Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma and can be separated into two subtypes based upon molecular features with similarities to germinal center B-cells (GCB-like) or activated B-cells (ABC-like). Here we identify gain of 3q27.2 as being significantly associated with adverse outcome in DLBCL and linked with the ABC-like subtype. This lesion includes the BCL6 oncogene, but does not alter BCL6 transcript levels or target-gene repression. Separately, we identify expression of BCL6 in a subset of human hematopoietic stem/progenitor cells (HSPCs). We therefore hypothesize that BCL6 may act by hit-and-run oncogenesis. We model this by transiently expressing Bcl6 within murine HSPCs, and find it causes mature B-cell lymphomas that lack Bcl6 expression and target-gene repression, are transcriptionally similar to post-GCB cells, and show epigenetic changes that are conserved from HSPCs to mature B-cells. Together these results suggest that Bcl6 may function in a hit-and-run role in lymphomagenesis.
Introduction
As the most common aggressive lymphoma afflicting nearly 30,000 Americans each year, diffuse large Bcell lymphoma (DLBCL) is highly heterogeneous. Current combination therapeutic regimens typically fail in nearly half of all patients with DLBCL, many of whom succumb to their disease. Given the inability to cure many patients with DLBCL, and the significant toxicity of current therapies, better treatment strategies are needed. We previously described a major molecular determinant of this biological and clinical heterogeneity, likely reflecting the cellular origin of tumors. Patients with tumors that have transcriptional profiles related to germinal center B-cells (GCB-like) have a better overall survival than those with tumors having a transcriptional profile related to post-GCB activated B-cells (ABC-like)1. This finding has been validated by several groups independently, and the molecular basis for this diversity in DLBCL has been partially deciphered in studies of distinctive genomic aberrations and somatic mutations in DLBCL subtypes.
Genomic studies have defined a subset of alterations that stratify between the two DLBCL subtypes2,3, with point mutations of histone modifying genes and B-cell receptor signaling components as the prevailing dominant drivers or accelerators of the disease4. However, these alterations are found in only a fraction of patients, and the relationship between more common genetic alterations and DLBCL subtypes remains largely obscure. For example, the most frequent somatic alteration observed in DLBCL, involving genetic translocation of BCL6, is arguably the most prominent and paradoxical5,6. BCL6 is a central regulator of germinal center development7,8, it is more highly expressed in the GCB-like subtype of DLBCL compared to the ABC-like subtype, and is associated with a favorable prognosis1,9. Yet genetic translocations of this gene are more prominent in the post-GCB subtype of the disease and associated with adverse outcome1,10. Recent findings have implicated Bcl6 in leukemia stem cell survival11,12 and show its activity may be altered by CREBBP or EP300 mutation3 at an early stage lymphoma development13,14. Separately, genetic and epigenetic aberrations in premalignant hematopoietic progenitors have recently been described in several hematological malignancies, including AML and CLL15–18. Together, these findings led us to postulate that BCL6 may promote tumorigenesis in a manner contrasting that of other traditional oncogenes which act in fully evolved tumor cells and require persistent activity due to oncogene addiction19.
Somatic DNA copy number alterations (SCNAs) perturb more of the cancer genome than any other somatic alteration, and can alter the gene dosage and subsequent expression of multiple genes in a single alteration20. The significance of SCNAs can be assessed from the patterns of broad and focal gains/losses across the genomes of a tumor cohort, allowing potential target genes within conserved regions of DNA copy number gain/loss to be identified. The integration of expression profiling data has additionally allowed putative driver genes within each lesion to be localized by their changes in transcript abundance resulting from altered gene dosage21. However, a subset of oncogenes with negative feedback loops may act in a ‘hit-and-run’ fashion; therein, transient expression of the oncogene may induce broad changes to the cancer genome, epigenome, or transcriptome, and be sufficient for oncogenesis in the absence of persistent expression. These ‘hit-and-run’ oncogenes may therefore not be detected by integrative analysis of DNA copy number and gene expression changes, and are difficult to identify in the absence of other genetic alterations targeting the same locus, such as genetic translocations or somatic mutations.
Here we use high resolution analysis of DNA copy number across a large cohort of DLBCL tumors to elucidate recurrent alterations in this disease. We identify gain of the BCL6 oncogene as being a potential ‘hit-and-run’ oncogene associated with poor outcome and the ABC-like DLBCL subtype. Using transgenic mouse models, we confirm that transient expression of Bcl6 is sufficient to induce aggressive mature B-cell lymphoma that appears transcriptionally similar to activated post-germinal center B-cells.
Results
Gain of 3q27.2 is associated with inferior outcome in DLBCL
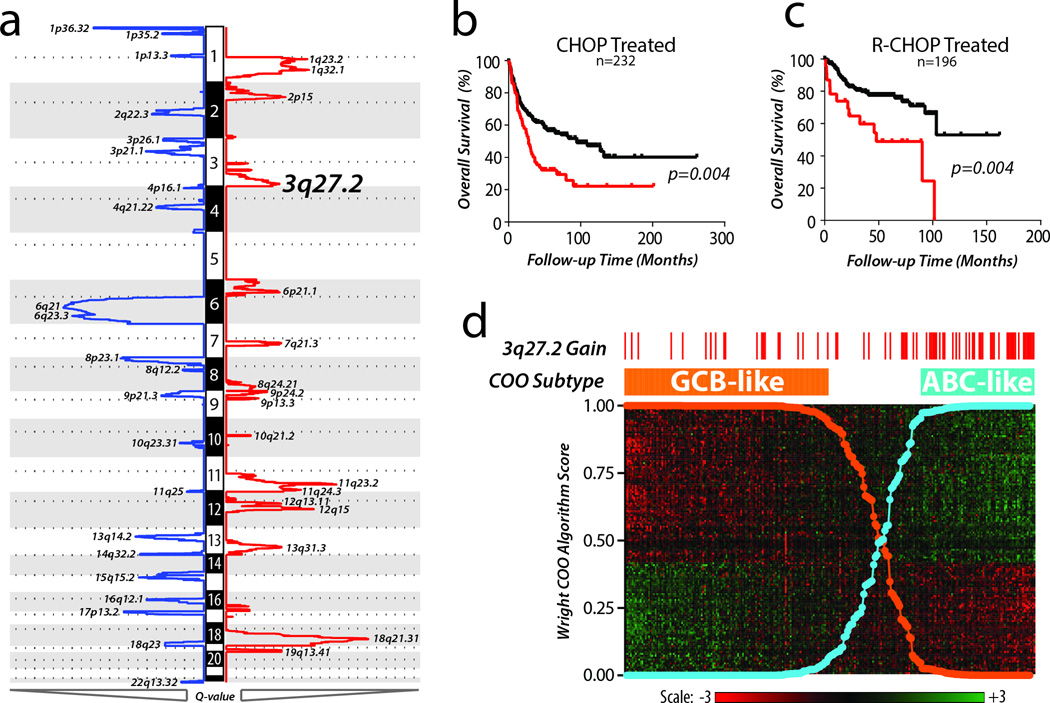
Using high-resolution DNA copy number profiles of 609 DLBCL tumors analyzed using the GISTIC method, we mapped the landscape of SCNAs in this disease. We identified 22 peaks of significant DNA copy number loss (GISTIC Q-value <0.25) and 17 peaks of significant DNA copy number gain (Figure 1a, Supplementary Table 1). We analyzed the association of each lesion with overall survival in cohorts of patients treated with combination chemotherapy (CHOP, n=232) or in combination with Rituximab (RCHOP, n=196). Gain of 3q27.2 was the most prognostic lesion and was associated with significantly decreased overall survival in both cohorts (Figure 1b–c, Supplementary Table 1). For 249 cases, matched gene expression profiling data was available and allowed for classification of samples into GCB-like and ABC-like subtypes using the previously defined method (Figure 1d). Gain of 3q27.2 was significantly over-represented in the ABC-like DLBCL subtype (Fisher P-value =8.1 × 10−8), suggesting that it may contribute to the genetic etiology of this subtype and its association with adverse outcome.
Figure 1. High resolution DNA copy number profiling of human DLBCL.
a) DNA copy number profiles from 609 primary DLBCL tumors were analyzed for significant alterations using the GISTIC algorithm. This algorithm uses the magnitude and frequency of alterations at each position in the genome to assign a GISTIC Q-value, with decreasing values indicating increasing significance. Significant peaks (GISTIC Q-value <0.10) of DNA copy number loss (blue) and gain (red) are annotated with their genomic location. b) In 232 patients treated with combination chemotherapy (CHOP) in the absence of Rituximab, presence of 3q27.2 gain (red) was associated with significantly worse overall survival than those with diploid copy number at this region (black). Log-rank P-values were 0.004 and 0.001 for CHOP-treated and R-CHOP-treated cohorts, respectively c) Gain of 3q27.2 (red) remained to be associated with significantly worse overall survival compared to diploid 3q27.2 (black) in 196 patients treated with combination chemotherapy plus Rituximab (R-CHOP). d) For 249 cases with matched gene expression profiling data, tumors were classified into GCB-like (Orange) and ABC-like subtypes (Blue) based upon the Wright 140 gene algorithm (heat map shown). Gain of 3q27.2, shown by red tick marks for each case in which it was detected, was significantly over-represented in the ABC-like subtype compared to the GCB-like subtype (Fisher P-value = <0.001).
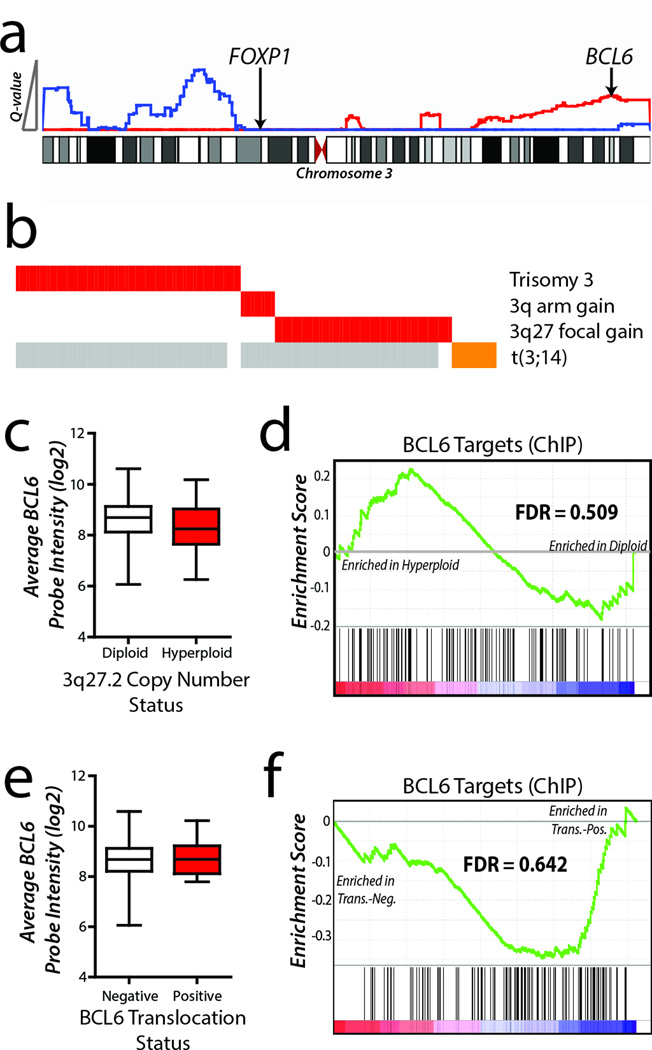
The peak of this alteration (chr3:180478352-199501827) contained 134 genes, including the lymphoma oncogene BCL622 but not a previously hypothesized target of chromosome 3 alterations, FOXP123 (Figure 2a). The significance of the 3q27.2 peak resulted from a combination of broad events (trisomy 3 and 3q arm-level gain) and recurrent focal gains of 3q27.2 over the BCL6 locus (Figure 2b). Furthermore, 3q27.2 DNA copy number gains were mutually exclusive of translocations targeting BCL6 in the 48 tumors for which such data were available24, suggesting that BCL6 is a likely target of these lesions (Figure 2b). However, there was no significant increase in either BCL6 expression or BCL6-target gene25 repression in cases with 3q27.2 gain compared to those cases with diploid copy number (Figure 2c–d). This same trend was also observed within the context of BCL6 translocations (Figure 2e–f), as previously described10. The absence of increased BCL6 transcript levels in tumors harboring these genomic alterations could not be attributed to the uniformly high expression of BCL6 within DLBCL, since BCL6 transcript levels were found to be significantly lower in DLBCL than in non-malignant B-cells (Supplementary Fig. 1). Together, these data led us to hypothesize that BCL6 may act in a ‘hit-and-run’ fashion and promote oncogenesis by transient over-expression during an early stage of hematopoietic differentiation or tumor evolution, and that its expression is no longer maintained or required in fully evolved tumor cells.
Figure 2. 3q27.2 gain targets BCL6 but does not alter its expression or target-gene repression.
a) GISTIC Q-values are shown for DNA copy number loss (blue) and gain (red) on chromosome 3. The peak of 3q27.2 gain included the BCL6 oncogene, but not the previously described target FOXP1. b) Significance of 3q27.2 gain was contributed to by broad gains of chromosome 3 or the 3q arm in 76 tumors, as well as focal gains of 3q27 in 52 tumors. These gains were mutually exclusive to BCL6 translocation in the 21 tumors for which matching DNA copy number and fluorescence in situ hybridization data were available. Grey bars represent data not available. c) Increased DNA copy number of BCL6 was not associated with increased transcript abundance compared to cases with no gain. Box plots represent the mean +/− the interquartile range with whiskers extending to the minimum and maximum value. d) Gene set enrichment analysis (GSEA) of BCL6 target genes showed no significant repression within cases possessing BCL6 DNA copy number gain (GSEA FDR = 0.509). e) For the 58 tumors with matching BCL6 translocation status and gene expression profiling data, BCL6 transcript abundance was not increased in tumors with BCL6 translocation compared to tumors without BCL6 translocation. Box plots represent the mean +/− the interquartile range with whiskers extending to the minimum and maximum value. f) Tumors with BCL6 translocation (Trans.-Pos.) showed no significant repression of BCL6 target genes by GSEA (GSEA FDR = 0.642) compared to tumors without BCL6 translocation (Trans.-Neg.).
BCL6 expression in human hematopoietic precursors
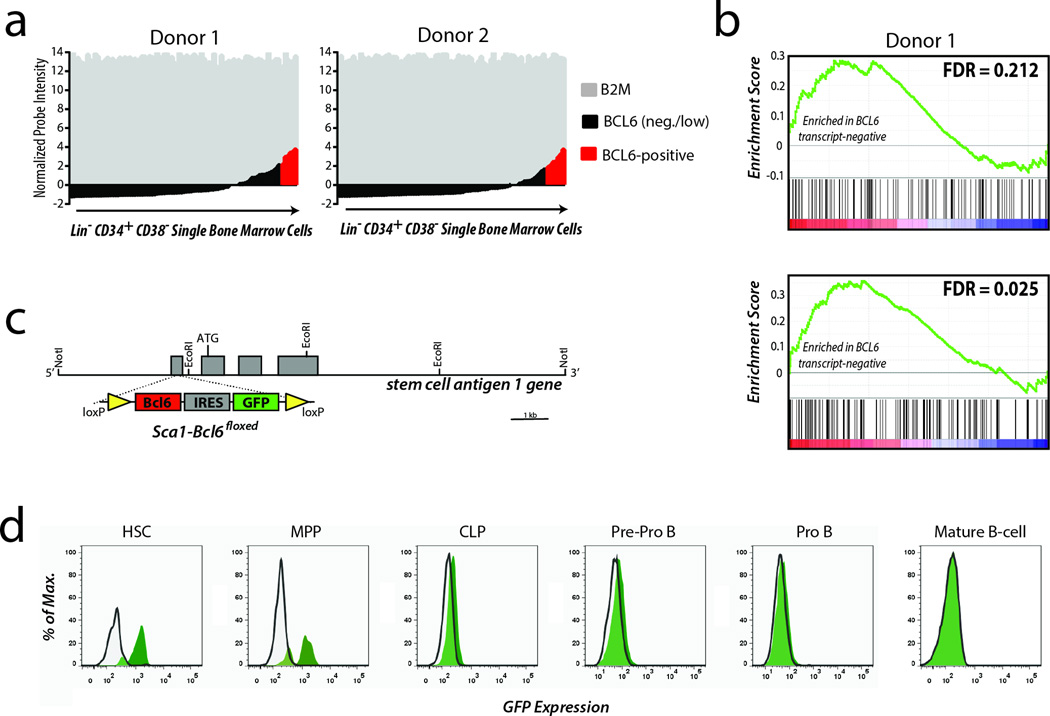
To investigate the potential for BCL6 to be acting at an early stage of hematopoietic differentiation, we performed gene expression profiling of 183 single sorted human hematopoietic stem/precursor (HSPC) cells from bone marrow aspirates of 2 healthy adults (Supplementary Fig. 2). Single cell profiling was employed in order to allow evaluation of small populations of cells possessing unique gene expression phenotypes that may not be discernible from analysis of bulk populations. Using the beta-2-microglobulin (B2M) gene as a reference for successful RNA amplification and gene expression microarray analysis, we observed that BCL6 transcript was detectable in 6.5% (6/92, donor 1) to 7.7% (7/91, donor 2) of single HSPCs (Figure 3a). Notably, HSPCs expressing BCL6 lacked significant expression of other markers of B-cell differentiation including Pax5, CD19, or CD20, among others. Nonetheless, gene set enrichment analysis found significant repression of BCL6-bound target genes25 within the population of cells with expressing BCL6 transcript levels compared to those cells not expressing BCL6 (Figure 3b). Thus, in a minor subset of adult human HSPCs, BCL6 expression is associated with an early transcriptional program, consistent with similar observations in human cord blood implicating this transcription factor in the commitment of specific progenitors to the lymphoid fate26.
Figure 3. Expression and activity of BCL6 in human and murine HSPCs.
a) Gene expression profiles of single human HSPCs showing expression of the control gene B2M were investigated for expression of BCL6. Positive expression values defined as being 2 standard deviations above the mean were observed in 11/183 single cells, with approximately equal proportions in each donor. b) Gene set enrichment analysis of BCL6 target genes in BCL6 transcript-positive cells compared to BCL6 transcript-negative cells in each donor showed an enrichment of target gene expression in BCL6 transcript-negative cells (GSEA FDRs 0.212 and 0.025 for Donor 1 and 2, respectively). This corresponds to repression of target genes in BCL6 transcript-positive cells. c) Transient Bcl6 expression within HSPCs was achieved by placing a floxed (yellow loxP sites) Bcl6 cDNA with IRES-GFP reporter under control of the of the promoter for the HSPCspecific gene, stem-cell-antigen 1 (Sca1). d) Tracking of the GFP marker for Bcl6 transgene expression during hematopoietic development shows expression is restricted to HSPCs and not in Pro B or mature B-cells. Images are representative of 4 independent experiments. For gating schema, see Supplementary Fig. 3.
We therefore hypothesized that BCL6 may contribute to lymphomagenesis at a stage of development prior to B-cells reaching full maturity. Because BCL6 shows highly conserved patterns of expression between humans and mice27, we tested the hypothesis by generating a murine strain that transiently expressed Bcl6 specifically within HSPCs by placing a floxed Bcl6 cDNA with an IRES-GFP marker under control of the promoter for the Ly6A locus encoding Stem cell antigen-1 (Sca1) (Sca1-Bcl6floxed, Figure 3c)28. As expected, using flow cytometric analysis of the co-expressed GFP marker, we detected expression in hematopoietic stem cells (HSC) and multipotential progenitors (MPP), with expression declining in common lymphoid progenitors (CLP) towards barely-detectable expression in pre-proB cells and no detectable expression in proB cells or later stages of B-cell development (Figure 3d; Supplementary Fig. 3). Therefore, this transient expression of Bcl6 during early hematopoietic development provided a means to evaluate the potential for Bcl6 to contribute to lymphoma development via a hit-and-run mechanism.
Transient Bcl6 expression induces mature B-cell lymphoma
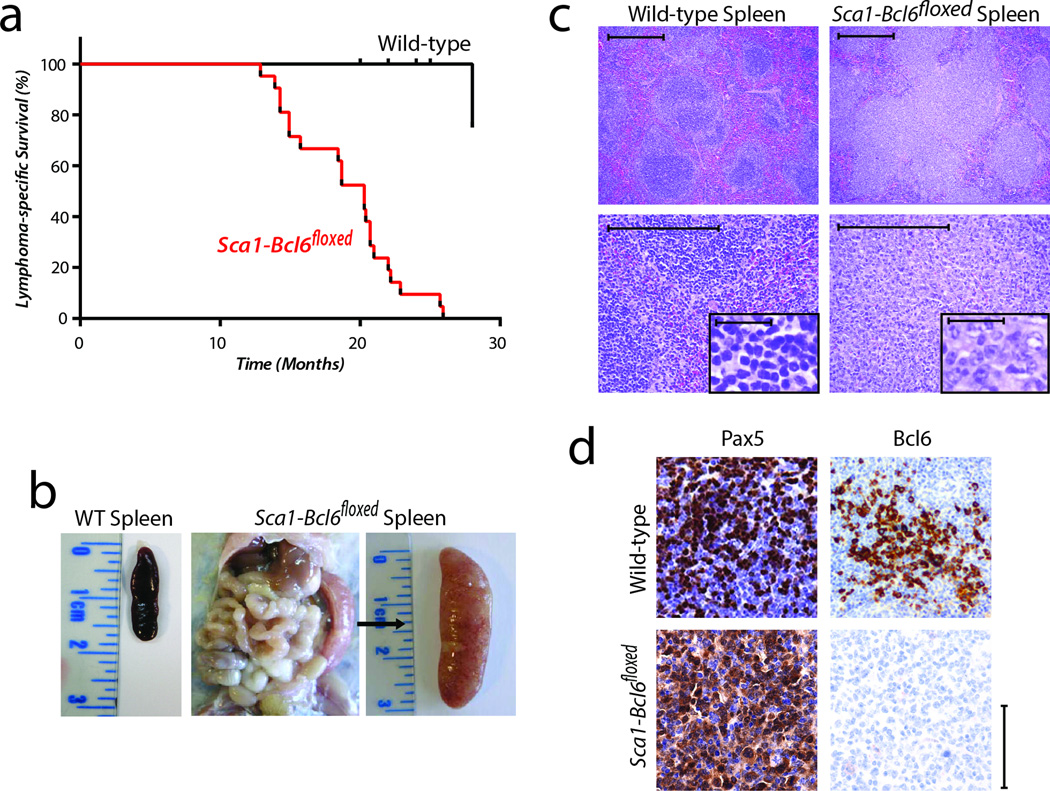
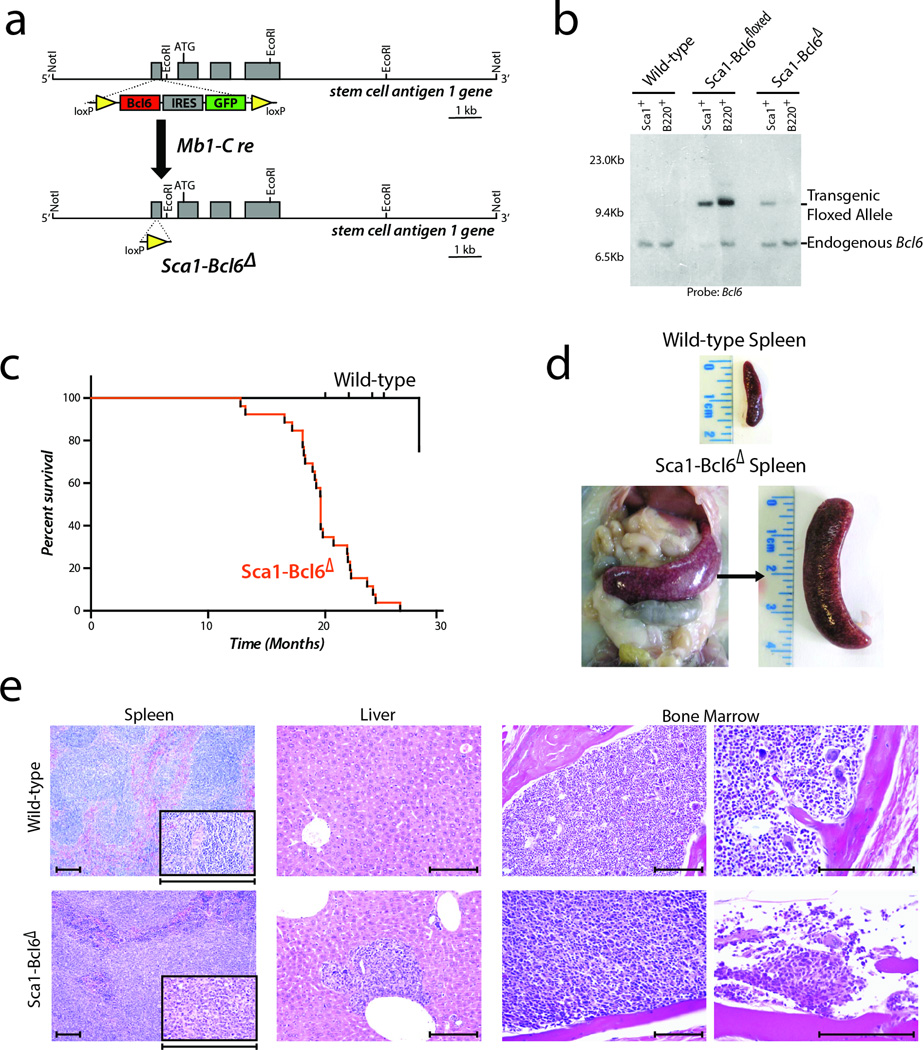
Sca1-Bcl6floxed mice are viable and develop normally, with a typical hematopoietic system early in post-gestational development, and normal germinal center development in response to a T-cell dependent immunogen (Supplementary Fig. 4). However, by 4 weeks of age, increased numbers of hematopoietic stem cells, Lin-Sca1+cKit+ (LSK) and lineage restricted progenitors (Lin-CD48+CD150+), and decreased numbers of common lymphoid progenitors (Lin-Sca1+CD127+cKit+) can be detected (Supplementary Fig. 5). In 8-week old mice, we also noted changes in multiple lymphocyte compartments within the bone marrow, thymus, spleen and peripheral blood (Supplementary Fig. 6). In an ageing mouse colony, Sca1-Bcl6floxed mice exhibit significantly shortened lifespan compared to wild-type (WT) littermates due to the development of B-cell lymphomas in 50% (21/42) of mice (Figure 4a). These lymphomas are manifested as expanded and confluent white pulp nodules composed of pleomorphic large B-cells resulting in splenomegaly (Figure 4b–c). The lymphoma cells lack Bcl6 expression but express hallmarks of B-cell identity, such as the Pax5 transcription factor (Figure 4d). These results therefore provide the first indication that transient expression of the Bcl6 oncogene in HSPCs can induce aggressive malignancies of mature B-cells.
Figure 4. Expression of the Bcl6 oncogene in hematopoietic progenitor cells (HSPCs) causes aggressive malignancy of mature B-cells that lack Bcl6 protein expression.
a) Lymphoma-specific survival of Sca1-Bcl6floxed mice (red, n=21), showing a significantly (Log-rank P-value <0.001) shortened lifespan compared to wild-type mice (black, n=20) as a result of mature B-cell malignancies. b) Example of splenomegaly observed in 50% (21/42) of Sca1-Bcl6floxed mice compared. A spleen from a wild-type mouse is shown for reference. c) Hematoxylin and eosin staining of wild-type spleens and tumor-bearing spleens from Sca1-Bcl6floxed mice shows loss of normal architecture resulting from effacement with cells morphologically resembling lymphocytes (above = 10×, below = 40×, inset = 400×). Images are representative of ≥3 replicates. Scale bar represents 200µm for all panels. d) Immunohistochemistry shows that lymphocytes from Sca1-Bcl6floxed tumors bear markers of B-cell identity (Pax5), but lack protein expression of Bcl6. Wild-type spleens obtained from immunized mice. Scale bar represents 100µm for large panels, 50µm for inset.
Sca1-Bcl6floxed DLBCL tumors resemble post-GCB cells
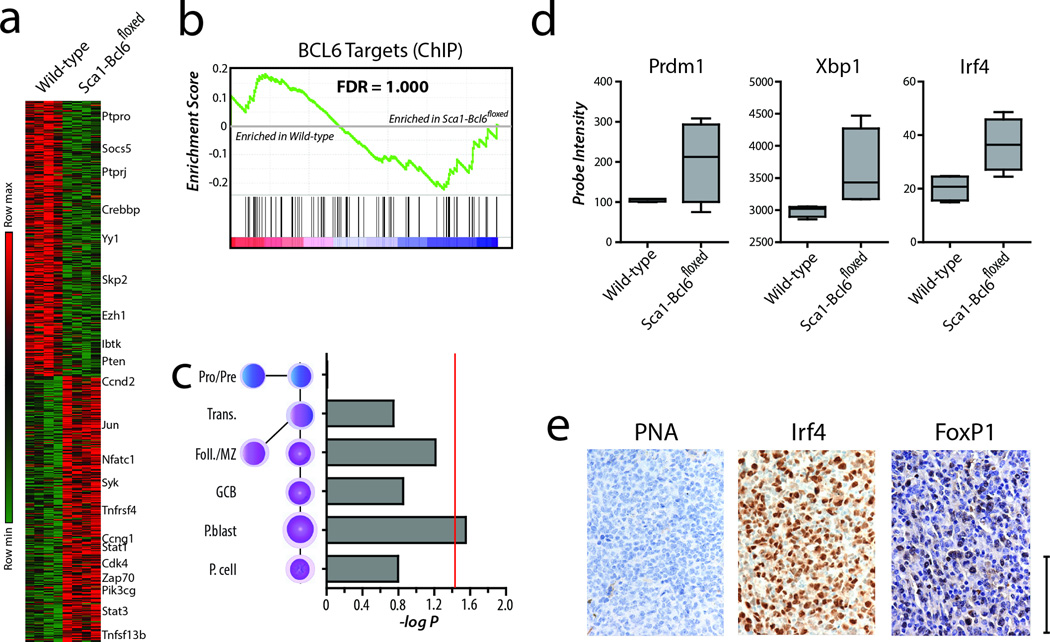
We next sought to evaluate the relationship of this model with the trends observed in human DLBCL by assessing whether these malignancies resembled cells at a GCB or post-GCB (ABC/plasmablast) stage of differentiation. Using transcriptome analysis, we identified 750 significantly repressed and 720 significantly induced genes (T-test FDR <0.25) in tumor-bearing spleens from Sca1-Bcl6floxed mice compared to spleens from WT mice, including increased expression of multiple genes with roles in oncogenesis (Figure 5a, Supplementary Data 1). However, BCL6 target genes were not significantly repressed within these tumors (GSEA FDR = 1.000, Figure 5b). This is consistent with our findings from human DLBCL tumors, and further supports a hit-and-run role for Bcl6 in generating these aggressive B-cell malignancies. Human DLBCL tumors can be reliably classified into GCB or ABC subtypes by gene expression profiling1, and this can be achieved in murine models due to recent mapping of the transcriptional signatures of normal murine B-cell development29,30. Using the broad transcriptional signature of tumors from Sca1-Bcl6floxed mice compared to signatures of normal stages of murine B-cell development, we found these tumors to most significantly align with the post-germinal center ABC/plasmablast stage of differentiation (Hypergeometric enrichment P-value = 0.028, FDR = 0.017, Figure 5c). These changes included increased transcript abundance of the post-germinal center transcription factors Irf4, Prdm1 and Xbp1 (Figure 5d). In addition, immunohistochemical staining of tumors from Sca1-Bcl6floxed mice showed no staining for the GCB marker PNA, but strong staining for Irf4 and the ABC-like DLBCL marker FoxP1 (Figure 5e). These data therefore demonstrate that transient expression of the Bcl6 oncogene within HSPCs of Sca1-Bcl6floxed mice is capable of inducing aggressive Bcell tumors that align with a differentiation stage comparable to human ABC-like DLBCL.
Figure 5. Tumors from Sca1-Bcl6floxed mice resemble post-germinal center B-cells.
a) Differential gene expression analysis of tumor-bearing spleens of four Sca1-Bcl6floxed mice compared to spleens from four wild-type mice show significant differences. These differences include genes that are involved in B-cell signaling, and cell cycle regulation. b) Gene set enrichment analysis identified no significant repression or derepression of Bcl6 target genes in Sca1-Bcl6floxed tumor-bearing spleens compared to spleens from wild-type mice (GSEA FDR = 1.000). This is in line with what is observed in human DLBCL that possess amplification of the BCL6 coding region. c) Analysis of differentially expressed genes in Sca1-Bcl6floxed tumor-bearing spleens with relation to gene expression signatures of normal murine B-cell differentiation including Pro/Pre-B, transitional (Trans.) B-cells, follicular and marginal zone (Foll./MZ) Bcells, germinal center B-cells (GCB), plasmablasts (P.blast) and plasma cells (P. cell). This shows significant enrichment of the normal plasmablast signature (Hypergeometric enrichment P-value = 0.028, FDR = 0.17). Red line represents a hypergeometric enrichment P-value of 0.05. d) Tumors from Sca1-Bcl6floxed mice show increased expression of transcription factors that are associated with post-germinal center stages of B-cell differentiation. Box plots represent the mean +/− the interquartile range with whiskers extending to the minimum and maximum value. e) Immunohistochemical staining shows negative expression of the germinal center marker peanut agglutinin (PNA), but positive staining for the post-germinal centre transcription factor Irf4, and the ABC-like DLBCL marker FoxP1. Images are representative of ≥3 replicates. All panels are 60×, scale bar represents 100µm.
Evidence for HSPCs as lymphoma-initiating cells
To exclude the potential contribution to lymphomagenesis of persistent ectopic Bcl6 expression in the mature B cell compartment we crossed the Sca1-Bcl6floxed mice with an mb1-Cre mouse strain. The resulting strain, Sca1-Bcl6Δ, maintains expression of Bcl6 under the Sca1 promoter in HSPCs, but deletes the exogenous floxed Bcl6 cDNA upon B-lineage commitment via Cre-recombinase driven by the promoter from mb1 locus encoding the immunoglobulin-associated alpha chain Cd79a (Figure 6a). To validate the efficient deletion of the exogenous floxed Bcl6 cDNA, we sorted B220+ cells from bone marrow of young Sca1-Bcl6Δ mice and cultured under conditions to allow the isolation and expansion of a pure population of B220+c-Kit+ proB cells. Southern blot analysis of DNA from these cells confirmed uniform and efficient deletion of the exogenous floxed Bcl6 cDNA at the pro-B stage, and therefore all subsequent stages, of B-cell differentiation (Figure 6b, Supplementary Fig. 7).
Figure 6. Cre-mediated deletion of the exogenous Bcl6 allele in precursor B-cells does not alter formation of mature B-cell malignancies.
a) Diagramatic representation of the Sca1-Bcl6Δ mice, showing expression of the transgenic Bcl6 allele within HSPCs under control of the Sca1 promoter, followed by Cre-mediated deletion at an early Pro-B cell stage upon Mb1 expression. b) Southern blot analysis confirms absence of the transgenic Bcl6 allele within mature (B220+) B-cells of Sca1-Bcl6Δ mice. Image is representative of 3 replicate experiments. c) Lymphoma-specific survival of Sca1-Bcl6Δ mice (n=13) demonstrates a significantly (Log-rank P-value <0.001) shorter lifespan compared to wild-type mice (n=20). d) Example of splenomegaly observed in Sca1-Bcl6Δ mice, with spleen from a wild-type mouse is shown for reference. Images are representative of 13 mice. e) Effacement of spleen (100×, inset 400×) by malignant B-cells and infiltrates in the liver (200×) and bone marrow (left 200×, right 400×) result in loss of normal architecture. Images representative are of 13 mice. Scale bar represents 200µm.
Importantly, Sca1-Bcl6Δ mice recapitulate the phenotype observed in Sca1-Bcl6floxed mice. These mice have a shortened survival compared to wild-type littermates (Figure 6c) due to aggressive B-cell malignancy in 56.50% (13/24) of mice, manifesting as splenomegaly resulting from complete effacement of normal architecture by diffuse B-cell infiltration (Figure 6d–e). Malignant B-cells are primarily IgM+ but show evidence of heavy-chain class-switch in a subset of tumors (Supplementary Fig. 8). These mice also showed infiltration of malignant cells into the liver and bone marrow, resulting in disruption of normal architecture (Figure 6e). Tumors showed increased clonality of immunoglobulin rearrangements (Supplementary Fig. 9), and significant similarity to Sca1-Bcl6floxed tumors at the transcriptional level (Supplementary Fig. 10). In line with Sca1-Bcl6floxed tumors, Sca1-Bcl6Δ tumors also expressed markers of B-cell identity and a post-germinal center stage of differentiation (Supplementary Fig. 10). To identify the tumor repopulating cells for Sca1-Bcl6Δ lymphomas, we purified LSK and B220+ cells and transplanted them into sub-lethally irradiated syngeneic recipient mice. Each of the mice transplanted with LSK cells developed a DLBCL that was phenotypically identical to the primary disease. In contrast, the B220+ cells were incapable of inducing lymphoma in secondary recipients, even when injected in a 10 or 100-fold higher number than the LSK cells (Supplementary Table 2), despite these cells being able to transplant disease in other models31. This indicates that Bcl6-induced DLBCL in this model is propagated by transformed HSPC cells but not mature tumor cells, and confirm that activity of the Bcl6 oncogene restricted to HSPCs can induce malignancies in mice that are of a post-germinal center stage of differentiation.
p53 loss in Sca1-Bcl6floxed mice does not promote lymphoma
Prior studies have shown that Bcl6 acts in myeloid leukemia stem cells and normal B-cell development by inactivation of p53 and subsequent sensing of DNA damage11,12. We therefore evaluated whether the inactivation of p53 and the accumulation of secondary genetic alterations play a role in this model by crossing Sca1-Bcl6floxed mice with heterozygous (p53+/−) or homozygous (p53−/−) p53 knock-out mice. This showed that decrease or loss of p53 did not facilitate B-cell lymphoma development, but instead resulted in myeloid neoplasia (Supplementary Fig. 11). B-cell lymphoma development in Sca1-Bcl6floxed mice therefore may not proceed via suppression of p53 and the accumulation of secondary genetic lesions as observed in myeloid malignancies. Exome sequencing of Sca1-Bcl6floxed tumors revealed the accumulation many somatic variants, but none that were recurrent across tumors or that had been implicated in lymphomagenesis. We also noted an absence of DNA copy number abnormalities that are associated with p53 deregulation in lymphoma32 (Supplementary Fig. 12, Supplementary Data 2). Together these data show that p53 dysfunction in combination with Bcl6 promotes myeloid malignancy, as previously described11.
Epigenetic changes associated with transient Bcl6 expression
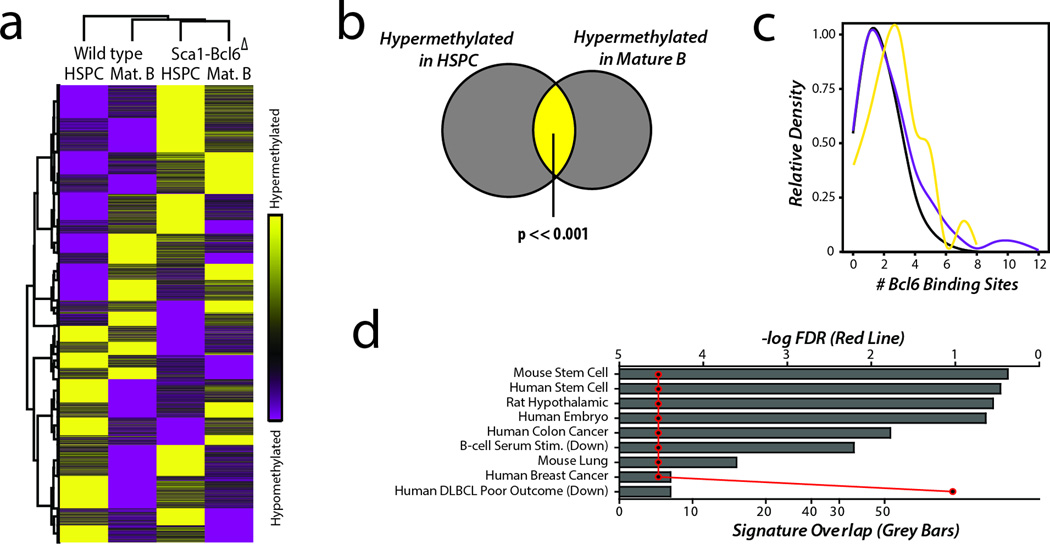
Bcl6 mediates suppression of target genes via recruitment of factors that epigenetically modify chromatin. We hypothesized that this may elicit changes in DNA methylation, a mark that is capable acting in gene silencing memory33, and therefore investigated whether this may be a potential mechanism for Bcl6-mediated hit-and-run oncogenesis. Using genome-wide DNA methylation profiling by reduced representation bisulfite sequencing (RRBS) of populations of HSPCs and mature B-cells from wild type and Sca1-Bcl6Δ mice, we identified broad epigenetic changes associated with the expression of Bcl6 in HSPCs (Figure 7a, Supplementary Data 3). Importantly, a significant subset of these changes were found to be maintained from HSPCs to mature B-cells in Sca1-Bcl6Δ mice, resulting in these populations being epigenetically more similar to each other than to their comparative population from wild-type mice (Figure 7a–b). Genomic regions found to have significant changes in DNA methylation contained a significantly higher representation of Bcl6 DNA binding motifs compared to those regions that showed no change in DNA methylation (Figure 7c), but were not significantly enriched for genes found to be bound by Bcl6 using ChIP-seq analysis of mature human B-cells25 (Hypergeometric enrichment FDR = 0.990). This trend was particularly notable in genes found to be hypermethylated in Sca1-Bcl6Δ HSPCs and mature B-cells compared to their wild type counterparts. Intriguingly, concordantly hypermethylated genes in HSPCs and mature B-cells from Sca1-Bcl6Δ mice were significantly enriched for gene sets associated with murine and human stem cells and poor outcome in human DLBCL (Figure 7d; Supplementary Table 3). Although this analysis was not performed on post-GCB tumor B-cells, we suggest that these results implicate epigenetic alterations that are imparted during transient Bcl6 expression and maintained thereafter as a mechanism for Bcl6 hit-and-run oncogenesis.
Figure 7. Bcl6 expression in HPCs induced broad epigenetic changes.
a) Unsupervised hierarchical clustering of methylation ratio data from 11258 promoter regions revealed that HSPCs and mature Bcells (Mat. B) from Sca1-Bcl6Δ mice are epigenetically more similar to each other than to their respective counterparts from wild-type mice, again suggesting that Bcl6 acts via an epigenetic mechanism that persists from HSPCs to mature B-cells. Each condition represents a pool of biological replicates from 6–9 mice. b) HSPCs and mature B-cells from Sca1-Bcl6Δ mice show significant hypermethylation of a large number of genes compared to the same subsets from wild-type mice (Supplementary Data 3). These included a significant overlap of 470 genes, suggesting that Bcl6 creates an epigenetic signature in the HSPCs that is maintained through differentiation and can be found in the mature B-cell compartment. c) Kernel density plot of the number of BCL6 DNA binding sequence motifs identified in genomic regions with significant hypermehtyaltion (yellow) or hypomethylation (purple) in both HSPCs and mature Bcells from Sca1-Bcl6Δ compared to wild-type mice (P-value < 0.05) and in regions with no change in methylation (black) between Sca1-Bcl6Δ and wild-type mice (p>0.95). Hypermethylated regions showed a significant (P-value <0.001) increase in the abundance of BCL6 binding motifs compared to regions with no change in methylation. Hypomethlated regions also showed a significant, but less dramatic, increase in abundance of BCL6 binding motifs (P-value <0.001). P-values calculated by Mann Whitney U test. d) Hypergeometric gene set enrichment analysis of the list of genes that were hypermethylated in both HPCs and mature B-cells of Sca1-Bcl6Δ mice showed significantly enrichment (Hypergeometric enrichment FDR < 0.25) of multiple gene sets including those associated with murine and human stem cells, and with poor outcome in DLBCL. The number of overlapping genes between those with conserved hypermethylation and those present in listed genes sets are shown by grey bars (bottom X-axis), with the corresponding FDR for the hypergeometric enrichment shown by the red line (top X-axis).
Discussion
It is well established that cancer arises via the stepwise acquisition of somatic alterations that transition clones via one or more pre-malignant states to a malignant state34. But deconvolution of the stepwise events taking place during tumor cell evolution is difficult because of the many genetic alterations that become clonally dominant by the time of their interrogation within the clinically manifested tumor, and the multitude of avenues by which any given tumor can evolve. However, research within the cancer stem cell field has led to a growing appreciation for the potential of oncogenic events to be acquired by tumor cell precursors that exist at an earlier differentiation state than the evolved tumor clone16,35. While several prior studies have implicated aberrations within HSPCs as important for driving neoplasms of mature B-cells including CLL17 and FL13,36, there has not yet been any suggestion that a similar mechanism may be relevant for DLBCL.
By high-resolution genomic analysis of a large number of human DLBCL tumors, we identified DNA copy number gain of 3q27.2 as being important for DLBCL disease biology because of its significant association with adverse outcome and the aggressive ABC-like disease subtype. Like many SCNAs, the minimal region of significant copy number gain on 3q27.2 contained multiple genes. However, the propensity for the BCL6 oncogene to be targeted by other genetic alterations in DLBCL, such as point mutations and translocation that deregulate its expression24,6,37, and the mutual exclusivity of BCL6 translocation with its DNA copy number gain, strongly suggested that BCL6 was a target of this alteration. Integrative analysis of DNA copy number and transcriptome data further suggested that BCL6 may act in a hit-and-run fashion, wherein its transient over-expression is promoted by genetic alteration but is not maintained in the evolved tumor cell population.
The BCL6 gene encodes a POZ/Zinc finger transcriptional repressor that regulates gene expression in a manner that is independent of the location of its binding site, via interaction with co-repressors that recruit histone deacetylases and induce epigenetic remodeling and heterochromatin formation38. The best characterized role of BCL6 is within normal B-cell activation and germinal center formation, but its expression is not limited to the B-cell compartment and contributes to the normal function of other cellular populations including multiple T-cell subsets and macrophages22,39–42. Recently, a role for BCL6 has also been described within progenitor populations during normal hematopoiesis and in the function of cancer stem cells in myeloid leukemia11,12,26. Laurenti et al. identified BCL6 transcript expression in human cord blood HSPCs and found that extinguishing this expression resulted in decreased numbers of mature B-cells, suggesting that Bcl6 expression in HSPCs has a critical role in early B-cell development26. Our observation of BCL6 expression in a subset of adult human bone marrow HSPCs, and the associated coordinate repression of BCL6 target genes within these cells, adds to growing evidence for a role of BCL6 in normal early human hematopoiesis. It is therefore plausible that genetic deregulation of BCL6 within these cells by DNA copy number gain or translocation could potentially contribute to the pathogenesis of diseases like leukemia and lymphoma. The oncogenic function of BCL6 may also potentially act in the germinal center, with repression occurring thereafter as a result of processes that occur during normal B-cell differentiation. However, transgenic mice expressing Bcl6 in mature B-cells under the control of the immunoglobulin promoter required repeated immunization in order to induce lymphoma43. In contrast, immunization was not required to induce lymphoma in Sca1-Bcl6floxed or Sca1-Bcl6Δ mice that expressed Bcl6 transiently within HSPCs, suggesting that the lymphomagenic potential of Bcl6 may be stronger within this compartment.
As patterns of BCL6 expression are tightly conserved between humans and mice27, we modeled this hypothesis by expression of BCL6 transiently within murine HSPCs. The lymphomas that developed in these mice were histologically similar to human DLBCL and transcriptionally similar to the differentiation stage of human ABC-like tumors in which BCL6 SCNAs were identified. Also in line with our observations in human ABC-like DLBCL, the murine lymphomas did not show over-expression of Bcl6 or coordinate target gene repression, suggesting that Bcl6 was acting in a hit-and-run fashion. This was further confirmed by Cre-mediated deletion of the exogenous Bcl6 allele upon B-cell maturity, which did not alter the development or phenotype of these tumors. Because BCL6 can alter the activity of the p53 tumor suppressor gene44, and this is critical for leukemia stem cell survival in chronic myeloid leukemia12, we investigated whether the lymphomagenic potential of transient Bcl6 expression was via repression of p53 and the accumulation of secondary genetic alterations. Crossing of Sca1-Bcl6floxed mice with p53+/− and p53−/− mice did not accelerate the development of DLBCL, but instead promoted myeloid malignancies. These myeloid malignancies may have masked the development of lymphoid malignancies that have longer latency periods, but shows that combined deregulation of Bcl6 and p53 has a relatively more profound role in myeloid compared to lymphoid tumorigenesis. Furthermore, lymphomas from Sca1-Bcl6floxed mice did not show patterns of DNA copy number change or somatic mutation that are associated with p53 malfunction in lymphoma32, indicating that BCL6 may be acting hit-and-run oncogenesis within this model in a manner that is not promoted by p53 dysfunction. While the exact mechanism by which transient Bcl6 expression promotes oncogenesis remains to be defined, we found some evidence that suggests Bcl6 may function by inducing epigenetic changes that were conserved from HSPCs to mature B-cells in Sca1-Bcl6Δ mice. In addition, the reduced polyclonality of B-cell lymphomas from these mice suggested that there may be additional genetic, epigenetic or microenvironmental factors following VDJ recombination that confer a growth advantage to some clones.
Our observations in the mouse model described here are potentially relevant to human disease, providing some evidence for hit-and-run oncogenesis in ABC-like DLBCLs that could be linked to BCL6. Several lines of evidence support such a linkage. First, genetic aberrations targeting BCL6 in human DLBCL do not result in its significant over-expression or coordinate repression of its target genes. Second, we observed that genes with conserved Bcl6-induced hypermethylation between HSPC and mature B cells of Sca1-Bcl6Δ mice are significantly enriched for markers distinguishing human DLBCL patients with fatal/refractory disease from those that are cured. Third, correlation between DLBCL subtype and immunoglobulin heavy-chain isotype highlights an important paradox between the differentiation stage and the isotype of the B-cell receptor. Specifically, despite similar levels of AID expression10, ABC-like DLBCL do not exhibit evidence of productive heavy chain class-switching45,46 or ongoing somatic mutation47. This suggests that the mature activated B-cell phenotype of ABC-like DLBCL is disconnected from their less mature immunoglobulin genotypes. Finally, in patients presenting with relapsed/refractory disease, DLBCL subtypes exhibit differential sensitivity to salvage chemotherapy regimens as part of autologous stem and progenitor cell transplantation studies48, raising the hypothesis that differences in therapy might modulate BCL6 expression through epigenetic mechanisms. While our model of hit-and-run oncogenesis is not compatible with those genes that induce ‘oncogene-addiction’ (eg. MYC49), it may be compatible with other genes that are able to induce long lasting epigenetic changes. Whether a subset of human ABC-like DLBCL tumors have their roots in genetic aberrations arising in early HSPCs or in a more mature stem-like lymphoid subpopulation requires further study. Definitive staging of somatic genetic lesions is not obvious because tumors can exhibit phenotypes of one stage of development but contain translocations from prior stages. For example, while BCL6, MYC, BCL2, and BCL1 translocations are all found in mature B-cell lymphomas, these lesions are distinguished by hallmarks such as immunoglobulin recombination signal sequences and junctional additions at translocation breakpoints, suggesting their distinct derivation from early or late events during B-cell development44,50. Separately, evidence for lineage plasticity of mature lymphomas17,36 complicates inferences of cell-of-origin that are based on either gene expression profiles or genotypes alone. It is therefore unclear as to the specific stage of hematopoietic or B-cell differentiation prior to evolved DLBCL that BCL6 acts, and the hierarchy of genetic events contributing to this disease will only be definitively defined by isolating human hematopoietic and tumor cell precursors from patients and identifying the minimal set of genetic lesions they harbor.
Based upon our observations we propose a model wherein an oncogene may act in a hit-and-run fashion within early tumor cell precursors and is no longer required in the evolved tumor cell progeny. Evolved tumor cells may in turn become reliant on alternate survival pathways that are not present within their precursors. We propose that in human ABC-like DLBCL, genetic alterations of BCL6 may act in a hit-and-run fashion in early precursors, while evolved tumor cells develop reliance on alternative oncogenic mechanisms such as nuclear-factor κB and B-cell receptor signaling pathways51–52. This may provide some explanation for the lower sensitivity of mature ABC-like DLBCL cell-lines to BCL6 inhibition53, despite the high prevalence of BCL6 gene alterations, and the failure of some modern targeted therapies to clear tumor stem cells, despite being effective agents against evolved tumor cells. As a consequence, targeted treatment strategies may need to be altered to accommodate combinations of agents that target oncogenic pathways that are active at both the early and late stages of tumor development. Our findings therefore have important implications for understanding and therapeutically targeting tumor cells.
Methods
Integrative analysis of human DLBCL and normal B-cells
All human data was obtained from public databases and associated with informed consent obtained as part of the original source studies. DNA copy number data was obtained from 609 primary DLBCL tumor specimens23,32,54–56, and analyzed by GISTIC257. This represented all publicly-available high-resolution (>244,000 markers) at the time this study was undertaken. Survival annotations were available for 232 CHOP-treated patients23,32 and 196 R-CHOP treated patients32,54, as described previously. These groups of patients were analyzed separately due to the improved outcome of patients treated with Rituximab. BCL6 translocation status determined by fluorescence in situ hybridization in 58 tumors with matched gene expression microarray data24. Raw.cel files from Affymetrix U133 plus 2.0 gene expression microarray data of 163 tumors with matched DNA copy number data and/or BCL6 translocation status23,24,32, and for normal B-cell subsets and DLBCL tumors58, were RMA normalized using the ExpressionFileCreator module of GenePattern59, and batch corrected using ComBat60. Cell of origin (COO) subtype was classified using the Wright algorithm of 140 genes61. These subtypes aligned with prior classification23 for 125/163 cases, with the remaining discordances being transition between GCB and Unclassified or ABC and unclassified, but not GCB and ABC. As a sanity-check, COO was found to significantly stratify survival (Log-rank P-value <0.001, Supplementary Fig. 13). For BCL6 expression, the probe intensities of 5 probes specific for BCL6 were averaged (203140_at, 215990_s_at, 228758_at, 236439_at, 239249_at). Comparison of BCL6 expression between was performed using a 2-sided T-test. Gene set enrichment analysis (GSEA) was performed for Bcl6 target genes using a previously described set of ChIP-validated target genes25 and GSEA-P software62.
Single cell gene expression profiling of human HSPCs
Bone marrow from two healthy donors (1 male, 1 female) were obtained purchased from AllCells, LLC. (Emeryville, CA) and sorted on a FACS ARIA II instrument as PI−, Lin−, CD34+, CD38−. Single cells were sorted into Terasaki plates containing alternating rows of wells containing media or Milteny SuperAmp lysis buffer for a total yield of 96 single cells in lysate per donor; wells containing media were visualized under a microscope to provide visual confirmation that single cells were sorted into each well (Supplementary Fig. 2). For all 4 donors, bulk population samples were further sorted into Terasaki plates in additional groups (no further sort, HSC (CD90+, CD45RA−), MPP (CD90−, CD45RA−) and L-MPP (CD90−, CD45RA+)), containing 200–10,000 cells. All antibodies were obtained from BD Biosciences. The lysates were shipped to Miltenyi Biotec (Auburn, CA) and processed using their SuperAmp microarray service, where they amplified the lysates using their SuperAmp technology and hybridized probes made from amplified material onto SurePrint G3 Human Gene Expression 8×60K Microarrays (Aglient). Raw data were quantile normalized in R (Bioconductor preprocessCore library), and replicate microarray probes (representing the same gene) were log2 transformed and combined as the geometric mean. Single cells were classified as expressing BCL6 if their intensity value was 2 standard-deviations above the mean for that donor.
Generation of Bcl6 transgenic mouse strains
All animal work has been conducted according to relevant national and international guidelines and it has been approved by the Bioethics Committee of University of Salamanca and by the Bioethics Subcommittee of Consejo Superior de Investigaciones Cientificas (CSIC). The Bcl6 floxed vector was generated by inserting the mouse Bcl6–IRES-eGFP cassette flanked by loxP sites into the ClaI site of the pLy6 vector. The transgene fragment was excised from its vector by restriction digestion with NotI, purified and injected (2 ng/mL) into CBAxC57BL/6J fertilized eggs. Transgenic mice were identified by Southern blot analysis of tail snip DNA after EcoRI digestion, using Bcl6 cDNA to detect the transgene. Two independent transgenic lines were generated and analyzed. Sca1-Bcl6floxed mice were bred to mb1-Cre (Cd79atm1(cre)Reth) mice to generate Sca1-Bcl6Δ mice. Upon signs of disease, mice were sacrificed and subjected to standard necropsy procedures. All major organs were examined under the dissecting microscope. Tissue samples were taken from homogenous portions of the resected organ and fixed immediately after excision. Differences in Kaplan-Meier survival plots of transgenic and WT mice were analyzed using the log-rank (Mantel-Cox) test. To test the rearrangement in Sca1-Bcl6Δ mice different samples were analyzed by Southern blot analysis after EcoRI digestion and using Bcl6 cDNA as probe. PCR was also used for confirmation, with the following primers: ClaI-F2, 5'-TATAAATCTGGCTTGATCAGG-3' and ClaI-R2, 5'-CTGAGGAATTCATGTCTGCC-3'.
Generation of Bcl6 floxed p53−/− and Bcl6 floxed p53+/− mice
The heterozygous p53+/− mice63 have been described previously. Heterozygous p53+/− mice were bred to Bcl6 floxed mice to generate compound heterozygotes. F1 animals were crossed to obtain null p53−/− mice hemyizygous for Sca1-Bcl6floxed mice. Tumor phenotype was assessed in the first generation of p53 heterozygous and homozygous mice.
Flow cytometry
Nucleated cells were obtained from total mouse bone marrow (flushing from the long bones), peripheral blood, thymus, or spleen. In order to prepare cells for flow cytometry, contaminating red blood cells were lysed with RCLB lysis buffer and the remaining cells were then washed in PBS with 1% FCS. After staining, all cells were washed once in PBS with 1% FCS containing 2 mg/mL propidium iodide (PI) to allow dead cells to be excluded from both analyses and sorting procedures. The samples and the data were acquired in an AccuriC6 Flow Cytometer and analyzed using Flowjo software. Specific fluorescence of FITC, PE, PI and APC excited at 488 nm (0.4 W) and 633 nm (30 mW), respectively, as well as known forward and orthogonal light scattering properties of mouse cells were used to establish gates. Nonspecific antibody binding was suppressed by preincubation of cells with CD16/CD32 Fc-block solution (BD Biosciences). For each analysis, a total of at least 50,000 viable (PI−) cells were assessed.
The following antibodies were used for flow cytometry: anti-B220 (RA3-6B2), CD3 (145-2C11), CD4 (RM4-5, 1:500), CD8a (53-6.7, 1:500), CD11b/Mac1 (M1/70, 1:200), CD19 (1D3), CD49b (DX5), CD117/c- Kit (2B8, 1:200), CD127/IL-7Rα (A7R34, 1:50), CD135/Flt3 (A2F10.1), Flt3 (A2B10), Ly-6G/Gr1 (RB6-8C5), IgD (11-26c.2a), IgM (R6-60.2), Sca1/Ly-6A/E (E13-161.7, 1:50), CD21 (7G6), CD22 (Lyb-8.2)(Cy34.1), CD23 (B3B4), CD25 (PC61), CD48 (HM48-1), CD150 (TC15-12F12.2) and Ter119 (TER119) antibodies. Unspecific antibody binding was suppressed by preincubation with CD16/CD32 (2.4G2) Fc-block solution (PharMingen). The different hematopoietic progenitors and B cell stages were defined by flow cytometry as shown in Supplementary Figure 6. All antibodies were purchased from BD Biosciences. All antibodies were used at a 1:100 dilution unless otherwise indicated.
V(D)J recombination
Immunoglobulin rearrangements were amplified by PCR using the primers listed in Supplementary Table 449.. Cycling conditions consisted of an initial heat-activation at 95°C followed by 31–37 cycles of denaturation for 1m at 95°C, annealing for 1m at 65°C for heavy chains or 62°C for light chains, and elongation for 1m45s at 72°C. This was followed by a final elongation for 10m at 72°C. To determine the DNA sequences of individual V–(D)J rearrangements, the PCR fragments were isolated from the agarose gel and cloned into the pGEM-Teasy vector (Promega); the DNA inserts of at least ten clones corresponding to the same PCR fragment were then sequenced.
Immunohistochemistry (IHC)
Tissue samples were taken from homogenous portions of the resected organ by the pathologist and fixed immediately after excision. Samples of each organ were processed into paraffin, sectioned and examined histologically including routinely standard haematoxylin and eosin, and immunohistochemical techniques. Transgenic mice samples were sectioned, dewaxed, and heated in 10 mmol/L sodium citrate buffer for 30 min. Slides were incubated with primary antibodies. The antibodies used included: Bcl6 (N-3, Santa Cruz, 1:100); Pax5 (C-20, Santa Cruz, 1:125), CD21 (A-3, Santa Cruz, 1:125); IgG (A85-I, BD Biosciences, 1:20). Samples were centrally reviewed by a panel of pathologists and diagnosed using uniform criteria based on clinical, histological, immunophenotypical, and molecular characteristics. For comparative studies, age-matched mice were used.
Analysis of germinal-center formation
Sheep red blood cells (1–2 × 108 cells) were injected into the peritoneum of control, Sca1-Bcl6floxed mice and Sca1-Bcl6Δ mice. Ten days later, the spleens were analyzed by immunohistochemistry. The spleens of control, Sca1-Bcl6floxed mice and Sca1-Bcl6Δ mice were isolated, embedded in OCT compound (Sakura) and snap-frozen on dry ice. Cryosections of the spleen were stained with a FITC–anti-IgD antibody (1:100 dilution, BD Biosciences) and biotinylated PNA (1:100 dilution, clone B-1075, Vector Laboratories). FITC–anti-IgD was detected with an alkaline-phosphatase-coupled anti-FITC antibody (Roche), which was visualized by incubation with Fast Red (Sigma). Biotinylated PNA was detected with horseradish peroxidase-conjugated streptavidin (Zymed) followed by incubation with diaminobenzidine (DAB; Sigma).
Pro-B cell culture
Iscove's modified Dulbecco's medium supplemented with 50 µM β-mercaptoethanol, 1 mM L-glutamine, 2% heat-inactivated fetal calf serum and 0.03% (w/v) primatone RL (Sigma) was used for Pro-B cell-culture experiments. Pro-B cells isolated by MACS-sorting for B220+ (Milteny Biotec) from bone marrow of 2-week-old mice were cultured on -irradiated ST2 cells in IMDM medium containing IL-7 (R&D Systems). The cells were maintain in culture for 1 week and then collected for Southern blot experiments.
Gene expression microarray analysis of murine tumors
Tumor-bearing spleens were harvested from Sca1-Bcl6floxed and Sca1-Bcl6Δ mice and healthy spleens were harvests from control mice; cells were not sorted prior to RNA extraction for this analysis. Total RNA was isolated in two steps using TRIzol (Life Technologies) followed by Rneasy Mini-Kit (Qiagen) purification following the manufacturer’s RNA Clean-up protocol with the optional On-column DNase treatment. The integrity and the quality of the RNA were verified by electrophoresis and its concentration measured. Samples were analyzed using Affymetrix Mouse Genome 430 2.0 arrays. Data was normalized as described above, and data sets containing the WT and either the Sca1-Bcl6floxed or Sca1-Bcl6Δ samples were filtered separately by median absolute deviation to derive the most variably expressed 10,000 genes for each comparison for use in GSEA and differential gene expression analysis. Differential gene expression analysis was performed using the ComparativeMarkerSelection module of GenePattern59 with T-test statistic, and correcting for multiple hypothesis testing using 1000 permutations. Genes were deemed to be significantly differentially expressed with a False Discovery Rate (FDR) Q-value <0.25 and a fold change ≥1.25. Differentially expressed genes were tested for enrichment of genes associated with normal murine B-cell differentiation states. Gene expression signatures that are specifically up-regulated in pre/pro-B cells, transitional B-cells, follicular or marginal zone B-cells, germinal center B-cells, plasmablasts, and plasma cells were assessed for their overlap with that were up-regulated within tumor specimens using hypergeometric enrichment analysis29,30. The relative over-abundance of a specific differentiation state within a tumor sample compared to a wild-type spleen is therefore detected by significant enrichment of the signature that is definitive of that differentiation state.
DNA methylation profiling
EpiQuest library construction
EpiQuest libraries were prepared from 200–500 ng mouse genomic DNA obtained from primary cells (Sca1+Lin− cells from bone marrow, and B220+ cells and Gr1+Mac1+ from peripheral blood) purified from Sca1-Bcl6Δ mice and/or wild-type mice. DNA were pooled from 6–9 mice to provide one pooled replicate per condition, as performed previously65,66.The DNA was digested with 60 units of TaqI and 30 units of MspI (New England Biolabs) sequentially. Size-selected TaqI-MspI fragments (40–120 bp and 120–350 bp) were filled in and 3'-terminal-A extended, extracted with a DNA Clean & Concentrator kit (Zymo Research). Ligation to pre-annealed adapters containing 5'-methylcytosine instead of cytosine was performed using the Illumina DNA preparation kit and protocol. Purified, adaptor ligated fragments were bisulphite-treated using the EZ DNA Methylation-Direct Kit (Zymo Research). Preparative-scale PCR (18 cycles) was performed and purified PCR products were subjected to a final size selection on a 4% NuSieve 3:1 agarose gel. SYBR-green-stained gel slices containing adaptor-ligated fragments of 130–210 bp or 210–460 bp in size were excised. Library material was recovered from the gel using a Zymoclean Gel DNA Recovery Kit (Zymo Research) and sequenced on an GAIIx genome analyzer (Illumina), yielding between 43,930,708 and 60,139,994 total reads for each condition.
Sequence alignments and data analysis
Sequence reads from bisulphite-treated EpiQuest libraries were identified using standard Illumina base-calling software and then analyzed using a Zymo Research proprietary computational pipeline. Residual cytosines (Cs) in each read were first converted to thymines (Ts), with each such conversion noted for subsequent analysis. A reference sequence database was constructed from the 36-bp ends of each computationally predicted MspI-TaqI fragment in the 40–220-bp size range. All Cs in each fragment end were then converted to Ts (only the C-poor strands are sequenced in the RRBS process; The converted reads were aligned to the converted reference by finding all 12-bp perfect matches and then extending to both ends of the treated read, not allowing gaps (reverse complement alignments were not considered). The number of mismatches in the induced alignment was then counted between the unconverted read and reference, ignoring cases in which a T in the unconverted read is matched to a C in the unconverted reference. For a given read, the best alignment was kept if the second-best alignment had 2 more mismatches; otherwise the read was discarded as non-unique. The mean CpG coverages ranged between 5–11× and total number of unique mapped reads ranged between 3,378,764–10,295,957 for each condition. The methylation level of each sampled cytosine was estimated as the number of reads reporting a C, divided by the total number of reads reporting a C or T. A bioinformatics pipeline was used to score epigenetic alterations according to strength and significance, and links them to potentially affected genes. To that end, we collected a comprehensive set of regions of interest, which includes promoters, CpG islands and repetitive elements. For each of these regions, the number of methylated and unmethylated CpG observations is determined, and a p-value is assigned using Fisher's exact test. Once all p-values are calculated, multiple-testing correction is performed separately for each region type using the q-value method, which controls the false discovery rate to be below a user-specified threshold (typically 10%). The software pipeline is implemented in Python (alignment processing module) and R (statistical analysis module). Unsupervised clustering was performed on methylation ratio data from each condition using Kendall tau rank correlation.
Exome sequencing
Next generation sequencing libraries were prepared using NEBnext DNA sample prep kit (New England Biolabs) and exome capture performed using SureSelect XT Mouse All Exon (Agilent). Sequencing was performed using and Genome Analyzer II instrument (Illumina) with 75bp paired-end reads, and one sample per lane. Raw sequences were trimmed by 2bp at their 5’ ends to remove GC bias, and aligned to the murine genome (mm9) using Burrows-Wheeler Aligner (BWA) with default parameters67. Somatic mutations and DNA copy number alterations were called with reference to age- and gender-matched controls using VarScan 2.068. Somatic nucleotide variants were discarded if they; (i) did not have a significant p-value (P≤0.05) determined by VarScan 2.0, (ii) were not determined to be somatic alterations by VarScan 2.0, (iii) were present in ≥10% of reads within the control sample, (iv) were not observed in both strands during sequencing, and (v) were not in the coding region of a gene.
Bone marrow transplantation experiments
In order to determine the nature of the lymphomagenic cell, bone marrow transplantation experiments were performed. BM LSK or splenic B220+ cells were isolated and highly purified from either male Sca1-Bcl6Δ (C57BL/6 x CBA) or male wild-type mice (C57BL/6 x CBA,). Mice were 12 months old. The sorting purity of these cells was analyzed by FACS and determined to be over 98%. In each cohort these cells were injected intravenously into sublethally irradiated (4 Gy) secondary recipient 12-week old male syngenic mice (C57BL/6 x CBA). Diseased recipient mice were sacrificed and assessed for B-cell lymphoma development.
Bcl6 DNA binding sequence motif analysis
DNA sequences were obtained for genomic regions that were (i) significantly hypermethylated in both HPCs and mature B-cells from Sca1-Bcl6Δ mice compared to identical populations from wild-type mice, (ii) significantly hypomethylated in both HPCs and mature B-cells from Sca1-Bcl6Δ mice compared to identical populations from wild-type mice, and (iii) were not differentially methylated between Sca1-Bcl6Δ mice populations and those from wild-type mice (Supplementary Data 3). Genomic regions were searched for BCL6 DNA binding sequence motifs (TTTNNNGNNATNCTTT)69 using the CisFinder70 identify motifs function, with an FDR of 0.2 and a matching threshold of 0.75..
Supplementary Material
Acknowledgments
We are grateful to Dr. Meinrad Busslinger for continuous and generous help and ideas over the years with this project, Dr. Takeshi Tokuhisa for the mouse Bcl6 cDNA, Prof. Michael Reth for the mb1-cre mice, and Dr. E. Dzierzak for the Sca1 promoter. Research in ISG group is supported partially by FEDER and by MICINN (SAF2009-08803 and SAF2012-32810), Junta de Castilla y León (CSI13A08 and proyecto Biomedicina 2009-2010), MEC OncoBIO Consolider-Ingenio 2010 (Ref. CSD2007-0017), NIH grant (R01 CA109335-04A1) and by Group of Excellence Grant (GR15) from Junta de Castilla y Leon. MRG and AA are Special Fellows of the Leukemia and Lymphoma Society. Funding for single cell human HSPC studies was provided by NIH grant (U01HL099999) to RM and AA. AM is supported by NCI R01 CA104348, the Chemotherapy Foundation, the Sam Waxman Cancer Research Foundation, the G&P Foundation and is a Leukemia and Lymphoma Society Scholar. Research at C.C.´s lab is partially supported by FEDER, Fondo de Investigaciones Sanitarias (PI080164), Proyectos Intramurales Especiales (CSIC) and Junta de Castilla y León (SA060A09 and proyecto Biomedicina 2009–2010). AO research is supported by a grant from the Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo, Madrid, Spain (IISCIII-RTICC RD06/0020/0035-FEDER).
Footnotes
Authorship Contributions
MRG and CVD designed and conducted the majority of experiments, analyzed the data and wrote the manuscript. IRC, BD CLL, IGH, EAE, ECS, RJ, JAMC, FJGC, MBGC, SZ, YN, ISL, AM, RM and CC performed experiments and analyzed the data. BP generated Bcl6-floxed mice, and IGR characterized the Bcl6-floxed and p53 mutant mice. AO analyzed flow cytometry data. TF and OB prepared tissue sections and helped analyze mouse histopathology. SZ and YN performed immunohistochemical characterization of mouse tissues and YN provided expert classification of DLBCL tumors as a trained hematopathologist. JI and WCC provided primary human DLBCL tumor BCL6 genotypes and corresponding gene expression profiles. AAA and ISG conceived the project, designed research, analyzed data, prepared the manuscript, and contributed equally as senior authors in supervising the project. All authors commented upon and edited the final manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Alizadeh A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 2.Morin R, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasqualucci L, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaffer A, Young R, Staudt L. Pathogenesis of human B cell lymphomas. Ann. Rev. of Immunol. 2012;30:565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaganti S, et al. Involvement of BCL6 in chromosomal aberrations affecting band 3q27 in B-cell non-Hodgkin’s lymphoma. Genes. Chrom. Cancer. 1998;23:323–327. [PubMed] [Google Scholar]
- 6.Ye B, et al. Alterations of a zing finger-encoding gene, BCL-6, in diffuse large B-cell lymphoma. Science. 1993;262:747–750. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- 7.Bihui H, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat. Genet. 1997;16:161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- 8.Dent A, Shaffer A, Yu X, Allman D, Staudt L. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 9.Lossos I, et al. Expression of a single gene, BCL-6, stronly predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2001;98:945–951. doi: 10.1182/blood.v98.4.945. [DOI] [PubMed] [Google Scholar]
- 10.Lossos I, Akasaka T, Martinez-Climent J, Siebert R, Levy R. The BCL6 gene in B-cell lymphomas with 3q27 translocations is expressed mainly from the rearranged allele irrespective of the partner gene. Leukemia. 2003;17:1390–1397. doi: 10.1038/sj.leu.2402997. [DOI] [PubMed] [Google Scholar]
- 11.Duy C, et al. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature. 2011;473:384–388. doi: 10.1038/nature09883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurtz C, et al. BCL6-mediated repression of p53 is critical for leukemia stem cell survival in chronic myeloid leukemia. J. Exp. Med. 2011;208:2163–2174. doi: 10.1084/jem.20110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weigert O, Weinstock DM. The evolving contribution of hematopoietic progenitor cells to lymphomagenesis. Blood. 2012;120:2553–2561. doi: 10.1182/blood-2012-05-414995. [DOI] [PubMed] [Google Scholar]
- 14.Green M, et al. Hierarchy in somatic mutations arising during genomic evolution and progression of follicular lymphoma. Blood. 2013;121:1604–1611. doi: 10.1182/blood-2012-09-457283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alizadeh A, Majeti R. Surprise! HSC are aberrant in chronic lymphocytic leukemia. Cancer Cell. 2011;20:135–136. doi: 10.1016/j.ccr.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Jan M, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kikushige Y, et al. Self-renewing hematopoietic stem cell is the primary target in pathogenesis of human chronic lymphocytic leukemia. Cancer Cell. 2011;20:246–259. doi: 10.1016/j.ccr.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Welch J, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein B. Addiction to oncogenes – the achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 20.Zack T, et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 2013;45:1134–1140. doi: 10.1038/ng.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green M, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basso K, Dalla-Favera R. Roles of BCL6 in normal and transformed germinal center B-cells. Immunol. Rev. 2012;247:172–183. doi: 10.1111/j.1600-065X.2012.01112.x. [DOI] [PubMed] [Google Scholar]
- 23.Lenz G, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc. Natl. Acad. Sci. 2008;105:13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iqbal J, et al. Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia. 2007;21:2332–2343. doi: 10.1038/sj.leu.2404856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basso K, et al. Integrated biochemical and computational approach identifies BCL6 direct target genes controlling multiple pathways in normal germinal center B cells. Blood. 2010;115:975–984. doi: 10.1182/blood-2009-06-227017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurenti E, et al. The transcriptional architecture of early human hematopoiesis identifies multilevel control of lymphoid commitment. Nat. Immunol. 2013;14:756–763. doi: 10.1038/ni.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shay T, et al. Convervation and divergence in the transcriptional programs of the human and mouse immune systems. Proc. Natl. Acad. Sci. USA. 2013;110:2946–2951. doi: 10.1073/pnas.1222738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles C, Sanchez MJ, Sinclair A, Dzierzak E. Expression of the Ly-6E.1 (Sca-1) transgene in adult hematopoietic stem cells and the developing mouse embryo. Development. 1997;124:537–547. doi: 10.1242/dev.124.2.537. [DOI] [PubMed] [Google Scholar]
- 29.Green M, et al. Signatures of murine B-cell development implicate Yy1 as a regulator of the germinal center-specific program. Proc. Natl. Acad. Sci. USA. 2011;108:2873–2878. doi: 10.1073/pnas.1019537108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero-Camarero I, et al. Germinal center protein HGAL promotes lymphoid hyperplasia and amyloidosis via BCR-mediated Syk activation. Nat. Comm. 2013;4 doi: 10.1038/ncomms2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams J, Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones. Cancer Res. 2008;68:4018–4021. doi: 10.1158/0008-5472.CAN-07-6334. [DOI] [PubMed] [Google Scholar]
- 32.Monti S, et al. Integrative analysis reveals an outcome-associated and targetable pattern of p53 and cell cycle deregulation in diffuse large B cell lymphoma. Cancer Cell. 2012;22:359–372. doi: 10.1016/j.ccr.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raynal N, et al. DNA methylation does not stably lock gene expression but instead serves as a molecular mark for gene silencing memory. Cancer Res. 2012;72:1170–1181. doi: 10.1158/0008-5472.CAN-11-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowell P. The clonal evolution of tumor cell populations. Science. 1996;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 35.Huntlet B, Gilliand D. Leukaemia stem cells and the evolution of cáncer stem cell research. Nat. Rev. Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 36.Hart J, et al. Transmission of a follicular lymphoma by allogeneic bone marrow transplantation – evidence to support the existence of lymphoma progenitor cells. Br. J. Haematol. 2006;136:163–172. doi: 10.1111/j.1365-2141.2006.06398.x. [DOI] [PubMed] [Google Scholar]
- 37.Pasqualucci L, Migliazza A, Basso K, Houldsworth J, Chaganti R, Dalla-Favera R. Mutations of the BCL6 proto-oncogene disrupts its negative auto-regulation in diffuse large B-cell lymphoma. Blood. 2003;101:2914–2923. doi: 10.1182/blood-2002-11-3387. [DOI] [PubMed] [Google Scholar]
- 38.Dent A, Vanaswala F, Toney L. Regulation of gene expression by the poto-oncogene BCL-6. Crit. Rev. Oncol. Hematol. 2002;41:1–9. doi: 10.1016/s1040-8428(01)00164-0. [DOI] [PubMed] [Google Scholar]
- 39.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nurieva R, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1008. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichii H, et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat. Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 42.Toney L, et al. BCL-6 regulates chemokine gene transcription in macrophages. Nat. Immunol. 2000;1:214–220. doi: 10.1038/79749. [DOI] [PubMed] [Google Scholar]
- 43.Phan R, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-center B cells. Nature. 2004;432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 44.Cattoretti, et al. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphoma in mice. Cancer Cell. 2005;7:445–455. doi: 10.1016/j.ccr.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 45.Ruminy P, et al. The isotype of the BCR as a surrogate for the GCB and ABC molecular subtypes in diffuse large B-cell lymphoma. Leukemia. 2011;25:681–688. doi: 10.1038/leu.2010.302. [DOI] [PubMed] [Google Scholar]
- 46.Lenz G, et al. Aberrant immunoglobulin class switch recombination and switch translocations in activated B cell-like diffuse large B cell lymphoma. J. Exp. Med. 2007;204:633–643. doi: 10.1084/jem.20062041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lossos I, et al. Ongoing immunoglobulin somatic mutation in germinal center B cell-like but not in activated B cell-like diffuse large cell lymphomas. Proc. Natl. Acad. Sci. USA. 2000;97:10209–10213. doi: 10.1073/pnas.180316097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thieblemont C, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: A Bio-CORAL study. J. Clin. Oncol. 2011;29:4079–4087. doi: 10.1200/JCO.2011.35.4423. [DOI] [PubMed] [Google Scholar]
- 49.Jain M, et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 50.Tsai A, Lu H, Raghavan S, Muschen M, Hsieh C, Lieber M. Human chromosomal translocations at CpG sites and a theoretical basis for their lineage and stage specificity. Cell. 2008;135:1130–1142. doi: 10.1016/j.cell.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Compagno M, et al. Mutations of multiple genes cause deregulation of NF-kB in diffuse large B-cell lymphoma. Nature. 2009;459:717–720. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis R, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerchietti L, et al. A peptomimetic inhibitor of BCL6 with potent antilymphoma effects in vitro and in vivo. Blood. 2009;113:3397–3405. doi: 10.1182/blood-2008-07-168773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scandurra M, et al. Genomic lesions associated with a different clinical outcome in diffuse large B-Cell lymphoma treated with R-CHOP-21. Br. J. Haem. 2010;151:221–231. doi: 10.1111/j.1365-2141.2010.08326.x. [DOI] [PubMed] [Google Scholar]
- 55.Green M, et al. Integrative genomic profiling reveals conserved genetic mechanisms for tumorigenesis in common entities of non-Hodgkin’s lymphoma. Genes Chrom. Cancer. 2011;50:313–326. doi: 10.1002/gcc.20856. [DOI] [PubMed] [Google Scholar]
- 56.Kato M, et al. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459:712–716. doi: 10.1038/nature07969. [DOI] [PubMed] [Google Scholar]
- 57.Mermel C, Schumacher S, Hill B, Meyerson M, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12 doi: 10.1186/gb-2011-12-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brune V, et al. Origin and pathogenesis of nodular lymphocyte – predominant Hodgkin lymphoma as revealed by global gene expression analysis. J. Exp. Med. 2008;205:2251–2268. doi: 10.1084/jem.20080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov J. GenePattern 2.0. Nat. Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 60.Johnson W, Rabinovic A, Li C. Adjusting batch effects in microarray expression data using Empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 61.Wright G, Tan B, Rosenwald A, Hurt E, Wiestner A, Staudt L. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. USA. 2003;100:9991–9996. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov J. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics. 2007;23:3251–3253. doi: 10.1093/bioinformatics/btm369. [DOI] [PubMed] [Google Scholar]
- 63.Jacks T, et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 64.Kwon K, et al. Instructive role of the transcription factor E2A in early B lymphopoiesis and germinal center B cell development. Immunity. 2008;28:751–762. doi: 10.1016/j.immuni.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 65.Taylor K, et al. Ultradeep bisulfite sequencing analysis of DNA methylation patterns in multiple gene promoters by 454 sequencing. Cancer Res. 2007;67:8511–8518. doi: 10.1158/0008-5472.CAN-07-1016. [DOI] [PubMed] [Google Scholar]
- 66.Seisenberger S, et al. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol. Cell. 2012;48:849–862. doi: 10.1016/j.molcel.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koboldt D, et al. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baron B, et al. BCL6 encodes a sequence-specific DNA binding protein. Genes Chrom. Cancer. 2003;13:332–224. doi: 10.1002/gcc.2870130314. [DOI] [PubMed] [Google Scholar]
- 70.Sharov A, Ko M. Exhaustive search for over-represented DNA sequence motifs with CisFinder. DNA Res. 2009;16:261–273. doi: 10.1093/dnares/dsp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.