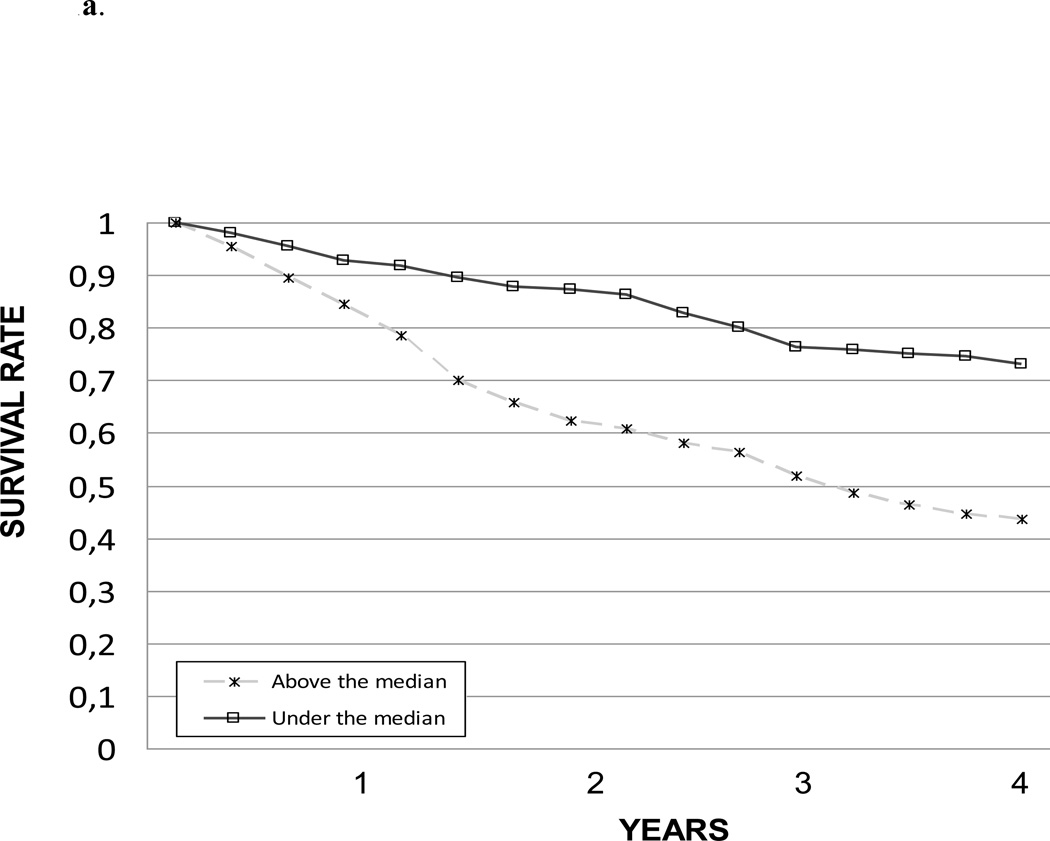
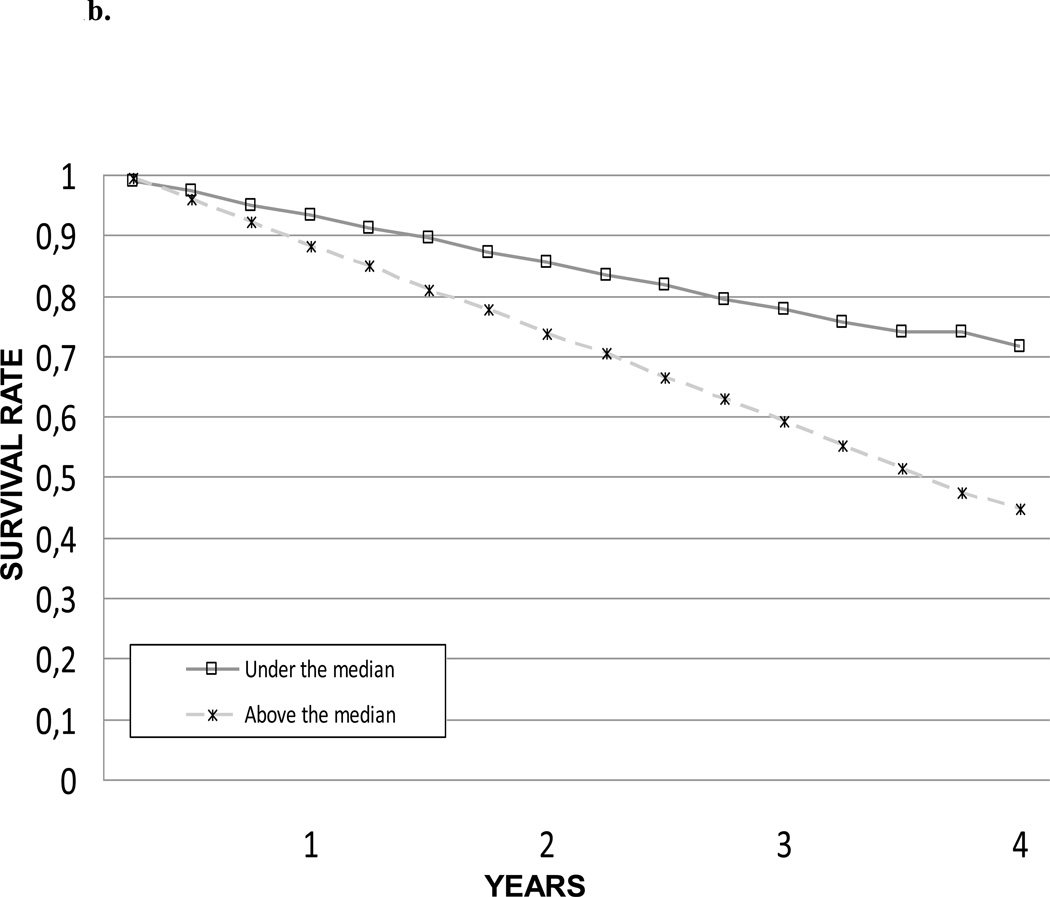
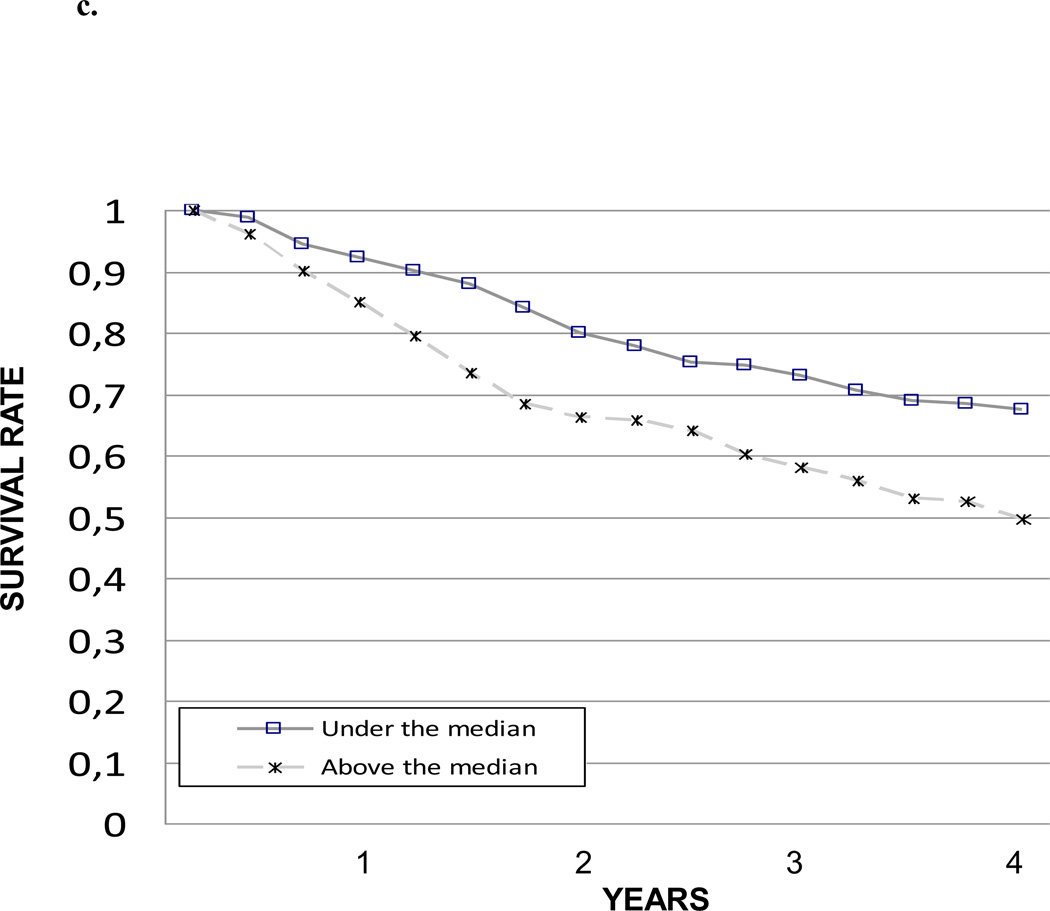
The impact of IL-6, CRP and TNF-α levels on 4-year survival was also tested comparing the survival curves obtained with the Kaplan-Meier method. Survival curves for the different IL-6, CRP and TNF-α levels are shown in Figure 1a, 1b and 1c, respectively. Survival curves for the summary composite score of inflammation are shown in Figure 2.
Figure 1.
Figure 2.
Survival according to inflammation composite score.
DISCUSSION
In the present study, we investigated the role of blood levels of three inflammatory markers (CRP, IL-6 and TNF-α) and their combination, as predictors of 4-year mortality in a sample of community-dwelling individuals aged 80 years and older. Baseline blood concentrations of all three markers were significantly higher in participants who died during the 4 years than in those who survived.
In our data, levels of CRP, IL-6 and TNF-α were linearly correlated but their association with mortality differed. Several earlier studies with old populations have indicated CRP as a possible predictor of mortality (10, 23) and it has been suggested that this association mainly reflects the activity of TNF-α and IL-6 (23). In the present study, however, TNF-α was not associated with mortality when age, gender and other clinical and functional variables where considered in the analysis. In fact, in the fully adjusted model, only baseline plasma concentration of CRP and IL-6 emerged as strong independent predictors of mortality, whereas the association between TNF-α and mortality lost significance.
Our findings extend the results of previous studies on the association between acute phase proteins and cardiovascular disease (24–25) by demonstrating a clear association between IL-6 and CRP levels and mortality. Furthermore, the risk associated with inflammation is a general phenomenon that is not limited to specific subgroups, such as those already affected by cardiovascular disease or those with cardiovascular risk factors.
The octogenarians and nonagenarians enrolled in this study represent an interesting and new model to investigate the biological and non-biological determinants of aging and longevity, as well as their interactions. Furthermore, studies performed in a specific and well-defined geographic region – such as the Sirente Mountain Community – can be particularly useful in interpreting and disentangling all the complex interactions involved in the development of disability status and longevity (16).
A major limit of inflammatory biomarkers is the lack of specificity. In fact, it is difficult to discriminate if the inflammatory status is due to the aging process or is diseased-related. Among most common inflammatory indices, such as IL-6, TNF-α and CRP, it is not possible to differentiate between inflammation markers producted as a result of senescence or induced by comorbidities; we cannot exclude that the inflammatory status might be, at least in part, a consequence of pathological processes. Inflammation may be due to infectious causes of atherosclerosis (26). In particular, acute infection may alter the risk associated with atheroma by impairing normal endothelial function thus leading to expression of tissue-factor, cell-surface adhesion molecules and induction of procoagulant activity (27). Greater C-reactive protein levels identify persons at risk of progression of cardiovascular disease (24, 25). High levels of circulating IL-6 have been reported in severe congestive heart failure (28) and are prognostic indicators in multiple myeloma (29). High IL-6 levels may also reflect cellular damage, such as oxidative stress (30). Lastly, IL-6 may counterregulate levels of TNF-α so that high IL-6 levels may reflect damage from other cytokines. However, the association between plasma IL-6 and CRP baseline concentrations with 4-year mortality in our analysis remained significant even after adjusting for comorbidities and drug use.
Some methodological issues may have influenced our results. First, in this longitudinal observational study, results may be confounded by unmeasured factors. In the absence of randomization, it is likely that there are significant, not considered differences between the evaluation groups that may have biased the study results and conclusions. Second, even though inflammation scores similar to that adopted in the present paper have been previously used in observational research investigating the effect of inflammation on mortality (10, 22), the predictive value and the potential for clinical use of such measures have not yet been investigated. Another limitation of the present study is the lack of any data concerning the cause of death. Finally, the ilSIRENTE sample was composed by persons aged 80 years or older, so our results may not be applicable to other age groups.
In conclusion, we showed that in a representative sample of very old and frail elderly individuals the risk of death is directly associated with the three inflammatory markers measured. Our findings support the hypothesis of a strong implication of low-grade inflammatory status in the process of living an extremely long life.
Supplementary Material
ACKNOWLEDGMENTS
The “Invecchiamento e Longevità nel Sirente” (ilSIRENTE) study was supported by the “Comunità Montana Sirentina” (Secinaro, L’Aquila, Italy). We thank all the participants for their enthusiasm in participating to the project and their patience during the assessments. We are grateful to all the persons working as volunteers in the “Protezione Civile” and in the Italian Red Cross of Abruzzo Region for their support. We sincerely thank the “Comunità Montana Sirentina”, and in particular its President who promoted and strongly supported the development of the project.
The ilSIRENTE Study Group is composed as follows:
Steering Committee: R. Bernabei, F. Landi
Coordination: A. Russo, M. Valeri, G. Venta
Writing Panel: C. Barillaro, M. Cesari, P. Danese, L. Ferrucci, G. Onder, M. Pahor, V. Zamboni, E. Capoluongo
Participants: Comune di Fontecchio: P. Melonio, G. Bernabei, A. Benedetti; Comune di Fagnano: N. Scarsella, A. Fattore, M. Fattore; Comune di Tione: M. Gizzi; Comune di Ovindoli: S. Angelosante, E. Chiuchiarelli; Comune di Rocca di Mezzo: S. Pescatore; Comune di Rocca di Cambio: G. Scoccia; Comune di Secinaro: G. Pizzocchia; Comune di Molina Aterno: P. Di Fiore; Comune di Castelvecchio: A. Leone; Comune di Gagliano Aterno: A. Petriglia; Comune di Acciano: A. Di Benedetto; Comune di Goriano Sicoli: N. Colella; Comune di Castel di Ieri: S. Battista; RSA Opera Santa Maria della Pace: A. De Santis, G. Filieri, C. Gobbi, G. Gorga, F. Cocco, P. Graziani.
Reference list
- 1.Franceschi C, Vallesin S, Lescai F, et al. Neuroinflammation and the genetics of Alzheimer's disease: the search for a pro-inflammatory phenotype. Aging Clin. Exp. Res. 2001;13:163–170. doi: 10.1007/BF03351475. [DOI] [PubMed] [Google Scholar]
- 2.Abbatecola AM, Ferrucci L, Grella R, et al. Diverse effect of inflammatory markers on insulin-resistance syndrome in the elderly. J Am Geriatr Soc. 2004;52:399–404. doi: 10.1111/j.1532-5415.2004.52112.x. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri M, Ferrucci L, Ragno E, et al. Chronic inflammation and the effect of IGF-1 on muscle strenght and power in older persons. Am J Physiol.: Endocrinol. Metab. 2003;284:481–487. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 4.Reuben DB, Cheh AI, Harris TB, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr. Soc. 2002;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- 5.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor I, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Stork S, Feelders RA, Van Den Beld AW, et al. Prediction of mortality risk in the elderly. Am J Med. 2006;119:519–525. doi: 10.1016/j.amjmed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 7.Bataille R, Klein B. C-reactive protein levels as a direct indicator of interleukin-6 levels in humans in vivo. Arthritis Rheum. 1992;35:982–983. doi: 10.1002/art.1780350824. [DOI] [PubMed] [Google Scholar]
- 8.Lio D, Scola L, Crivello A, et al. Inflammation, genetics, and longevity: further studies on the protective effects in meno f IL-10-1082 promoter SNP and its interaction with TNF- α-308 promoter SNP. J Med. Genet. 2003;40:496–499. doi: 10.1136/jmg.40.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruunsgaard H, Andersen Ranberg K, Jeune B, et al. A high plasma concentration of TNF- α is associated with dementia in centenarians. J Gerontol Med. Sci. 1999;54:357–364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- 10.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 11.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volpato S, Guralnik JM, Ferrucci L, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the Women’s Health and Aging Study. Circulation. 2001;103:947–953. doi: 10.1161/01.cir.103.7.947. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disabilityin older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 14.Schaap LA, Pluijm SM, Deeg DJ, et al. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526. doi: 10.1016/j.amjmed.2005.10.049. e9-17. [DOI] [PubMed] [Google Scholar]
- 15.Bruunsgaard H, Andersen Ranberg K, Hjelmborg JB, et al. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003b;115:278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 16.Landi F, Russo A, Cesari M, et al. The ilSIRENTE study: a prospective cohort study on persons aged 80 years and older living in a mountain community of Central Italy. Aging Clin Exp Res. 2005;17:486–493. doi: 10.1007/BF03327416. [DOI] [PubMed] [Google Scholar]
- 17.Morris JN, Fries BE, Bernabei R, et al. RAI – Home Care assessment manual. Washington, DC: InterRAI Coporation; 1996. [Google Scholar]
- 18.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49:M174–M182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 19.Morris JN, Fries BE, Steel K, et al. Comprehensive clinical assessment in community setting: applicability of the MDS-HC. J Am Geriatr Soc. 1997;45:1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- 20.Landi F, Tua E, Onder G, et al. Minimum data set for home care: a valid instrument to assess frail older people living in the community. Med Care. 2000;38:1184–1190. doi: 10.1097/00005650-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 22.Jylha M, Paavilainen P, Lehtimaki T, Goebeler S, Karhunen PJ, Hervonen A, Hurme M. Interleukin-1 receptor antagonist, interleukin-6, and c-reactive protein as predictors of mortality in nonagenarians: the vitality 90+ study. J Gerontol A Biol Sci Med Sci. 2007 Sep;62(9):1016–1021. doi: 10.1093/gerona/62.9.1016. [DOI] [PubMed] [Google Scholar]
- 23.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North. Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 24.Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. NEJM. 1994;331:417–424. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- 25.Haverkate F, Thompson SG, Pyke SDM, et al. Production of C-reactive protein and risk of coronary events in stable and unstable angina. Lancet. 1997;349:462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 26.Vallance P, Collier J, Bhagat K. Infection, inflammation, and infarction:does acute endothelial dysfunction provide a link? Lancet. 1997;349:1392–1392. doi: 10.1016/S0140-6736(96)09424-X. [DOI] [PubMed] [Google Scholar]
- 27.Visseren FLJ, Bouwman JJM, Bouter KP, Diepersloot RJA, De Groot PhG, Erkelens DW. Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb Haemost. 2000 Aug;84(2):319–324. [PubMed] [Google Scholar]
- 28.Torre-Amione G, Kapadia S, Benedict C, et al. Proinflammatory cytokines levels in patientswith depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 29.Pelliniemi TT, Irijala K, Mattila K, et al. Immunoreactive interleukin-6 and acute phase proteins as prognostic factors in multiple myeloma. Blood. 1995;85:765–771. [PubMed] [Google Scholar]
- 30.Baeuerle PA, Rupec RA, Pahl HL. Reactive oxygen intermediates as second messengers of a general pathogen response. Path Biol. 1996;44:29–35. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.