Abstract
There is an emerging consensus that people consuming large amounts of fish with selenium:mercury ratios below 1 may be at higher risk from mercury toxicity. As the relative amount of selenium increases compared to mercury, risk may be lowered, but it is unclear how much excess selenium is required. It would be useful if the selenium:mercury ratio was relatively consistent within a species, but this has not been the case in our studies of wild-caught fish. Since most people in developed countries and urban areas obtain their fish and other seafood commercially, we examined selenium:mercury molar ratios in commercial fish purchased in stores and fish markets in central New Jersey and Chicago. There was substantial interspecific and intraspecific variation in molar ratios. Across species the selenium:mercury molar ratio decreased with increasing mean mercury levels, but selenium variation also contributed to the ratio. Few samples had selenium:mercury molar ratios below 1, but there was a wide range in ratios, complicating the interpretation for use in risk management and communication. Before ratios can be used in risk management, more information is needed on mercury:selenium interactions and mutual bioavailability, and on the relationship between molar ratios and health outcomes. Further, people who are selenium deficient may be more at risk from mercury toxicity than others.
Keywords: Mercury, Selenium, Selenium:mercury molar ratios, Risk balancing
1. Introduction
Mercury is considered a global environmental problem because it is ubiquitous and undergoes biomethylation to methylmercury which in turn bioaccumulates and bioamplifies up the food chain. In aquatic food chains, the highest bioaccumulation of mercury generally occurs in fish-eating species, and in large-sized or older organisms (Sormo et al., 2011). All forms of mercury are toxic to probably all forms of life, but methylmercury has higher bioavailability from food and greater toxicity than elemental or inorganic species of mercury. The primary source of mercury exposure in humans is from fish consumption (Rice et al., 2000), and levels of methylmercury in some fish are high enough to cause toxic effects in the fish themselves and in top-level predators, including humans, who consume the fish (WHO, 1989). People who consume large amounts of such fish are at risk from chronic exposure to methylmercury (Grandjean et al., 1997; IOM, 2006; Gochfeld, 2003; Hites et al., 2004; Burger et al., 2007).
Effects from high methylmercury exposure include neurodevelopmental deficits (Steuerwald et al., 2000; NRC, 2000, Trasande et al., 2005), developmental and behavioral deficits in infants (JECFA, 2003; Stringari et al., 2008), and poorer cognitive test performance from fetal and childhood exposure (Oken et al., 2008; Freire et al., 2010). Methylmercury exposure in adults can counteract the cardioprotective effects of fish consumption (Rissanen et al., 2000; Guallar et al., 2002), promote development of cardiovascular disease (Choi et al., 2009; Roman et al., 2011), and result in neurological and locomotary deficits (Hightower and Moore, 2003; Zahir et al., 2005).
However, fish and seafood are an important source of protein and other nutrients (Brunner et al., 2009; NRC, 2000). Fish are not only a low-fat source of protein, but some species also contain high levels of omega-3 (n-3) polyunsaturated fatty acids (PUFAs) that are associated with positive pregnancy outcomes (Kris-Ethereton et al., 2002; Daviglus et al., 2002), better child cognitive test performances (Oken et al., 2008), lowered asthma rates in children (Hodge et al., 1996), and lower incidences of cardiovascular disease (Virtanen et al., 2008; Ramel et al., 2010). Some fish also contain high levels of selenium, an essential trace element that, among other functions, plays an antioxidant role and may confer some protection against mercury (Kaneko and Ralston, 2007; Ralston, 2009; Ralston and Raymond, 2010). Human, and particularly pre-natal, exposure to methylmercury can be lowered by reducing mercury in the environment (e.g. cutting emissions from coal-fired power plants), harvesting fish from low-mercury environments, or by modifying human fish consumption behavior. In the United States, many states have responded to high mercury levels in freshwater fish by issuing consumption advisories, and the U.S. Food and Drug Administration (U.S. FDA, 2001) has issued advisories for saltwater fish. EPA also issues guidance and warnings about high mercury levels in fish (U.S. FDA -EPA, 2004, 2005). However, advisories are often ignored or misunderstood (Burger, 2000). The FDA warnings about fish consumption may have resulted in decreased fish consumption, especially canned fish (Shimshack et al., 2007). However, commercial statistics indicate that fish species with high mercury levels actually make up only a small share of seafood consumption, at least in the United States (Groth, 2010).
Determining the toxicity of methylmercury to humans and other vertebrates is not always clearcut since a number of factors affect uptake, toxicokinetics, and toxicity, including co-occurrence with other metals and vitamins, nutritional status and probably genetic susceptibility (Haley, 2005; Beyrouty and Chan, 2006; Ralston, 2008; Borderias and Sanchez-Alonso, 2011). From the mid-1960s to the early 1980s some studies showed that selenium could protect against mercury toxicity (Pařizek and Ošťádalová, 1967; Lindh and Johansson, 1987), and also suggested that mercury might protect against selenium toxicity. Although most mercury toxicity has been attributed to binding to sulphur, mercury also binds to selenium with a high affinity.
Low levels of selenium are associated with increased coronary heart disease (Seppanen, 2004), while higher (but subtoxic) levels of selenium are associated with lower levels of nonfatal heart attacks (Mozaffarian, 2009). High maternal exposure to methylmercury in animals inhibits selenium-dependent enzyme activity in the brain while selenium supplementation is protective (Berry and Ralston, 2008). Sormo et al. (2011) have proposed that selenium moderates mercury toxicity in free-ranging fish (Sormo et al., 2011). Selenium and mercury interact in complex ways to influence egg hatchability and chick defects in ducks (Heinz et al., 2011).
Mercury acts on multiple endpoints. Mercury and methylmercury are irreversible selenoenzyme inhibitors that impair selenoprotein form and function (Watanabe et al., 1999; Carvalho et al., 2008). Therefore one proposed mechanisms of toxicity is whether binding to mercury produces a relative selenium-deficiency, resulting in inadequate synthesis of seleno-enzymes or inhibition of their activity (Ralston, 2008, 2009; Ralston et al., 2008). Selenoenzymes play an important role in antioxidant defenses, which may explain the oxidative damage attributable to methylmercury (Cabanero et al., 2007; Pinheiro et al., 2009; Ralston and Raymond, 2010). The toxicokinetics and toxicodynamics of the selenium and mercury interactions require extensive study as effects differ depending on the forms or species of selenium and of mercury (Dang and Wang, 2011; Khan and Wang, 2009), administration methods (Klimstra et al., 2011), and relationship among them (Falnoga et al., 2006; Farina et al., 2011). There is a limit to the protection of selenium on mercury toxicity, and selenium itself can be highly toxic (Klimstra et al., 2011).
Ralston and others have suggested that selenium:mercury molar ratios below 1:1 are hazardous and that as the ratio rises above 1 there is increasing protectiveness (Ralston, 2008), and that these ratios should be an important consideration for risk assessment (Raymond and Ralston, 2004, 2009; Peterson et al., 2009; Ralston and Raymond, 2010). Watanabe (2002) argued that the practical implications of the modifying effect of selenium on mercury toxicity are unclear because of the variability in toxicodynamics.
We have proposed (Burger and Gochfeld, 2012, Burger et al., 2012) that the great intraspecific variability in the molar ratio makes this metric less useful, than just focusing on methylmercury concentration itself. Future research may delineate how the ratio can be used, but sufficient data are not yet available to do so.
In this paper, we examine the variability in the selenium:mercury molar ratio in commercial fish collected from supermarkets and fish markets in central New Jersey and Chicago-area, Illinois. We were particularly interested in whether the intraspecific variation in the selenium:mercury ratio was sufficiently low to allow its use in a regulatory context, in the issuance of consumption advisories, and in risk management. To be useful, there should be little variation in selenium:mercury molar ratios within a species, allowing this information to influence advisories and consumer selection. Thus we are testing the hypothesis that there is great variability in the selenium:mercury molar ratio within commercial, saltwater fish, as we found for self-caught fish Burger and Gochfeld, 2012).
Although there are hundreds of papers on mercury levels in fish and other seafood, until recently there have been relatively few papers that also report selenium levels, and fewer still that report selenium:mercury molar ratios. This paper is part of a series to understand the variation in selenium:mercury molar ratios in freshwater fish (Burger, 2012) and marine fish (Burger and Gochfeld, 2012, Burger et al., 2012). This work has generally dealt with self-caught wild-caught fish, where we could determine the collection location, and the size of the fish. However in this paper we examined molar ratios in commercial fish (where neither fish size nor collecting location was known). Most people obtain their fish commercially, and this paper begins to address the utility of selenium:mercury molar ratios in commercial fish. There are large public health consequences of methylmercury exposure and toxicity, especially in the developing brain (Transande et al., 2005), and understanding the factors that might ameliorate some of these effects is therefore an important economic and public health concern.
2. Materials and methods
Fish samples were purchased from grocery stores and fish markets in central New Jersey in 2003-2004 (Burger et al., 2004; Burger and Gochfeld, 2005), and in Chicago, Illinois in 2004 (Burger and Gochfeld, 2006). In the former two cases, we collected samples of the most popular fish purchased in supermarkets (Burger et al., 2004). Fish samples were transported in coolers to the Environmental and Occupational Health Sciences Institute (EOHSI) of Rutgers University for element analysis. All fish were analyzed individually for total mercury and selenium. At EOHSI, a 2 g (wet weight) sample of skinless fish muscle was digested in ultrex ultrapure nitric acid in a microwave (MD 2000 CEM), using a digestion protocol of three stages of ten min each under 3.5, 7, and 10.6 kg/cm2 at 80X power. Digested samples were subsequently diluted in 100 ml deionized water. All laboratory equipment and containers were washed in 10% HNO3 solution and deionized water rinse prior to each use. Mercury was analyzed by the cold vapor technique using the Perkin Elmer FIMS-100 mercury analyzer, with an instrument detection level of 0.2 ng/g, and a matrix level of quantification of 0.002 ug/g. Selenium was analyzed by graphite furnace atomic absorption, with Zeeman correction. All concentrations are expressed in parts per million (ppm= μg/g) on a wet weight basis (1 μg Hg= 0.005 μmol; 1 μg Se= 0.013 μmols).
A DORM-2 Certified dogfish tissue was used as the calibration verification standard. Recoveries between 85-115 % were accepted to validate the calibration. All specimens were run in batches that included blanks, a standard calibration curve, two spiked specimens, and one duplicate. The accepted recoveries for spikes ranged from 85 % to 115%. Ten percent of samples were digested twice and analyzed as blind duplicates. Further methods can be obtained from Burger and Gochfeld (2011).
Mean selenium:mercury molar ratios were calculated from the average selenium and average mercury levels in each fish species (see Table 1). There were very few values below the highly sensitive method detection limits (MDL), and for calculations, non-detects were set at half the MDL. We calculated molar ratio by dividing the concentration (micrograms per gram) by the molecular weight. For each species we divided the mean selenium concentration (μg/g) by 78.96 and the mean mercury concentration (μg/g) by 200.59, and calculated the ratio (Se:Hg). We also calculated the ratio for each individual fish. The ratios reported here were calculated from total selenium and total mercury. Many studies have shown that most of the mercury in most fish tissues is methylmercury, and 90% is a reasonable approximation of this proportion, which does not vary by age of the fish (Cabanero et al., 2007; Scudder et al., 2009). However, since all forms of mercury may bind to all forms of selenium, the total molar ratio is probably most informative. We note that some papers report the mercury:selenium molar ratio which is the reciprocal of the selenium:mercury ratio, but there is a preponderance of papers using selenium:mercury, and for our emphasis on selenium variability, this provides more interpretable graphs.
Table 1.
Total mercury and selenium levels (ppm, wet weight)(ug/g) and molar ratios in fish species collected from fish markets and grocery stores in New Jersey and Chicago, Illinois. Given are arithmetic means ± SE, standard deviation and Kendall Tau correlation coefficients.
| Common Name | n | Mercury ug/g Mean ± SE |
Selenium ug/g Mean ± SE |
Hg nmol/g wet wt. |
Se nmol/g wet wt. |
Se:Hg Ratio (Means)a |
Se:Hg Ratio Correlation with Hg tau (p) |
Hg:Sebb |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| New Jersey Markets | ||||||||
| Yellow fin tuna | (49) | 0.65 ± 0.1 | 0.75 ± 0.05 | 3.22 | 9.44 | 2.93 | −0.7 (<0.0001) | 0.34 |
| Chilean Sea Bass | (7) | 0.38 ± 0.06 | 1.02 ± 0.12 | 1.87 | 12.89 | 6.90 | −0.7 (0.02) | 0.15 |
| Bluefish | (50) | 0.26 ± 0.02 | 0.51 ± 0.04 | 1.31 | 6.51 | 4.96 | −0.5 (<0.0001) | 0.20 |
| Red Snapper | (4) | 0.24 ± 0.01 | 0.91 ± 0.09 | 1.20 | 11.56 | 9.66 | −0.3 (NS) | 0.10 |
| Croaker | (14) | 0.14 ± 0.02 | 0.77 ± 0.11 | 0.72 | 9.79 | 13.64 | −0.6 (0.006) | 0.07 |
| Cod | (7) | 0.11 ± 0.01 | 0.70 ± 0.13 | 0.54 | 8.87 | 16.47 | −0.2 (NS) | 0.06 |
| Porgy | (16) | 0.10 ± 0.01 | 0.95 ± 0.11 | 0.47 | 11.97 | 25.27 | −0.6 (0.002) | 0.04 |
| Flounder | (54) | 0.05 ± 0.001 | 0.31 ± 0.03 | 0.23 | 3.94 | 17.18 | −0.5 (<0.0001) | 0.06 |
| Whiting | (16) | 0.04 ± 0.004 | 0.93 ± 0.14 | 0.17 | 11.73 | 67.21 | −0.3 (0.08) | 0.01 |
| Shrimp (small) | (12) | 0.02 ± 0.001 | 0.16 ± 0.03 | 0.07 | 2.08 | 27.78 | 0.0 (NS) | 0.04 |
| Scallops | (12) | 0.01 ± 0.001 | 0.05 ± 0.01 | 0.06 | 0.68 | 10.55 | −0.1 (NS) | 0.09 |
| Shrimp (large) | (12) | 0.01 ± 0.01 | 0.23 ± 0.03 | 0.05 | 2.89 | 57.92 | −0.2 (NS) | 0.02 |
|
| ||||||||
| Kruskal Wallis X2 (p) | 203 (<0.0001) | 145 (<0.0001) | 145 (<0.0001) | |||||
|
| ||||||||
| Chicago Markets | ||||||||
| Swordfish | (18) | 1.31 ± 0.19 | 0.63 ± 0.05 | 6.54 | 8.03 | 1.23 | −0.67 (<0.0001) | 0.81 |
| Orange Roughy | (19) | 0.57 ± 0.06 | 0.75 ± 0.04 | 2.84 | 9.46 | 3.33 | −0.68 (<0.0001) | 0.30 |
| Walleye Pollock | (18) | 0.51 ± 0.13 | 0.47 ± 0.03 | 2.53 | 5.95 | 2.35 | −0.74 (<0.0001) | 0.43 |
| Tuna Steak | (18) | 0.35 ± 0.06 | 0.82 ± 0.03 | 1.72 | 10.41 | 6.05 | −0.84 (<0.0001) | 0.17 |
| Canned Tuna (White) | (21) | 0.31 ± 0.03 | 0.83 ± 0.04 | 1.54 | 10.57 | 6.89 | −0.66 (<0.0001) | 0.15 |
| Grouper | (18) | 0.26 ± 0.06 | 0.59 ± 0.06 | 1.29 | 7.46 | 5.80 | −0.93 (<0.0001) | 0.17 |
| Canned Tuna (Light) | (19) | 0.10 ± 0.02 | 0.89 ± 0.05 | 0.49 | 11.32 | 22.96 | −0.84 (<0.0001) | 0.04 |
| Canned Tuna (Gourmet) | (18) | 0.06 ± 0.01 | 1.02 ± 0.05 | 0.30 | 12.89 | 42.96 | −0.83 (<0.0001) | 0.02 |
| Salmon | (18) | 0.03 ± 0.01 | 0.35 ± 0.03 | 0.15 | 4.45 | 28.86 | −0.62 (0.0004) | 0.03 |
|
| ||||||||
| Kruskal Wallis X2 (p) | 104 (<0.0001) | 100 (<0.0001) | 103 (<0.0001) | |||||
The Se/Hg molar ratios are calculated on unrounded mean Hg and Se values.
The correlations for Hg:Se ratio with mercury and length are the same as Se:Hg ratio correlations with mercury and length, only positive.
We used Kruskal-Wallis non-parametric one way analysis of variance to compare molar ratios among species, and Kendall Rank Correlation yielding a tau statistic to determine associations among variables. We used non-parametric statistics because they are more conservative, less sensitive to the shape of the distribution, and less likely to lead to a type I error.
3. Results
3.1. Differences among species in selenium:mercury ratios
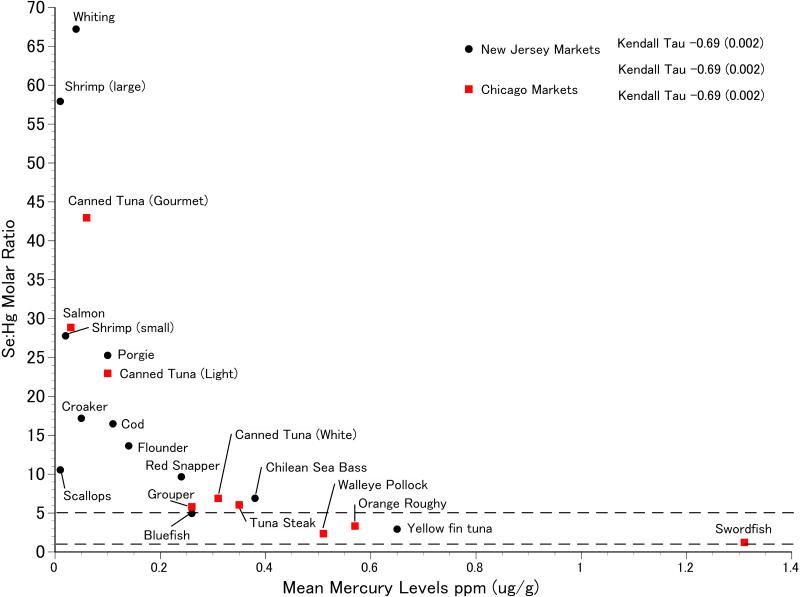
The mean selenium:mercury ratio (ratio of mean selenium to mean mercury) in commercial fish and other seafood varied greatly among fish species from 2.9 to 67 for New Jersey fish and 1.23 to 43 for the Chicago fish (Table 1). The mean selenium:mercury molar ratio was significantly and negatively correlated with mean mercury levels for all fish species (Fig. 1). Since many samples were obtained as fillets, we did not have fish length as in our previous studies.
Figure 1.
Mean selenium:mercury molar ratio as a function of mean mercury levels for commercial fish collected in New Jersey and Illinois.
3.2. Differences within species in selenium:mercury ratios
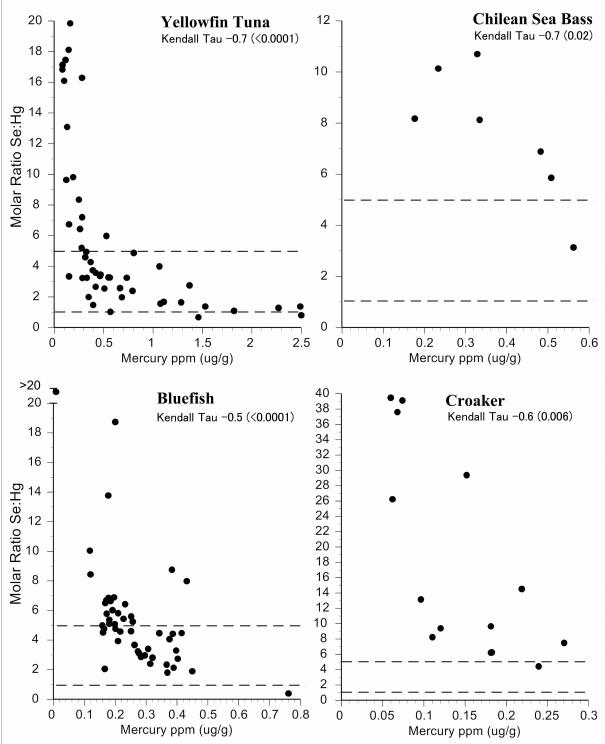
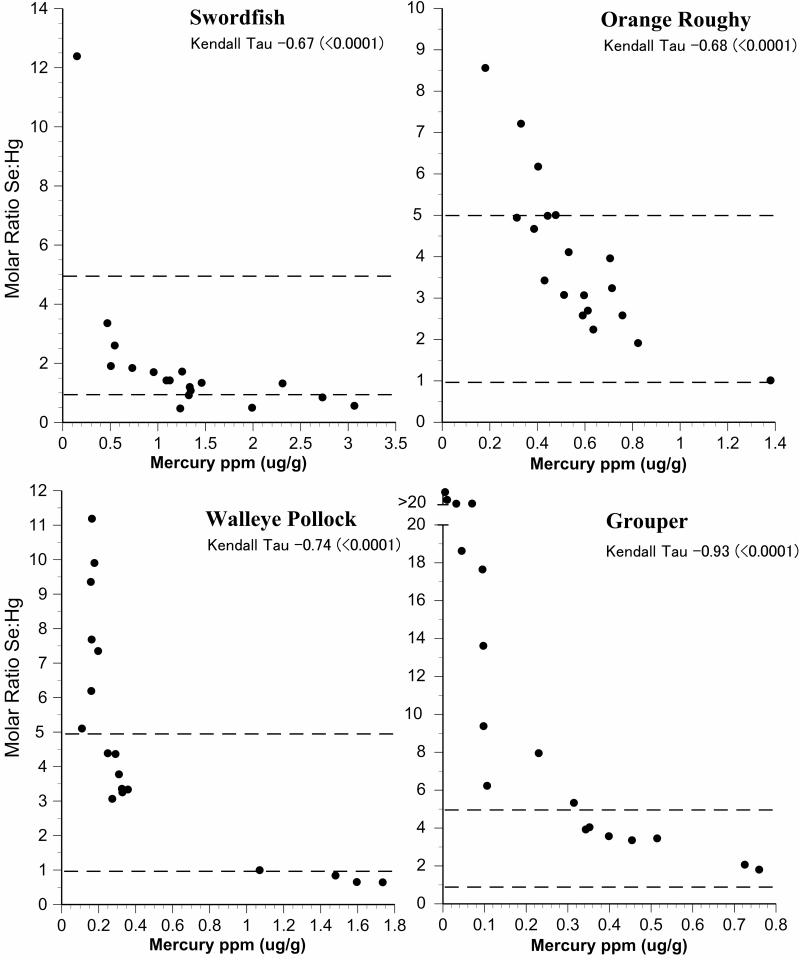
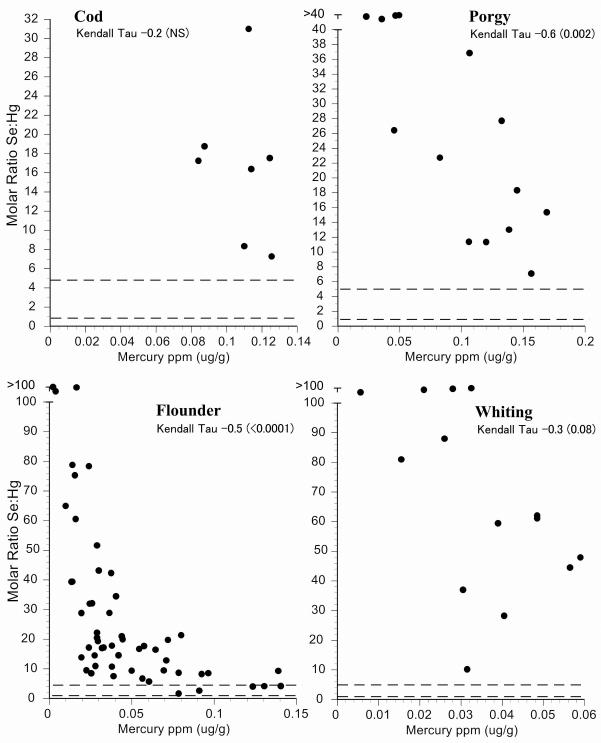
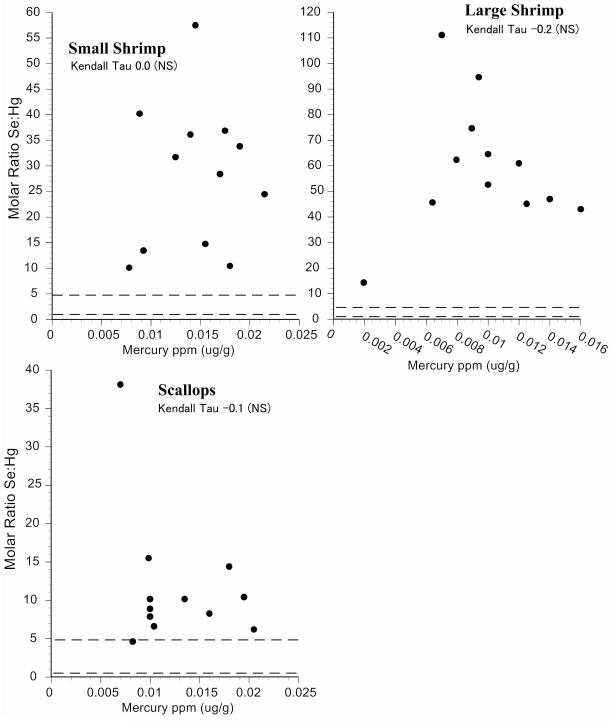
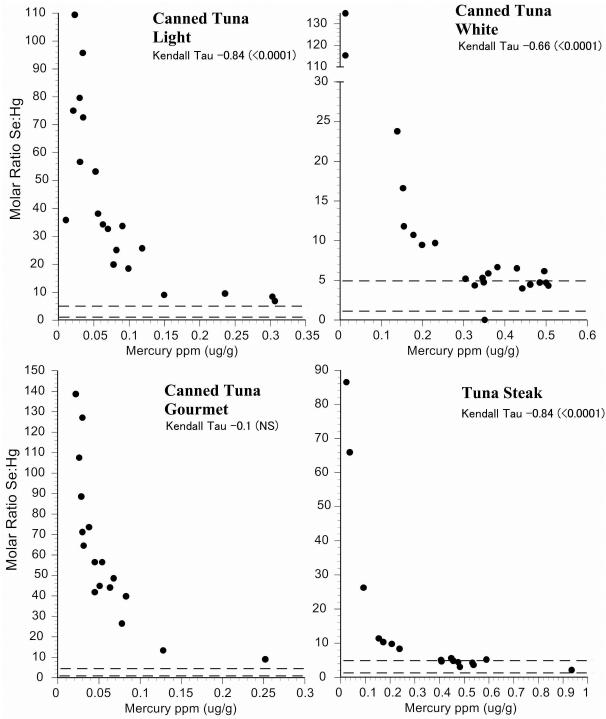
Intraspecific variation in selenium:mercury ratios provides some indication of the reliability of the mean selenium:mercury ratio for risk management and for consumer decisions. We examined individual variation in the selenium:mercury molar ratios within species by plotting them against mean mercury levels (Fig.2-6). On these graphs, the dotted horizontal lines correspond to molar ratios of 1:1 and 5:1. A ratio of 1:1 is a level below which mercury toxicity is likely to occur (Ralston et al., 2008). We make no assumptions about any level above which no toxicity is likely, and the ratio of 5:1 is shown only for convenience. It may well turn out that a higher level is required for relative protection against mercury toxicity in some organs or tissues (Lemire et al., 2010). All individual molar ratios are shown so that values are available for comparison with fish from elsewhere (both marine and freshwater).
Figure 2.
Relationship between selenium:mercury molar ratio and mercury levels for individual fish for yellow fin tuna, sea bass, bluefish, and croaker. Given are Kendall-Tao correlations. Dotted line indicates a molar ratio of 1 and 5.
Figure 6.
Relationship between selenium:mercury molar ratio and mercury levels for individual fish for swordfish, pollock, grouper, orange roughy. Given are Kendall-Tao correlations. Dotted line indicates a molar ratio of 1 and 5.
For New Jersey commercial fish species, most selenium:mercury molar ratios were between 1 and 5 (Figs. 2,3). Only four individual fish had ratios below 1. The molar ratios for shrimp and scallops were generally above 5 (Fig 4). The correlation between selenium:mercury molar ratios and mercury levels (shown on each figure) are not always significant. In some cases, there is no relationship between the molar ratio and mercury levels (e.g. shrimp, scallops, cod, whiting). For commercial fish collected in Chicago, only canned white tuna, swordfish, and walleye pollock had molar ratios below 1. (Figs. 5,6).
Figure 3.
Relationship between selenium:mercury molar ratio and mercury levels for individual fish for cod, porgy, flounder, and whiting. Given are Kendall-Tao correlations. Dotted line indicates a molar ratio of 1 and 5.
Figure 4.
Relationship between selenium:mercury molar ratio and mercury levels for individual fish for shrimp and scallops. Given are Kendall-Tao correlations. Dotted line indicates a molar ratio of 1 and 5.
Figure 5.
Relationship between selenium:mercury molar ratio and mercury levels for individual fish for canned tuna and tuna steak. Given are Kendall-Tao correlations. Dotted line indicates a molar ratio of 1 and 5.
4. Discussion
4.1. Species-specific differences in selenium:mercury molar ratios
There were substantial interspecific differences in the selenium:mercury molar ratios. This is consistent with our studies of wild-caught freshwater and marine fish (Burger, 2012; Burger and Gochfeld, 2012; Burger et al., 2012). Most commercial fish, as well as scallops and shrimp, are captured from marine or estuarine waters, and some commercial species in this study were also taken by anglers.
We expected that the mean selenium:mercury molar ratios would vary among species because of the known variation in mercury in fish. Selenium usually varies less because it is an essential trace element (i.e. a deficiency state has been identified), known to be regulated in the body, and is toxic at high levels (Eisler, 2000). Mercury levels are usually correlated with fish size (weight or length), both within and among species (Storelli et al., 2002). Further, predatory fish, particularly those that are large, old, and are at the top of the food chain tend to have the highest levels of mercury (Power et al., 2002). These factors predict that selenium:mercury ratios in species of fish that are large should be low, which is hypothesized to confer the least protective effect against mercury toxicity. Theoretically, consumers that understood the importance of molar ratios, and with knowledge of mercury and selenium levels, could then select fish that have a high selenium: molar ratio. However, whether this risk communication is clearer or more useful than knowing that eating large predatory fish increases risk from mercury, remains unknown (and is untested). Also it is not clear that a particular molar ratio confers the same level of benefit in different species of fish or at different levels of mercury, or against different endpoints.
In the present study, there was significant interspecific variation, and species with the highest mercury levels had the lowest selenium:mercury molar ratios (refer to Table 1 and Table 2). Although this seems tautological, it would be possible for selenium and mercury to be correlated within fish. Further, top level predators (swordfish, tuna, bluefish, walleye pollock, orange roughy) had mean selenium:mercury molar ratios below 5. Species with relatively high selenium:mercury ratios included porgy, whiting, light canned tuna, and salmon and shrimp.
The factors that contribute to a low selenium:mercury molar ratio are clear in some cases; tuna, bluefish and swordfish are large, top-level predators with high average mercury levels. The factors contributing to high selenium:mercury molar ratios are less predictable on the basis of mercury concentrations alone. For example, there were large differences in mean selenium:mercury molar ratios for fish known to have low mercury levels; flounder and whiting had very similar mercury levels, but very different selenium:mercury molar ratios, driven by differences in selenium levels (whiting had three times higher levels of selenium than flounder). The larger question is why there was variation in selenium levels. This requires further study of toxicokinetics in the fish themselves.
The selenium:mercury molar ratios in shrimp bear comment because the smallest shrimp had a lower ratio than did the larger shrimp. Fish generally have the opposite relationship in that within a species, the larger individuals have higher mercury levels, and lower selenium:mercury molar ratios (see next section). This may be an artifact of very low mercury levels, and relatively low selenium levels in shrimp.
4.2. Variations in selenium:mercury molar ratios within a species
All the papers that describe selenium:mercury molar ratios in fish do so using statistical measures, such as median, mean, standard deviation, and standard error for each fish species. This provides an overview of the relative values of these ratios, although a species may have a relatively high selenium:mercury molar ratio that sounds protective, with some individual fish having low ratios, even below 1. Further study may reveal that certain subsets of a species have consistent differences in ratio.
Our main objective in this paper was to examine variability within each species in the selenium:mercury ratios to consider whether these ratios in commonly-eaten, commercial fish and shellfish might be useful in communicating potential toxicity of mercury from fish consumption. Variation within most species of commercial fish was quite large, even for species where we had smaller sample sizes.
There are many reasons for the variability in selenium:mercury ratios within a species. Since the ratio is a function of both mercury and selenium levels, then the ratio should reflect differences in mercury levels (since selenium should have some regulation and therefore more consistent levels within a species) (Eisler, 2000). This aspect needs further examination, as few papers report selenium levels in fish, or examine variation within a species.
Other reasons for variation within a species include 1) mercury levels are not regulated; levels reflect bioaccumulation with age, and biomagnification up the food chain (Downs et al., 1998; Swanson et al., 2003); 2) mercury levels increase with age, while selenium levels do not (McIntyre and Beauchamp, 2007; Burger and Gochfeld, 2011), 3) differences in trophic level and foraging location (locally or geographically) affect mercury uptake (Power et al., 2002), 4) mercury levels in prey foods vary, even if the prey are at the same trophic level, due to feeding in different habitats (Snodgrass et al., 2000), and 5) fish have different migratory paths and time in residence in contaminated waters, which result in different mercury uptake and levels within a species (Burger, 2009). These same factors could influence selenium uptake as well, since selenium is readily absorbed from the gastrointestinal tract.
4.3. Selenium:mercury molar ratios and making risk decisions
Advice on healthy fish consumption choices is particularly important for women during their reproductive years (Abelsohn et al., 2011). Lemire et al., (2011) provided evidence that selenium may be neuroprotective in Amazon basin dwellers with high mercury levels. Whether selenium is a protective factor against developmental neurotoxicity of methylmercury would be important for women of child-bearing age (Choi et al., 2008). Popular articles and websites provide encouragement or caution regarding fish consumption during pregnancy. The relationships between fish consumption, fish oils, omega-3 fatty oils, selenium and other metals or vitamins, needs to be clearer for women making such choices (Kris-Etherton et al., 2002).
Selenium does bind to mercury and can confer some protection against mercury toxicity, while at the same time excess selenium compensates selenoenzyme synthesis for any selenium sequestered by mercury (Ralston, 2009). However, selenium toxicity from supplementation is a concern (MacFarquhar et al., 2010; Aldosary et al., 2012). Also, the amount of selenium required to protect against any particular concentration of mercury is unclear, and as this paper has shown, variation within species makes it difficult to use the mean selenium:mercury molar ratio as a measure of reduced mercury toxicity. While the mean molar ratio for all species was above 1, some individuals of six species/types had individuals with levels below 1, and many had a significant proportion of fish with ratios in the 1-5 range. There is no consensus as to how much selenium is not needed to reduce the risk of mercury toxicity, nor is it clears whether there is a linear relationship between the hypothesized protective ratio and toxicity. Moreover, a potential role for selenium supplements is an obvious area for study.
There are three problems with using molar ratios at present: 1) it is unclear what if any, molar ratio is protective for people or populations, 2) it is unclear whether the same ratio is protective for all organs or endpoints (e.g. liver, brain), and 3) sensitive populations (such as fetuses and neonates) may suffer ill effects from only one meal with high mercury (Ginsberg and Toal 2000) due to a peak exposure exceeding some threshold at a critical developmental window. Fish with different characteristics are not evenly distributed in time and space. Anglers often target the largest fish available and some legal size limits (i.e. for striped bass) force anglers to retain the largest fish, which can result in a several days of consumption of fish high in mercury, with a relatively low selenium:mercury ratio. People buying and selling fish often do not know where the fish were caught, or how large they were. The great variability in selenium and mercury concentrations complicates the process of developing fish advisories. Because of this variation, the advice to avoid fish high in mercury is still the most useful information for the public. Making available information on mercury, selenium and omega-3 fatty acid concentrations could also help consumers. Consuming fish is a matter of balancing several factors: health benefits versus harm from contaminants, red meat vs fish, depleting fish populations vs eating fish, availability and cost, pleasure and aesthetics of fishing, and personal preferences (Gochfeld and Burger, 2005; Burger et al., 2005; Conover et al., 2009; Stern and Korn, 2011).
4.4. Risk management, risk communication and the food industry
Understanding the potential positive effects of selenium on mercury toxicity has become an important issue with risk management, risk communication, and food policy. Public health messages about the benefits of fish consumption conflict with messages about dangerous effects of mercury and other contaminants in fish, which extend to state and federal policies and practices regarding consumption advice and advisories (Hughner et al., 2008). Advice is often stated as women of child bearing age should focus on species with high omega-3 content, while avoiding fish species with a high merthylmercury content (Zeilmaker et al., 2011), but that is oversimplified because a range of factors affect mercury toxicity. The U.S. FDA (2001, 2003, 2005) warnings about mercury have the potential to reduce fish consumption, and there is some indication that fish consumption has decreased (Shimshack et al., 2007). A clear framework for public health action about fish consumption seems appropriate for the general public (Frieden, 2010), and a wide range of scientists should be included in such a formulation, including health professionals, biologists, toxicologists, and food production scientists, as well as risk manager, public policy makers, and communications professionals.
For the most part, people consuming fish only once a week or less often, will not reach hazardous intake levels (such as the EPA’s Reference Dose of 0.1 μg/kg/day of methylmercury. People who consume fish almost daily are likely to exceed the Reference Dose. Hence, considerations of the potential ameliorating effects of selenium intake on mercury toxicity might be important. Even with the great variation among individuals of a species, there are still some generalizations: 1) fish high in mercury have lower selenium:mercury molar ratios than fish low in mercury, 2) as fish of a given species increase in size, the selenium:mercury molar ratio decreases as mercury increases, 3) larger fish species higher on the food chain tend to have lower molar ratios, and 4) because of the variation and the above generalizations, it is be prudent to vary fish species consumed, and choose from among the many fish species that are low in mercury (for example below 0.1 ppm of methylmercury). Further, it is possible that people who are selenium deficient themselves, might be more at risk from mercury toxicity while consuming fish than people who are not selenium deficient.
Further, before the food industry, health professionals, or risk managers communicate findings regarding mercury toxicity and selenium:mercury molar ratios, it is necessary to develop much more data on how mercury and selenium vary in different species of fish and how they interact. The U.S. FDA provides information only on mercury levels, and other web sites post both mercury and PUFA data, but no sites give selenium levels. We suggest that using selenium:mercury ratios at this time in risk assessment, risk management or risk communication is premature because recent evidence suggests that there is no apparent threshold for the adverse effects of methylmercury exposure (Groth, 2010), the ratio of selenium to mercury that is protective might vary among individuals and tissues (Lemire et al., 2010), and very little is known about either the blood brain barrier or the ratio in the brain that might be protective. The emphasis on understanding selenium-mercury interaction and molar ratios has been a valuable stimulus for research and discussion, and there is an urgent need for more data on how these molar ratios vary and on the protectiveness of different ratios for different organs and endpoints. Meanwhile individuals who seldom eat fish can be encouraged to eat fish, while individuals who eat fish frequently (more than twice a week) should be encouraged to choose fish low in mercury, particularly if they are pregnant. Finally, high end consumers, who consume fish daily, are not just statistical outliers, but are people at risk who should be admonished to choose low mercury fish and eat smaller portions.
Acknowledgements
We particularly thank A. Stern for his role in our initial study of mercury in commercial fish, C. Jeitner, M. Donio and T. Pittfield for field, laboratory and manuscript assistance, and Sam Roe of the Chicago Tribune for collecting the samples in his region. B. Buckley, P. Copeland, M. Lémire, K. Mahaffey, D. Mergler, N. Ralston, C. Safina, R. Schoeny, A. Stern, and H. Zarbl provided valuable discussion on mercury, selenium, and the selenium-mercury interactions. This research was partly supported by the Office of Science, Research and Technology of the New Jersey Department of Environmental Protection, the NIEHS Center grant (P30ES005022), the Consortium for Risk Evaluation with Stakeholder Participation (Departmnt of Energy, # DE-FC01-06EW07053), Chicago Tribune, and EOHSI. This study of commercial fish was exempt under Rutgers University Animal Use protocols. The views and conclusions expressed in this paper are solely those of the authors, and do not reflect the funding agencies.
References
- Abelsohn A, Vanderlinden LD, Scott F, Archbold JA, Brown TL. Healthy fish consumption and reduced mercury exposure. Can. Family Physic. 2011;57:26–30. [PMC free article] [PubMed] [Google Scholar]
- Aldosary BM, Sutter ME, Schwartz M, Morgan BW. Case series of selenium toxicity from a nutritional supplement. Clin. Toxicol. 2012;50:57–64. doi: 10.3109/15563650.2011.641560. [DOI] [PubMed] [Google Scholar]
- Berry MJ, Ralston NVC. Mercury toxicity and the mitigating role of selenium. EcoHealth. 2008;5:456–459. doi: 10.1007/s10393-008-0204-y. [DOI] [PubMed] [Google Scholar]
- Beyrouty P, Chan HM. Co-consumption of selenium and vitamin E altered the reproductive and developmental toxicity of methylmercury in rats. Nereurotox. Teratol. 2006;28:49–58. doi: 10.1016/j.ntt.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Borderias AJ, Sanchez-Alonso I. First processing steps and the quality of wild and farmed fish. J. Food Sci. 2011;76:81–85. doi: 10.1111/j.1750-3841.2010.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner EJ, Jones PJS, Friel S, Bartley M. Fish, human health and marine ecosystem health: policies in collision. Intl. J. Epidemiol. 2009;38:91–100. doi: 10.1093/ije/dyn157. [DOI] [PubMed] [Google Scholar]
- Burger J. Consumption advisories and compliance: the fishing public and the deamplification of risk. J. Environ. Plan. Manage. 2000;43:471–488. [Google Scholar]
- Burger J. Risk to consumers from mercury in bluefish (Pomatomus saltatrix) from New Jersey: size, season and geographical effects. Environ. Res. 2009;109:803–811. doi: 10.1016/j.envres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J. Selenium:mercury molar ratios in fish from the Savannah River: implications for risk management. J. Risk Res. 2012 DOI:10.1080/13669877.2011.64929 Available online: 09 Jan 2012. [Google Scholar]
- Burger J, Gochfeld M. Heavy metals in commercial fish in New Jersey. Environ. Res. 2005;99:403–412. doi: 10.1016/j.envres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Mercury in fish available in supermarkets in Illinois: are there regional differences. Sci. Total Environ. 2006;367:1010–1016. doi: 10.1016/j.scitotenv.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci. Total Environ. 2011;409:1418–1429. doi: 10.1016/j.scitotenv.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M. Selenium and mercury molar ratios in saltwater fish from New Jersey: individual and species variability complicate use in human health fish consumption advisories. Environ. Res. 2012 doi: 10.1016/j.envres.2012.02.004. ePub Mar 9 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Donio M, Pittfield T. Selenium:mercury molar ratios in freshwater fish from Tennessee: individual, species and geographical variations have implications for management. EcoHealth. 2012;9:171–182. doi: 10.1007/s10393-012-0761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Stern AH, Gochfeld M. Mercury in commercial fish: optimizing individual choices to reduce risk. Environ. Health Perspect. 2005;113:266–271. doi: 10.1289/ehp.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Stern AH, Dixon C, Jeitner C, Shukla S, Burke S, Gochfeld M. Fish availability in supermarkets & fish markets in New Jersey. Sci. Tot. Environ. 2004;333:89–97. doi: 10.1016/j.scitotenv.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Burke S, Stamm T, Snigaroff R, Snigaroff E, Patrick D, Weston J. Mercury levels and potential risk from subsistence foods from the Aleutians. Sci. Total Environ. 2007;384:93–105. doi: 10.1016/j.scitotenv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Cabanero AI, Madrid Y, Camara C. Mercury-selenium species ratio in representative fish samples and their bioaccessibility by an in vitro digestion method. Biol. Trace Elem. Res. 2007;119:195–211. doi: 10.1007/s12011-007-8007-5. [DOI] [PubMed] [Google Scholar]
- Choi AL, Budtz-Jorgensen E, Jorgensen PJ, Steuerwald U, Debes F, Weihe P, Grandjean P. Selenium as a potential protective factor against mercury developmental neurotoxicity. Environ. Res. 2008;107:45–52. doi: 10.1016/j.envres.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AL, Budtz-Jorgensen E, Jorgensen PJ, Salonen JT, Tuomainen T, Murata K, Nielsen HP, Petersen MS, Askham J, Grandjean P. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ. Health Perspect. 2009;117:367–372. doi: 10.1289/ehp.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover DO, Munch SB, Arnott SA. Reversal of evolutionary downsizing caused by selective harvest of large fish. Proc. R. Soc. 2009;276:2015–2020. doi: 10.1098/rspb.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang F, Wang W. Antagonistic interaction of mercury and selenium in a marine fish is dependent on their chemical species. Environ. Sci. Technol. 2011;45:3116–3122. doi: 10.1021/es103705a. [DOI] [PubMed] [Google Scholar]
- Daviglus M, Sheeshka J, Murkin E. Health benefits from eating fish. Comments Toxicol. 2002;8:345–374. [Google Scholar]
- Downs SG, Macleod CL, Lester JM. Mercury in precipitation and its relation to bioaccumulation in fish: a literature review. Water, Air, Soil Pollut. 1998;108:149–187. [Google Scholar]
- Eisler R. Handbook of chemical risk assessment: health hazards to humans, plants, and animals. Vol. 3. CRC Press; Boca Raton, FL: 2000. Selenium. [Google Scholar]
- Falnoga I, Tusek-Znidaric M, Stegnar P. The influence of long-term mercury exposure on selenium availability to tissues: an evaluation of data. BioMetals. 2006;19:283–294. doi: 10.1007/s10534-005-8642-2. [DOI] [PubMed] [Google Scholar]
- Farina M, Aschner M, Rocha JBT. Oxidative stress in MeHg-induced neurotoxicity. Toxi. Appl. Pharmacol. 2011;256:405–417. doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden TP. A framework for public health action: the health pyramid. Am. J. Public Health. 2010;100:590–596. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Ramos R, Lopez-Expinosa M, Diez S, Vioque J, Ballester F, Fernandez M. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ. Res. 2010;110:96–104. doi: 10.1016/j.envres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Ginsberg GL, Toal BF. Development of a single-meal fish consumption advisory for methyl mercury. Risk Anal. 2000;20:41–7. doi: 10.1111/0272-4332.00004. [DOI] [PubMed] [Google Scholar]
- Gochfeld M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol. Environ. Safety. 2003;56:174–179. doi: 10.1016/s0147-6513(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Gochfeld M, Burger J. Good fish/bad fish: a composite benefit-risk by dose curve. Neurotoxicol. 2005;26:511–520. doi: 10.1016/j.neuro.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorenson N, Jorgensen PJ. Cognitive deficit in 7-year old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 1997;19:418–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Groth G., III Ranking the contributions of commercial fish and shellfish varieties to mercury exposure in the United States: implications for risk communication. Environ. Res. 2010;110:226–236. doi: 10.1016/j.envres.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van't Veer P, Bode P, Aro A, Gomez-Aracena J. Heavy metals and Myocardial Infarction Study Group: Mercury, fish oils, and the risk of myocardial infarction. N. Engl. J. Med. 2002;347:1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- Haley BE. Mercury toxicity: genetic susceptibility and synergistic effects. BE Haley/Med Ver. 2005;2:535–542. [Google Scholar]
- Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA. Species differences in the sensitivity of avian embryos to methylmercury. Archives of Environmental Contamination and Toxicology. 2011;56:129–138. doi: 10.1007/s00244-008-9160-3. [DOI] [PubMed] [Google Scholar]
- Hightower JM, Moore D. Mercury levels in high-end consumers of fish. Environ. Health Perspec. 2003;111:604–608. doi: 10.1289/ehp.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ. Global assessment of organic contaminants in farmed salmon. Sci. 2004;303:226–229. doi: 10.1126/science.1091447. [DOI] [PubMed] [Google Scholar]
- Hodge L, Salome CM, Peat JF, Haby MM, Zuan W, Woolcock AJ. Consumption of oily fish and childhood asthma risk. Med. J. Austr. 1996;164:137–140. doi: 10.5694/j.1326-5377.1996.tb122010.x. [DOI] [PubMed] [Google Scholar]
- Hughner RS, Maher JK, Childs NM. Review of food policy and consumer issues of mercury in fish. J. Am. College Nutrit. 2008;27:185–194. doi: 10.1080/07315724.2008.10719690. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Seafood Choices: Balancing benefits and risks. National Academy Press; Washington, DC: 2006. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) Sixty-first meeting. 2003 ftp://ftp.fao.org/es/esn/jecfa/jecfa61sc.pdf [accessed 6 August 2012]
- Kaneko JJ, Ralston NV. Selenium and mercury in pelagic fish in the central north Pacific near Hawaii. Biol. Trace Element Res. 2007;119:242–254. doi: 10.1007/s12011-007-8004-8. [DOI] [PubMed] [Google Scholar]
- Khan MAK, Wang F. Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environ. Toxicol. Chem. 2009;28:1567–1577. doi: 10.1897/08-375.1. [DOI] [PubMed] [Google Scholar]
- Klimstra JD, Yee JL, Heinz GH, Hoffman DJ, Stebbins KR. Interactions between methylmercury and selenomethionine injected into Mallard eggs. Environ. Toxicol. Chem. publ. 2011;31:579–584. doi: 10.1002/etc.1708. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acides, and cardiovascular disease. Circulat. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- Lemire M, Fillion M, Frenette B, Mayer A, Philibert A, Passon CJS, Guimaraes JRD, Babose F, Jr., Mergler D. Selenium and mercury in the Brazalian Amazon: opposing influences on age-related cataracts. Environ. Health Perspect. 2010;10:1584–1589. doi: 10.1289/ehp.0901284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire M, Fillion M, Frenette B, Passos CJ, Guimarães JR, Barbosa F, Jr, Mergler D. Selenium from dietary sources and motor functions in the Brazilian Amazon. Neurotoxicol. 2011;32:944–953. doi: 10.1016/j.neuro.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Lindh U, Johansson E. Protective effects of selenium against mercury toxicity as syudied in the rat liver and kidney by nuclear analytical techniques. Biol. Trace Element Res. 1987;12:109–120. doi: 10.1007/BF02796669. [DOI] [PubMed] [Google Scholar]
- McIntyre JK, Beauchamp DA. Age and trophic position dominate bioaccumulation of mercury and organoichlorines in the food web of Lake Washington. Sci. Total Environ. 2007;372:571–584. doi: 10.1016/j.scitotenv.2006.10.035. [DOI] [PubMed] [Google Scholar]
- MacFarquhar JK, Broussard DL, Melstrom P, Hutchinson R, Wolkin A, Martin C, Burk RF, Dunn JR, Green AL, Hammond R, Schaffner W, Jones TF. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010;170:256–261. doi: 10.1001/archinternmed.2009.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. Int. J. Environ. Res. Pub. Health. 2009;6:1894–1916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (NRC) Toxicological effects of methylmercury. National Academy Press; Washington DC: 2000. [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, Hu H, Gillman MW. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am. J. Epidemiol. 2008;167:1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pařizek J, Ostádalová I. The protective effect of small amounts of selenite in sublimate intoxication. Experientia. 1967;23:142–143. doi: 10.1007/BF02135970. [DOI] [PubMed] [Google Scholar]
- Peterson SA, Ralston NVC, Whanger PD, Oldfield JE, Mosher WD. Selenium and mercury interactions with emphasis on fish tissue. Environ. Bioindicat. 2009;4:318–334. [Google Scholar]
- Pinheiro MCN, de Nascimento JLM, Silveira LCL, daRocha JBT, Aschner M. Mercury and selenium – a review on aspects related to the health of human populations in the Amazon. Environ. Bioindicat. 2009;4:222–245. doi: 10.1080/15555270903143440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power M, Klein GM, Guiguer RRA, Kwan MKH. Mercury accumulation in the fish community of a sub-Arctic lake in relation to trophic position and carbon sources. J. Appl. Ecol. 2002;39:819–890. [Google Scholar]
- Ralston NVC. Selenium health benefit values as seafood safety criteria. Eco-Health. 2008;5:442–455. doi: 10.1007/s10393-008-0202-0. [DOI] [PubMed] [Google Scholar]
- Ralston NVC. Introduction to 2nd issue on special topic: selenium and mercury as interactive environmental indicators. Environ. Bioindicat. 2009;4:286–290. [Google Scholar]
- Ralston NVC, Raymond LJ. Dietary selenium’s protective effects against methylmercury toxicity. Toxicol. 2010;278:112–123. doi: 10.1016/j.tox.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Ralston NVC, Ralston CR, Blackwell JL, III, Raymond LJ. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicol. 2008;29:802–811. doi: 10.1016/j.neuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Ramel A, Martinez JA, Kiely M, Bandarra NM, Thorsdottir I. Moderate consumption of fatty fish reduces diastolic blood pressure in overweight and obese European young adults during energy restriction. Nutrit. 2010;26:168–174. doi: 10.1016/j.nut.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Raymond LJ, Ralston NVC. Mercury:selenium interactions and health implications. SMDJ Seychelles Med. Dental J. 2004;17:72–77. doi: 10.1016/j.neuro.2020.09.020. [DOI] [PubMed] [Google Scholar]
- Raymond LJ, Ralston NVC. Selenium’s importance in regulatory issues regarding mercury. Fuel Proc. Technol. 2009;90:1333–1338. [Google Scholar]
- Rice G, Swartout J, Mahaffey K, Schoeny R. Derivation of U.S. EPS's oral Reference Dose (RfD) for methylmercury. Drug Chem. Toxicol. 2000;23:41–54. doi: 10.1081/dct-100100101. [DOI] [PubMed] [Google Scholar]
- Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT. Fish oil-derived fatty acids, docosahexaenoic acid and docosaphentaenoic acid, and the risk of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Circulat. 2000;102:2677–2679. doi: 10.1161/01.cir.102.22.2677. [DOI] [PubMed] [Google Scholar]
- Roman HA, Walsh TL, Coull BA, Dewailly É, Guallar E, Hattis D, Mariën K, Schwartz J, Stern AH, Virtanen JK, Rice G. Evaluation of the cardiovascular effects of methylmercury exposures: current evidence supports development of a dose-response function for regulatory benefits analysis. Environ. Health Perspect. 2011;119:607–614. doi: 10.1289/ehp.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder BC, Chaser LC, Wentz DA, Bauch NJ, Brigham ME, Moran PW, Krabbenhoft DP. U.S. Dept of Interior, Report 2009-5109. Reston, Virginia: 2009. Mercury in fish, bed sediment, and water from streams across the United States, 1998-2005; p. 74. [Google Scholar]
- Seppanen D, Soininen P, Salonen JT, Lotjonen S, Laatikainen R. Does mercury preomote lipid peroxidation? An in vitro study concerning mercury, copper, and iron in peroxidation of low-density lipoprotein. Biol. Trace Elem. Res. 2004;101:117–132. doi: 10.1385/BTER:101:2:117. [DOI] [PubMed] [Google Scholar]
- Shimshack JP, Ward MB, Beatty KM. Mercury advisories: information, education, and fish consumption. J. Environ. Econ. Manage. 2007;53:158–179. [Google Scholar]
- Snodgrass JW, Jagoe CH, Bryan AL, Jr., Burger J. Effects of trophic status, and wetland morphology, hydroperiod and water chemistry on mercury concentrations in fish. Can. J. Fish Aquat. Sci. 2000;57:171–180. [Google Scholar]
- Sormo EG, Ciesielski TM, Overjordet IB, Lierhagen S, Eggen GS, Berg T, Jenssen BM. Selenium moderates mercury toxicity in free-ranging fish. Environ, Sci. Technol. 2011;45:6561–6566. doi: 10.1021/es200478b. [DOI] [PubMed] [Google Scholar]
- Stern AH, Korn LR. An approach for quantitatively balancing methylmercury risk and omega-3 benefit in fish consumption advisories. Environ. Health Perspect. 2011;119:1043–1046. doi: 10.1289/ehp.1002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuerwald U, Weihe P, Jorgansen PJ, Bjerve K, Brock J, Heinzow B, Budtz-Jorgensen E, Grandjean P. Maternal seafood diet, methylmercury exposure, and neonatal neurological function. J. Pediatr. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- Storelli MM, Stuffler RG, Marcotrigiano GO. Total and methylmercury residues in tuna-fish from the Mediterranean Sea. Food Addit. Contam. 2002;19:715–720. doi: 10.1080/02652030210153569. [DOI] [PubMed] [Google Scholar]
- Stringari J, Nunes AKC, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JBT, Aschner M, Farina M. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol. Appl. Pharmacol. 2008;227:147–154. doi: 10.1016/j.taap.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson HK, Johnston TA, Leggett WC, Bodaly RA, Doucett RR, Cunjak RA. Trophic positions and mercury bioaccumulation in rainbow smelt (Osmerus mordax) and native forage fishes in northwestern Ontario Lakes. Ecosyst. 2003;6:289–299. [Google Scholar]
- Trasande L, Landrigan PJ, Schechter C. Public health and economic consequences of methylmercury toxicity to the developing brain. Environ. Health Perspect. 2005;113:590–596. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. FDA (U.S. Food and Drug Administration) FDA consumer advisory. U.S. Food and Drug Administration; Washington, DC: 2001. Available: http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. accessed 1 December 2001. [Google Scholar]
- U.S. FDA (U.S. Food and Drug Administration) FDA consumer advisory. U.S. Food and Drug Administration; Washington, DC: 2003. Available: http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. accessed 1 January 2004. [Google Scholar]
- U.S. FDA -EPA (U.S. Food and Drug Administration & Environmental Protection Agency) What You Need to Know About Mercury in Fish and Shellfish. 2004 http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm110591.htm [accessed 6-January 2012]
- U.S. FDA -EPA (U.S. Food and Drug Administration & Environmental Protection Agency) What You Need to Know About Mercury in Fish and Shellfish. 2005 http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm110591.htm [accessed 16-August 2012]
- Virtanen JK, Mozaffarian D, Chiuve SE, Rimm EB. Fish consumption and risk of major chronic disease in men. Am. J. Clin. Nutr. 2008;88:1618–1625. doi: 10.3945/ajcn.2007.25816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe C. Modification of mercury toxicity by selenium: practical importance. Tohoku J. Exp. Med. 2002;196:71–77. doi: 10.1620/tjem.196.71. [DOI] [PubMed] [Google Scholar]
- Watanabe C, Yoshida K, Kasanuma Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain. Environ. Res. 1999;80:208–214. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Mercury-environmental aspects. WHO; Geneva, Switzerland: 1989. [Google Scholar]
- Zahir F, Rizwi SJ, Haq SK, Khan RH. Low does mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005;20:351–361. doi: 10.1016/j.etap.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Zeilmaker MJ, Hoekstra J, van Eijkeren JCH, deLong N, Hart A, Kennedy M, Owen H, Gunnlaugsdottir H. Fish consumption during child-bearing age: a quantitative risk-benefit analysis on neurodevelopment. Food Chem. Toxicol. 2011 doi: 10.1016/j.fct.2011.10.068. epub ahead of print. [DOI] [PubMed] [Google Scholar]