Abstract
Rats emit aversive taste reactivity (TR) behavior (i.e., gapes) following intraoral delivery of a cocaine-paired taste cue, and greater conditioned aversive TR in well-trained rats predicts greater drug-taking. Here, we used a between-groups design and tracked the development of this conditioned aversive TR behavior on a trial by trial basis in an effort to determine when the change in behavior occurs and at what point individual differences in cue reactivity become predictive of cocaine-seeking and cocaine-taking. The results demonstrate that conditioned aversive TR to a cocaine-predictive flavor cue appears very early in training (i.e., following as few as 1 to 2 taste-drug pairings), stabilizes quickly, and becomes predictive of terminal self-administration within 3 to 4 trials. Indeed, rats exhibiting high conditioned aversive TR to the cocaine-paired cue also exhibited greater goal-directed behavior, were faster to take drug, self-administered more cocaine, and exhibited greater seeking during periods of drug non-availability. High conditioned aversive TR, then, develops quickly and is associated with a greater motivation for drug.
Rats avoid intake of an otherwise palatable saccharin solution when paired with a drug of abuse such as morphine or cocaine (Bechara & van der Kooy, 1985; Booth, Pilcher, D’Mello, & Stolerman, 1977; Cappell & LeBlanc, 1973; Ferrari, O’Connor & Riley 1991). This finding originally was interpreted as a conditioned taste aversion (CTA) like that induced by the aversive agent, LiCl (LeMagnen, 1970). Rats also avoid intake of a saccharin conditioned stimulus (CS) when paired with a highly preferred sucrose solution. This phenomenon is referred to as an anticipatory contrast effect because the value of the saccharin cue is thought to pale in comparison to the value of the highly preferred sucrose solution (Flaherty & Checke, 1982). In 1997, this juxtaposition led us to propose that rats may avoid intake of a palatable saccharin cue when paired with a drug of abuse because the saccharin cue is devalued in anticipation of the highly rewarding drug of abuse (Grigson, 1997). In support, avoidance of a drug-paired saccharin cue, like a sucrose-paired saccharin cue, is similarly affected by the deprivation state of the rat (Gomez & Grigson, 1999; Grigson, Cornelius & Wheeler, 2001), the strain of the rat (at least when using a cocaine unconditioned stimulus, US) (Flaherty, Grigson, Checke & Hnat, 1991; Glowa, Shaw & Riley, 1994; Grigson & Freet, 2000), a history of chronic morphine treatment (Grigson, Cornelius, & Wheeler, 2001), and lesions of either the gustatory thalamus (Grigson, Lyuboslavsky, & Tanase, 2000; Schroy, Wheeler, Davidson, Scalera, Twining, & Grigson, 2005) or the gustatory cortex (Geddes, Han, Baldwin, Norgren & Grigson, 2008). That said, the two phenomena (i.e., suppression by drugs and suppression by sweets) have been dissociated. Thus, lesions of the trigeminal orosensory area, located just lateral to the gustatory thalamus, eliminate avoidance of a morphine- or a cocaine-paired saccharin cue, but not a sucrose-induced anticipatory contrast effect or a LiCl-induced CTA (Liang, Grigson, & Norgren, 2012; Liang, Norgren, & Grigson, 2012; Nyland, Liang, Norgren, & Grigson, 2010).
These data suggest that, while drug- and sucrose-induced suppression of intake of the saccharin cue have much in common, ultimately, different substrates mediate the comparison of two disparate sweets and a sweet and a drug of abuse. This is not so surprising, as sweets are not interchangeable with drugs and drugs are not interchangeable with sweets. Additionally, with further study, it is now clear that avoidance of a drug-paired saccharin cue involves both reward and aversion, and the resultant complexion depends upon a multitude of factors including, for example, the nature of the drug under study, the dose of the drug, the individual, and the sex of the individual. In accord, avoidance of a drug-paired saccharin cue is dose dependent and, at least for cocaine, is greater for male, than for female, rats (Cason & Grigson, 2013). Individual differences also are marked. Some rats more greatly avoid intake of a drug-paired saccharin cue than others (Gomez, Leo, & Grigson, 2000; Grigson & Twining, 2002; Wise, Yokel, & DeWit, 1976) and greater avoidance is associated with higher levels of the stress hormone, corticosterone at test (Gomez, Leo, & Grigson, 2000). Avoidance also is associated with a blunted dopamine response in the nucleus accumbens to the otherwise highly palatable saccharin cue (Grigson & Hajnal, 2007; Wheeler et al., 2011). Finally, intraoral delivery of the drug-paired cue leads to the onset of frank aversive taste reactivity behavior (i.e., gapes) and greater aversive taste reactivity behavior, and greater avoidance of the drug-paired cue, predict greater cocaine self-administration and greater drug-seeking following an extended period of abstinence (Grigson & Twining, 2002; Twining, Bolan, & Grigson, 2009; Wheeler, Twining, Jones, Slater, Grigson, & Carelli, 2008).
It was, then, evident that greater conditioned avoidance/aversion toward the drug-paired cue is associated with greater drug-taking and drug-seeking behavior. What was unclear until recently, however, was how quickly this conditioned change in behavior occurs over time. Using a within subjects design, Colechio, Imperio and Grigson, (2014) examined taste reactivity behavior to a cocaine-paired flavor cue (referred to as the CS+) and, on alternate days, to a saline-paired flavor cue (referred to as the CS−). The results revealed the conditioned development of gapes following a single pairing of the Kool-Aid flavored saccharin CS+ with the opportunity to self-administer cocaine, for some rats. Other rats failed to exhibit conditioned gaping behavior despite repeated pairings of the CS+ with the opportunity to self-administer cocaine. Importantly, these early individual differences in conditioned taste reactivity behavior predicted later drug-taking. Thus, the number of gapes elicited upon the third CS+ trial predicted a shorter latency to take cocaine, greater load-up on cocaine at the start of a daily session, and greater cocaine self-administration at the end of testing (Colechio, Imperio, & Grigson, 2014). That said, likely because of the mechanics of the within-subjects design, goal-directed behavior (i.e., a clear increase in responding on the active vs. the inactive empty spout operant) was not evident in the high gaping/high drug-taking subjects and measures of the motivation for drug were either not significant or not tested (e.g., seeking during periods of timeout). The present study sought to address these limitations by measuring taste reactivity behavior following the intraoral delivery of a cocaine- or saline-paired cue on a trial by trial basis using a between groups design. Further, additional measures of the motivation for drug were included by assessing goal-directed (i.e., seeking) behavior emitted not only during drug self-administration, but also during the intraoral infusion period (i.e., when no drug was available). Persistence in responding, or seeking on the active spout also was assessed during each mandatory timeout (TO) period when the active spout was retracted for 20 s immediately following each iv drug infusion.
Method
The subjects were 32 male Sprague-Dawley rats obtained from Charles River. Rats weighed between 246–277 grams when received. They were housed individually in standard, metal cages in a temperature-controlled (21 °C) animal care facility with a 12:12 hour light:dark cycle (lights on at 7:00 am). All experimental manipulations were conducted during the light phase of the cycle. The rats were maintained with free access to dry Purina rodent diet 5001 at the beginning of the experiment and free access to water, except where noted otherwise below.
Surgery
Self-administration catheter
Intra-jugular catheters were custom-made in our laboratory and implanted as described by Twining et al. (2009). General maintenance of catheter patency involved daily examination and flushing of catheters with heparinized saline (0.2 ml of 30 IU/ml heparin). Catheter patency was verified as needed using 0.2 ml of propofol (Diprivan 1%) administered intravenously.
Intraoral cannulae
Intraoral cannulae were custom-made in our laboratory as described by Colechio, Imperio, and Grigson (2014). PE 100 tubing was cut into 8-cm segments, flared at one end, and fitted with a nylon washer. The tubing was inserted into the cheek lateral to the first maxillary molar with the nylon washer (Product Components Corp.) flush against the cheek. The cannula was then secured at the excision point on the top of the head with a PTFE washer, VetBond, and superglue. Cannulae were maintained by daily inspection and flushing with distilled water.
Rats weighed 287–468 g on the day of surgery. They were anesthetized with intramuscular (i/m) ketamine (70 mg/kg) and xylazine (14 mg/kg). Each rat received 20,000 units of GPenicillin (subcutaneous, s/c) at the beginning of the procedure, and 5 mL saline (s/c) when it was complete. For each rat, a catheter was implanted in the right external jugular vein and intraoral cannulae were implanted bilaterally. When righting reflexes had returned, rats were returned to their home cages with mash and solid chow ad lib. Rats recovered for 14–20 days before habituation began. During this time, solid chow and water were available ad lib, and soft chow and s/c fluids (saline) were provided as needed. All rats returned to regular chow and achieved a stable body weight before the experiment began. Catheters and cannulae were flushed once (recovery and habituation) or twice (self-administration) daily beginning three days after implantation.
Apparatus
Each rat was trained in one of four identical operant chambers (MED Associates, St. Albans, VT). Each chamber measured 29.3 cm in length × 24.0 cm in width × 27.0 cm in height, and was individually housed in a light- and sound-attenuated cubicle. The front and back walls and the floor were clear Plexiglas. The side walls were made of aluminum. Each chamber was equipped with three retractable sipper spouts that entered through 1.3-cm diameter holes, spaced 16.4 cm apart (center to center). A cue light was located 6.0 cm above each spout. Each chamber was also equipped with a houselight (25 W), a tone generator (Sonalert Time Generator, 2900 Hz, Mallory, Indianapolis, IN), and a speaker for white noise (75 dB). Cocaine (or saline) reinforcement was controlled by an infrared motion detector circuit that monitored nosepokes to operate a syringe pump (Model PHM-100VS, Razel Scientific Instruments, Stamford, CT). A coupling assembly attached the syringe pump to the catheter assembly on the back of each rat and entered through a 5.0-cm diameter hole in the top of the chamber. This assembly consisted of a metal spring attached to a metal spacer with Tygon tubing inserted down the center, protecting passage of the tubing from rat interference. The tubing was attached to a 5-channel counterbalanced swivel assembly (Instech, Plymouth Meeting, PA) that, in turn, was attached to the syringe pump. A similar metal spring protected the Tygon tubing that connected the intraoral cannula to the swivel. An angled mirror was located below the floor, allowing for a view of the ventral surface of the rat. Video was collected via a camera (Basler) aimed at the mirror to record the orofacial responses that followed the intraoral infusion of the gustatory stimuli. Lighting for video was provided by two fluorescent lights (each 8W) located below the chamber, and a green light panel (CleverSys, Inc.) that served as the chamber’s ceiling. Events in the chamber and collection of data were controlled on-line with a Pentium computer that used programs written in the Medstate notation language (MED Associates).
Procedure
On the day before the first habituation session, ad lib water was removed at 16:00. Habituation sessions began two hours into the light phase. For the first three days the rats were given five min access to water in the operant chambers; each day water bottle placement changed (spout1, spout2, spout3). On the fourth day, rats received 10 min of intraoral (IO) dH2O infusions with the same parameters as would be used for infusion of the tastant during self-administration trials (each 0.2-ml infusion was 3.5 sec in duration and one infusion was delivered per minute). Each day rats were given overnight access to 20 mL dH2O at the front of the home cage, beginning about 75 min after returning to the home cage from the operant chamber.
Self-administration
Self-administration (SA) sessions consisted of a 30-min IO infusion period during which infusions of the tastant (CS, 0.2 mL grape-flavored Kool-Aid in 0.15% saccharin) were delivered at a rate of 1/min, followed by 120 min to self-administer cocaine (0.33 mg/infusion) or saline (0.2 mL/infusion). During the IO infusion period, the chamber was lit by the fluorescent lights and green panel lights, and the house light was illuminated. The beginning of SA opportunity was signaled by turning off the fluorescent lights and houselight, extending the middle (inactive) and right (active) empty spouts, and illuminating a cue light over the active (right) spout. The operant response was nose-pokes to break an infrared beam in front of the spouts. The operant schedule was as follows: two daily trials at FR1, eight daily trials at FR5, and six daily trials at FR10. Upon completion of the FR requirement, the i.v. infusions were administered, accompanied by retraction of the spouts, a tone (2.9 kHz, 70 dB), and illumination of the houselight/offset of the cue light (Backes & Hemby, 2008; Carelli, 2000; Kruzich, Grimm, Rustay, Parks & See, 1999; Nicola & Deadwyler, 2000; Schenk & Partridge, 2001; Sun & Rebec, 2003, 2005). Each infusion was followed by a 20-sec timeout period, during which contacts were recorded but did not count toward earning the next infusion. At the end of the 20-sec timeout period, the cue light was turned on, house light was turned off, the tone was turned off, and the middle (inactive) and right (active) empty spouts advanced.
Video collection and analysis
Video was recorded during IO infusion periods throughout the experiment. The video was collected at 100 frames/sec as a CVI file, which later was compressed to AVI format. Gapes were manually scored after the conclusion of the experiment. The reviewer was blind to the treatment condition (saline or cocaine access) of the rat.
Data Analysis
Dependent measures included: the number of gapes emitted to the CS, the latency to the first response on the active and the inactive spout, the number of responses emitted on the active and inactive spout, the latency to the first infusion, the number of load-up infusions, and the total number of infusions/session. A log10 transformation was used for the latency data in order to minimize the impact of a few extreme scores. Goal-directed behavior was defined as the difference between the number of responses at the active and inactive spouts, calculated for each rat on individual trials. Thereafter, the data were analyzed using 3 × 16 mixed factorial analysis of variance (ANOVAs) varying group (saline vs. high vs. low gapers) and Trial (1–16). In one case (i.e., self-administration behavior), the two-way ANOVA did not attain statistical significance. In this instance, planned one-way ANOVAs were conducted as a function of group on a trial by trial basis. Significant ANOVAs were followed up with post hoc Student Newman-Keuls (SNK) tests with p < 0.05. Statistical procedures were performed in Statistica7 (StatSoft) and SPSS 20 (IBM). Graphs have been prepared in Origin7.0 Pro.
Results
The final number of rats included in the analyses was 16 from replication 1 and 15 from replication 2, for a total of 31 (24 cocaine access, 7 saline access).
Taste Reactivity (TR)
As shown in Figure 1, panel A, rats who had access to cocaine were divided into two groups, High Gapers (n = 7) and Low Gapers (n = 17), based upon the number of gapes emitted/30 min during terminal Trials 15 and 16. These subjects fell into distinct groups, with High Gapers showing significantly more aversive TR during the last two taste-drug pairings than Low Gapers (116.43 ± 47.11 vs. 3.64 ± 1.04 gapes per session), as indicated by significant post-hoc assessment of a significant main effect of group, F(2, 28) = 10.53, p < 0.001). The Saline Controls (not shown in A) gaped an average of 2.48 ± 0.73 times per session, p < 0.001).
Figure 1.
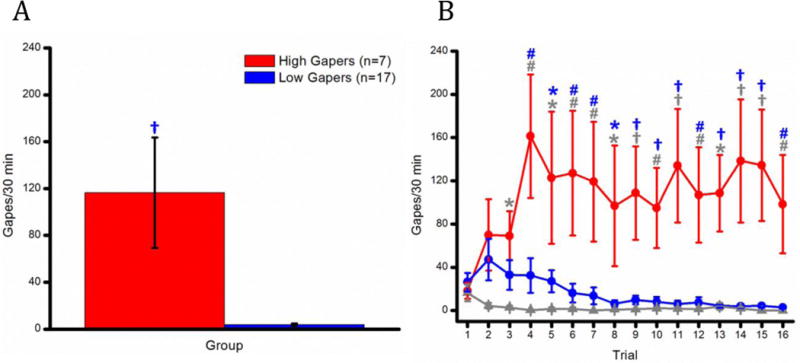
(A) Mean (+/− SEM) number of gapes/30 min for the High and the Low Gapers averaged across terminal Trials 15 and 16. (B) Mean (+/− SEM) number of gapes/30 min across Trials 1–16 for the High Gapers, the Low Gapers, and the Saline Controls. *, p <0.05; #, p <0.01; †, p <0.001.
Having divided rats in groups according to the number of gapes emitted at the end of the study, we next compared their taste reactivity behavior across each of 16 trials (see Figure 1B). We found a significant main effect of Group, F(2, 28) = 10.70, p < 0.001, and a significant Group × Trial interaction, F(30, 420) = 2.46, p < 0.001. Student Newman Keuls post-hoc tests revealed that the High Gapers evidenced significantly greater aversive TR than Saline rats beginning with Trial 3 (p < 0.05; gray symbols). High Gapers also emitted more gapes than Low Gapers beginning with Trial 4 (p < 0.01; blue symbols), and the behavior of Low Gapers did not differ from that of the Saline Controls, all ps > 0.05.
Self-Administration Behavior
The operant behaviors were then compared across the three groups (High gapers, n = 7; Low Gapers, n = 17; and Saline, n = 7). As described, following the overall mixed factorial ANOVA, post-hoc tests were conducted on significant effects using SNK tests. In one case, the two-way interaction missed significance. In this instance, the data were analyzed using one-way ANOVAs on a trial by trial basis and post hoc SNK tests were employed where appropriate. Figure 2 summarizes these data.
Figure 2.
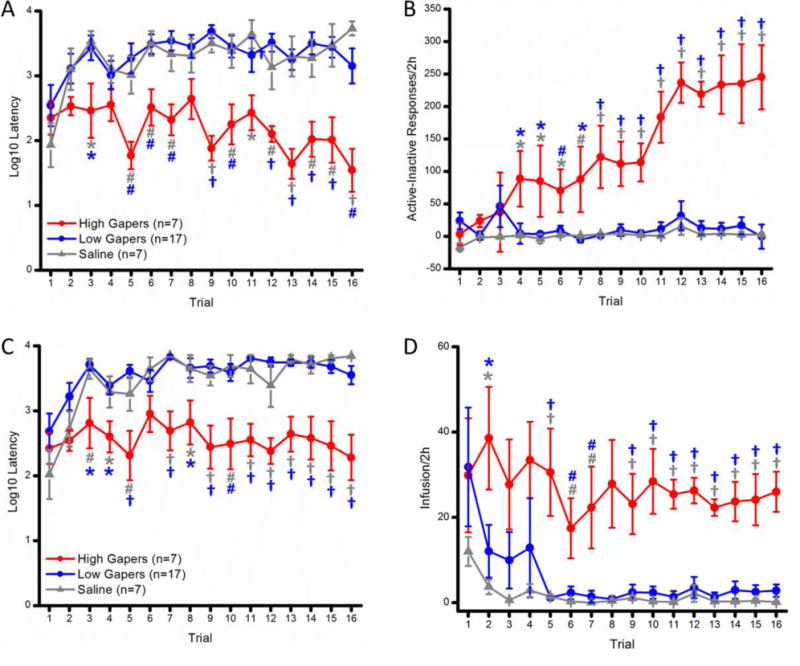
(A) Mean (+/− SEM) Log10 latency (seconds) to make the first response on the active empty spout across Trials 1–16 for the High Gapers, the Low Gapers, and the Saline Controls. (B) Mean (+/− SEM) Goal-Directed behavior (i.e., active minus inactive spout responses/2 h.) for High Gapers, Low Gapers, and Saline Controls across Trials 1 – 16. (C) Mean (+/− SEM) Log10 latency (seconds) to earn the first infusions of saline or cocaine across Trials 1–16 for the Saline Controls and the Low and High Gapers. (D) Mean (+/− SEM) number of infusions/2 h for the High Gapers, the Low Gapers, and the Saline Controls across Trials 1–16. *, p <0.05; #, p <0.01; †, p <0.001.
Latency to first response on the active spout
A mixed factorial ANOVA of the log10 response latencies revealed significant main effects of Group, F(2, 28) = 27.44, p < 0.001, and Trial, F(15, 420) = 2.46, p < 0.01, and a significant Group × Trial interaction, F(30, 420) = 1.51, p < 0.05. The results of SNK post-hoc tests showed that the High Gapers were faster than the Low Gapers (ps < 0.05) and the Saline Controls (ps < .05) to begin working for cocaine across nearly all trials, beginning with Trial 3 (see Figure 2A). Trials 4 and 8 were the exceptions, ps > 0.05.
Goal-directed behavior
Figure 2B shows the development of goal-directed behavior (i.e., active minus inactive spout responding during daily 2 h access periods to cocaine or saline) for High and Low Gapers. Significant main effects of Group, F(2, 28) = 28.40, p < 0.001, and Trial, F(15, 420) = 6.86, p < 0.001, and a significant Group × Trial interaction, F(30, 420) = 5.84, p < 0.001) were found. SNK Post hoc tests of the significant Group × Trial interaction confirmed that the High Gapers exhibited greater goal-directed behavior than both the Saline Controls and the Low Gapers, beginning with Trial 4, ps < 0.05. The degree of goal-directed behavior did not differ between the Low Gapers and the Saline Controls on any trial, ps > 0.05.
Latency to first infusion
A repeated-measures ANOVA of the log10 first infusion latencies revealed significant main effects of Group, F(2, 28) = 35.63, p < 0.001, and Trial, F(15, 420) = 5.84, p < 0.001, and a significant Group × Trial interaction, F(30, 420) = 1.51, p < 0.05. Thus, High Gapers were quicker, overall, than Low Gapers, p < 0.001, and Saline Controls, p < 0.001, to take their first infusion of the session (see Figure 2C). The results of SNK post-hoc tests clarified that this was true for every individual trial, beginning with Trial 3, except for Trial 6 (p < 0.01 vs. Saline; p < 0.05 vs. Low Gapers). The latencies to obtain the first infusion did not significantly differ for the Low Gapers vs. the Saline Controls on any individual trial.
Self-administered infusions
A repeated-measures ANOVA of the number of self-administered infusions for each 2-hr trial revealed significant main effects of Group, F(2, 28) = 17.82, p < 0.001, and Trial, F(15, 420) = 1.80, p < 0.05. Post hoc tests (SNK) of the significant main effect of Group revealed that High gapers took more infusions than Low Gapers and Saline Controls overall, ps < 0.05. The Group × Trial interaction, F < 1, did not attain statistical significance. Thus, planned 1-way ANOVAs (factor: Group) were conducted on a trial by trial basis. Post hoc SNK tests on significant main effects confirmed that High Gapers self-administered more infusions than Low Gapers or Saline Controls on nearly all trials, beginning with Trial 2, ps < 0.05. The number of infusions for the Low Gapers and the Saline Controls, on the other hand, did not differ on any individual trial, ps > 0.05.
Persistence in responding
One behavior associated with substance abuse or dependence in humans is compulsive drug-seeking. This can be quantified as the extent to which an animal persists in unrewarded responding for the drug. We used two measures to model this feature of addiction. First, we recorded the number of responses at both the active and the inactive spouts during the 30′ IO infusion period that preceded the 2-h self-administration opportunity for each trial, and calculated the goal-directed behavior as described above (i.e., as active minus inactive responses). As shown in Figure 3A, a mixed factorial ANOVA of the number of responses at the active minus inactive spout during each 30′ IO infusion period showed that, despite high variability in this behavior, High Gapers exhibited significantly more goal-directed responding than Low Gapers and Saline Controls. This conclusion was supported by a significant main effect of Group, F(2, 28) = 7.15, p < 0.01, and Trial, F(15, 420) = 2.89, p < 0.001, and a significant Group × Trial interaction, F(30, 420) = 2.21, p < 0.001. Post hoc Newman-Keuls tests on the significant 2-way interaction confirmed greater goal-directed responding during the IO period for the High Gapers vs. the Low Gapers on Trials 2 and 8–16, and vs. the Saline Controls on Trials 3, 8 – 14, and 16, all ps < 0.05.
Figure 3.
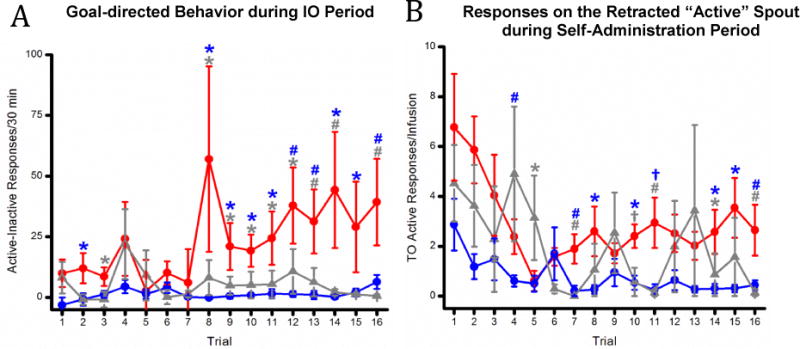
(A) Mean (+/− SEM) Goal-Directed behavior (active minus inactive responses) during the 30 min intraoral infusion period for Low Gapers, High Gapers, and the Saline Controls across Trials 1–17. (B) Mean (+/− SEM) responses on the active spout during the 20 second time out (TO) period for the Low Gapers, the Higher Gapers, and the Saline Controls across Trials 1–16. *, p <0.05; #, p <0.01; †, p <0.001.
For a second index of persistent seeking behavior, the number of active spout responses was measured during each 20 sec timeout period that followed each i.v. infusion during daily 2-h self-administration sessions. To control for the dependence of this behavior on the number of self-administered infusions, we divided the total number of time-out responses by the number of infusions taken during each session (see Figure 3B). A mixed factorial ANOVA of this ratio revealed significant main effects of Group, F(2, 28) = 5.81, p < 0.01, and Trial, F(15, 420) = 4.51, p < 0.001, and a significant Group × Trial interaction, F(30, 420) = 1.49, p < 0.05). Post hoc tests of the significant Group × Trial interaction revealed that High Gapers exhibited more time-out responding/infusion than Low Gapers on Trials 4, 7–8, 10–11, & 14–16, ps < 0.05. Additionally, High Gapers’ time-out responding was greater than Saline rats’ time-out responding on Trials 7, 10–11, 14, and 16, ps < 0.05; on Trial 5, they had fewer responses than Saline rats, p < 0.05. Taken together, these measures indicate that, in the absence of cocaine availability, the High Gapers show more persistent seeking behavior than do the Low Gapers or the Saline Controls.
Correlations
Table 1 shows the correlations (R2) between the number of gapes emitted to the cocaine-associated flavor cue on each trial beginning with Trial 3 and operant responding for and intake of cocaine, as assessed at the end of self-administration training (mean of last 2 trials). These data reveal that, in general, gapes to the CS begin to be significantly correlated with later cocaine seeking and taking very early in the rats’ experience. We also see that the rats’ early gapes (e.g., Trial 4) are significantly correlated with their TR over the course of the study. Thus, early aversive TR is correlated with later aversion to the cue as well as with increased cocaine-seeking and self-administration behavior.
Table 1.
Correlations (R2) between the number of gapes emitted to a cocaine-associated flavor cue and terminal behaviors.
| Terminal Behavior
|
|||||||
|---|---|---|---|---|---|---|---|
| Gapes/Each Trial ↓ | Gapes | Goal-directed behavior during IO period | LogLatency to First Active Spout Response | Goal-Directed Behavior | LogLatency to First Infusion | Infusions | Time-out Active Responses per Infusion |
| Trial 3 | 0.13 | 0.16* | 0.14 | 0.18* | 0.17* | 0.15 | 0.09 |
| Trial 4 | 0.82*** | 0.09 | 0.12 | 0.27** | 0.11 | 0.26* | 0.31** |
| Trial 5 | 0.65*** | 0.10 | 0.13** | 0.33 | 0.15 | 0.36** | 0.47*** |
| Trial 6 | 0.85*** | 0.11 | 0.17* | 0.23* | 0.12 | 0.24* | 0.31** |
| Trial 7 | 0.85*** | 0.09 | 0.16* | 0.27** | 0.12 | 0.29** | 0.38** |
| Trial 8 | 0.75*** | 0.09 | 0.18* | 0.32** | 0.20* | 0.34** | 0.33** |
| Trial 9 | 0.87*** | 0.15 | 0.23* | 0.24* | 0.15 | 0.24* | 0.34** |
| Trial 10 | 0.88*** | 0.13 | 0.22* | 0.37** | 0.20* | 0.41*** | 0.41*** |
| Trial 11 | 0.90*** | 0.05 | 0.14 | 0.22* | 0.08 | 0.22* | 0.38** |
| Trial 12 | 0.89*** | 0.03 | 0.11 | 0.19* | 0.05 | 0.19* | 0.27** |
| Trial 13 | 0.65*** | 0.02 | 0.08 | 0.16 | 0.04 | 0.17* | 0.29** |
| Trial 14 | 0.84*** | 0.02 | 0.10 | 0.14 | 0.04 | 0.14 | 0.29** |
| Trial 15 | 0.97*** | 0.05 | 0.16 | 0.24* | 0.09 | 0.25* | 0.38** |
| Trial 16 | 0.95*** | 0.01 | 0.11 | 0.21* | 0.08 | 0.24* | 0.25** |
p < 0.05.
p < 0.01.
p < 0.001.
Discussion
Some rats exhibited more gapes than others following the IO infusion of the flavor that had been paired with the opportunity to self-administer cocaine. This group difference emerged quickly, following just three flavor-drug pairings. Further, rats that emitted the greatest aversive taste reactivity behavior also exhibited a shorter latency to contact the active spout, a shorter latency to take the first infusion, greater goal-directed behavior (i.e., greater responding on the active vs. the inactive empty spout operant), greater cocaine self-administration across trials, and when drug was not available (i.e., when waiting for access to drug or when in time-out), greater persistence in seeking.
Using a within-subjects design, we previously demonstrated group differences in aversive TR following a single CS+ – drug pairing (Colechio et al., 2014). In that case, 1–3 flavor-drug pairings were required for conditioned aversive TR behavior to predict later cocaine self-administration behavior. The present results using a between-subjects design are consistent, showing group differences between High and Low Gapers by Trial 3 and the ability to predict later drug-taking behavior by Trial 3–4. The strength of these correlations, however, was not as strong in this between-groups study compared to those obtained in the earlier with-subjects (Colechio et al., 2014) study. That said, by using a simpler between-subjects paradigm, we now show reliable goal-directed behavior, exclusively in the High Gapers, and greater goal-directed behavior is correlated with greater aversive TR behavior. Finally, a greater motivation for drug was revealed for the High Gapers as they exhibited greater seeking on the active spout when drug was not available (i.e., during the intraoral infusion period and during periods of timeout). These between-group data, then, both confirm and extend our within-subjects data (Colechio et al., 2014).
When taken together, these data make it clear that conditioned aversive TR following the IO infusion of the drug-paired cue is highly predictive of later drug-seeking and drug-taking and, as such, provides an early indication of vulnerability to, or resilience from, drug. So what does the increase in aversive TR mean? On a very basic level, taste reactivity behavior (i.e., ingestion and rejection) is sensitive to innate differences in perceived palatability. As such, the IO delivery of increasing concentrations of sucrose, for example, is associated with a monotonic increase in appetitive TR behaviors (i.e., licks), but elicits no gapes (Grill & Norgren, 1978; Travers & Norgren, 1986). Alternatively, the intraoral infusion of an aversive gustatory stimulus, such as quinine (QHCl), leads to few licks and to a monotonic increase in gaping behavior as a function of increasing concentration (Grill & Norgren, 1978; Travers & Norgren, 1986). Appetitive TR is accompanied by increased dopamine (DA) release in the NAc shell and aversive TR is accompanied by suppression of DA release in the same region (Brown, McCutcheon, Cone, Ragozzino, & Roitman, 2011; McCutcheon, Ebner, Loriaux, & Roitman, 2012). Importantly, these taste reactivity behaviors also can change with changes in need state and as a function of learning. Thus, rats will exhibit appetitive taste reactivity to an otherwise aversive NaCl solution when intraorally infused during a time of high salt need (Loriaux, Roitman, & Roitman, 2011). Moreover, via classical conditioning, rats will emit aversive TR (i.e., gapes) following the intraoral delivery of an otherwise palatable gustatory stimulus that has been paired with LiCl-induced malaise (Grill & Norgren, 1978; Spector, Breslin, & Grill, 1988), which is known to cause decreased NAc DA (Mark, Blander, & Hoebel, 1991; McCutcheon, Ebner, Loriaux, & Roitman, 2012). As shown here, conditioned aversive TR also is evident when a palatable gustatory cue is paired with the opportunity to self-administer cocaine (Colechio et al., 2014; Wheeler, Twining, Jones, Slater, Grigson, & Carelli, 2008; Wheeler, Aragona, Fuhrmann, Jones, Day, Cacciapaglia, Wightman, & Carelli, 2011) and this too is associated with a decrease in NAc DA (Wheeler et al., 2008; Wheeler et al., 2011).
While this parallel can be drawn between conditioned aversion to a LiCl-paired cue and a drug-paired cue, there are many differences. First, and very importantly, conditioned avoidance of a LiCl-paired gustatory cue is associated with a conditioned place aversion (Chung, Barot, Kim, & Bernstein, 2011) and a decrease in instrumental responding for LiCl (White, Sklar, & Amit, 1977). In contrast, avoidance of a drug-paired cue is associated with a conditioned place preference (Reicher & Holman, 1977) and with the seeking and taking of drug (Colechio et al., 2014; Grigson & Twining, 2002; Wheeler et al., 2008). For this reason alone, it would be limiting, if not fully inaccurate, to interpret avoidance of a drug-paired cue as a mere CTA, akin to that induced by the aversive agent, LiCl (but see Arthurs, Lin, Amodeo, & Reilly 2012; Huang & Hsiao, 2008; Lin, Arthurs, Amodeo, & Reilly, 2012; Riley, 2011). Second, although all animals tested acquire a conditioned aversion to a LiCl-paired gustatory cue (Grigson, 1997), reliable individual differences are evident, and marked, in conditioned avoidance (i.e., reduced intake) and/or aversion (i.e., gapes) to a drug-paired cue. Thus, in paradigms that combine taste cues with self-administration opportunity, rats who most greatly avoid the tastant, self-administer the most drug and exhibit the greatest seeking following a period of abstinence (Grigson & Twining, 2002; Wise et al., 1976). Likewise, some rats exhibit high aversive taste reactivity behavior to the drug-paired cue, while others do not, and those exhibiting the highest aversive TR also are the quickest to take drug, take the most drug, and exhibit the greatest seeking behavior when the drug is not available (Colechio et al., 2014; Wheeler et al., 2008; Wheeler et al., 2011). None of this would be expected with a LiCl-induced CTA.
In TR-drug pairings, the tastant becomes a cue for experimenter-delivered drug (Parker, 1991, 1993 Parker, 1996; Parker & Carvell, 1986; Parker & Gillies, 1995) or for self-administered drug (Colechio et al., 2014; Wheeler et al., 2008; Wheeler et al., 2011). Drug-delivery, or exposure to cues for drug delivery, elicit approach and increased NAc DA (Hunt & Amit, 1987; Di Chiara, 2002). It has been shown that this also is true for a taste cue that accompanies contingent cocaine delivery (Wheeler et al, 2011). When IO tastant delivery precedes the passive injection of cocaine by just 2 min, the number of ingestive TR components is reduced, but the number of aversive components (including gapes) is not reliably elevated (Parker, 1993). However, at longer intervals (30–45 min), IO delivery of drug-paired tastant does elicit gapes from rats experienced with the association (Colechio et al., 2014; Wheeler et al., 2008; Wheeler et al, 2011). The gapes are associated with a QHCl-like ‘aversive’ electrophysiological profile in the NAc (Wheeler et al., 2008) and with a QHCl-like ‘aversive’ reduction in NAc DA (Wheeler et al., 2011). This finding is specifically due to the delay because, when simultaneously infused with drug, the same taste cue elicits appetitive TR and increased NAc DA (Wheeler et al., 2011). In this context, it is of interest to note that addicted humans also exhibit aversive affect (Sayette et al., 2003) and are less responsive to an alternative natural reward, seen as a reduced striatal BOLD response (Wilson, Sayette, Delgado & Fiez, 2008; Wilson et al., 2014), when having to wait for access to nicotine in the presence of smoking-related cues. The present study adds to these results by demonstrating that individual differences in aversive response to delayed-drug cues emerge in rats after relatively few experiences.
One possibility for the negative responses to delayed drug availability is that the drug cues, or even simply the expectation of delayed reward (i.e. Sayette’s told-yes condition), elicits conditioned withdrawal. The drug-related cues or explicit expectation that drug will be consumed would act as discriminative stimuli or occasion-setters, thus preparing the user’s body for impending ingestion of the drug. In the absence of drug, such preparation would be experienced as withdrawal. Such a hypothesis is feasible as conditioned withdrawal also is associated with an increase in circulating corticosterone (Nunez et al., 2007), blunted accumbens dopamine (Shaham & Stewart, 1995), and the onset of aversive TR following the intraoral infusion of a naloxone-paired taste cue (McDonald, Parker, & Siegel, 1997). Finally, were the cue to elicit the onset of such a conditioned aversive state, drug-taking would be the best correction for this state. In accord, as shown here and elsewhere, greater conditioned avoidance/aversion predicts greater drug-seeking and drug-taking in rats (Colechio et al., 2014; Grigson & Twining, 2002; Wheeler et al., 2008). Likewise, a smaller striatal response to monetary outcome in humans waiting to smoke, predicts selection of a cigarette over money in subsequent two-choice tests (Wilson et al., 2014).
Yet, still, why some and not others? One possibility is that early individual differences in response to the IO cue may be due to differences in rats’ initial or very early experiences with cocaine. For example, some rats may have had a more rewarding initial experience with the drug, as has been seen in some humans (Davidson, Finch & Schenk, 1993; Lambert, McLeod & Schenk, 2006), laying the foundation for them to experience a greater motivation to self-administer the drug (Epstein et al., 2009) and/or more cue-induced cocaine craving (Dudish-Poulsen & Hatsukami, 1997). Alternatively, the IO flavor may be best viewed as a natural reward. Natural rewards can be devalued by drugs (Grigson, 1997), but they also can be highly protective. As mentioned, human smokers with a strong striatal response to monetary reward selected money over a cigarette across repeated two-choice tests (Wilson, Delgado, McKee, Grigson, MacLean, Nichols, & Henry, 2014). Similarly, although female rats typically take more drug than male rats (Carroll, Morgan, Lynch, Campbell, Dess, 2002; Lynch, 2008), they self-administered less cocaine than males when access to the drug was preceded by brief access to a sweet saccharin cue (Cason & Grigson, 2013). Thus, the sweet may serve as enrichment, and enrichment is known to reduce responding for drug (Puhl, Blum, Acosta-Torres, & Grigson, 2012). As such, consideration also must be given to the possibility that pre-existing individual differences in responsiveness to natural rewards account for these individual differences in conditioned aversion or avoidance to the drug-paired cue and subsequent drug-taking behavior. Future studies will need to determine whether such pre-existing individual differences exist in this strain of rats and whether these individual differences in responsiveness to natural rewards and/or drug map onto other known indices of later drug vulnerability, e.g., sign-tracking and goal-tracking (Robinson & Berridge, 2008).
Acknowledgments
Financial Support: Support for this work was provided by NIH grant DA009815.
Footnotes
Conflict of Interest: All authors of this paper declare no conflict of interest.
References
- Arthurs J, Lin JY, Amodeo LR, Reilly S. Reduced palatability in drug-induced taste aversion: II. Aversive and rewarding unconditioned stimuli. Behavioral Neuroscience. 2012;126:433–444. doi: 10.1037/a0027676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes EN, Hemby SE. Contribution of ventral tegmental GABA receptors to cocaine self-administration in rats. Neurochemistry Research. 2008;33:459–467. doi: 10.1007/s11064-007-9454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DA, Pilcher CWT, D’Mello GD, Stolerman IP. Comparative potencies of amphetamine, fenfluramine and related compounds in taste aversion experiments in rats. British Journal of Pharmacology. 1977;61:669–677. doi: 10.1111/j.1476-5381.1977.tb07560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. European Journal of Neuroscience. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE. Aversive conditioning by psychoactive drugs: effects of morphine, alcohol and chlordiazepoxide. Psychopharmacologia. 1973;29:239–246. doi: 10.1007/BF00414038. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Activation of accumbens cell firing by stimuli associated with cocaine delivery during self-administration. Synapse. 2000;35:238–242. doi: 10.1002/(SICI)1098-2396(20000301)35:3<238::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;16:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Cason AM, Grigson PS. Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physiology & Behavior. 2013;112–113:96–103. doi: 10.1016/j.physbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A, Barot SK, Kim JJ, Bernstein IL. Biologically predisposed learning and selective associations in amygdalar neurons. Learning & Memory. 2011;18:371–374. doi: 10.1101/lm2053711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colechio EM, Imperio CG, Grigson PS. Once is too much: conditioned aversion develops immediately and predicts future cocaine self-administration behavior in rats. Behavioral Neuroscience. 2014;128:207–216. doi: 10.1037/a0036264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson ES, Finch JF, Schenk S. Variability in subjective responses to cocaine: initial experiences of college students. Addictive Behaviors. 1993;18:445–453. doi: 10.1016/0306-4603(93)90062-e. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural Brain Research. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Dudish-Poulsen SA, Hatsukami DK. Dissociation between subjective and behavioral responses after cocaine stimuli presentations. Drug & Alcohol Dependence. 1997;47:1–9. doi: 10.1016/s0376-8716(97)00054-9. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic-diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Archives of General Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari CM, O’Connor DA, Riley AL. Cocaine-induced taste aversions: effect of route of administration. Pharmacology, Biochemistry, & Behavior. 1991;38:267–271. doi: 10.1016/0091-3057(91)90277-9. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Checke S. Anticipation of incentive gain. Animal Learning & Behavior. 1982;10:177–182. [Google Scholar]
- Flaherty CF, Grigson PS, Checke S, Hnat KC. Deprivation state and temporal horizons in anticipatory contrast. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:503–518. [Google Scholar]
- Geddes RI, Han L, Baldwin AE, Norgren R, Grigson PS. Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced suppression of conditioned stimulus intake. Behavioral Neuroscience. 2008;122:1038–1050. doi: 10.1037/a0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowa JR, Shaw AE, Riley AL. Cocaine-induced conditioned taste aversions: comparisons between effects in LEW/N and F344/N rat strains. Psychopharmacology. 1994;114:229–232. doi: 10.1007/BF02244841. [DOI] [PubMed] [Google Scholar]
- Gomez F, Grigson PS. The suppressive effects of LiCl, sucrose, and drugs of abuse are modulated by sucrose concentration in food-deprived rats. Physiology & Behavior. 1999;67:351–357. doi: 10.1016/s0031-9384(99)00079-7. [DOI] [PubMed] [Google Scholar]
- Gomez F, Leo NA, Grigson PS. Morphine-induced suppression of saccharin intake is correlated with elevated corticosterone levels. Brain Research. 2000;863:52–58. doi: 10.1016/s0006-8993(00)02093-x. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: a reinterpretation. Behavioral Neuroscience. 1997;111:129–136. [PubMed] [Google Scholar]
- Grigson PS, Cornelius K, Wheeler DS. The suppressive effects of intraperitoneal cocaine are augmented when evaluated in nondeprived rats. Pharmacology Biochemistry & Behavior. 2001;69:117–123. doi: 10.1016/s0091-3057(01)00501-9. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Freet CS. The suppressive effects of sucrose and cocaine, but not lithium chloride, are greater in Lewis than in Fischer rats: evidence for the reward comparison hypothesis. Behavioral Neuroscience. 2000;114:353–363. doi: 10.1037//0735-7044.114.2.353. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Hajnal A. Once is too much: conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behavioral Neuroscience. 2007;121:1234–1242. doi: 10.1037/0735-7044.121.6.1234. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky P, Tanase D. Bilateral lesions of the gustatory thalamus disrupt morphine-but not LiCl-induced intake suppression in rats: evidence against the conditioned taste aversion hypothesis. Brain Research. 2000;858:327–337. doi: 10.1016/s0006-8993(00)01939-9. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behavioral Neuroscience. 2002;116:321–333. [PubMed] [Google Scholar]
- Grigson PS, Wheeler RW, Wheeler DS, Ballard SM. Chronic morphine treatment exaggerates the suppressive effects of sucrose and cocaine, but not lithium chloride, on saccharin intake in Sprague-Dawley rats. Behavioral Neuroscience. 2001;115:403–416. doi: 10.1037/0735-7044.115.2.403. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Huang ACW, Hsiao S. Re-examination of amphetamine-induced conditioned suppression of tastant intake in rats: the task-dependent drug effects hypothesis. Behavioral Neuroscience. 2008;122:1207–1216. doi: 10.1037/a0013511. [DOI] [PubMed] [Google Scholar]
- Hunt T, Amit Z. Conditioned taste aversion induced by self-administered drugs: paradox revisited. Biobehavioral Reviews. 1987;11:107–130. doi: 10.1016/s0149-7634(87)80005-2. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Grimm JW, Rustay NR, Parks CD, See RE. Predicting relapse to cocaine-seeking behavior: a multiple regression approach. Behavioral Pharmacology. 1999;10:513–521. doi: 10.1097/00008877-199909000-00009. [DOI] [PubMed] [Google Scholar]
- Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- Liang N, Grigson PS, Norgren R. Pontine and thalamic influences on fluid rewards: II. Sucrose and corn oil conditioned aversions. Physiology & Behavior. 2012;105:589–594. doi: 10.1016/j.physbeh.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N, Norgren R, Grigson PS. Pontine and thalamic influences on fluid rewards: III. Anticipatory contrast for sucrose and corn oil. Physiology & Behavior. 2012;105:595–606. doi: 10.1016/j.physbeh.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Arthurs J, Amodeo LR, Reilly S. Reduced palatability in drug-induced taste aversion. I. Variations in the initial value of the conditioned stimulus. Behavioral Neuroscience. 2012;126:423–432. doi: 10.1037/a0027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loriaux AL, Roitman JD, Roitman MF. Nucleus accumbens shell, but not core, tracks motivational value of salt. Journal of Neurophysiology. 2011;106:1537–1544. doi: 10.1152/jn.00153.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Research. 1991;551:308–310. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Frontiers in Neuroscience. 2012;6:1–10. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Deadwyler SA. Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. Journal of Neuroscience. 2000;20:5526–5537. doi: 10.1523/JNEUROSCI.20-14-05526.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyland JE, Liang NC, Norgren R, Grigson PS. Lesions of the thalamic trigeminal taste area dissociate natural from drug reward. Appetite. 2010;54:667–667. doi: 10.1016/j.appet.2010.04.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA. Taste reactivity responses elicited by cocaine-, phencyclidine-, and methamphetamine-paired sucrose solutions. Behavioral Neuroscience. 1993;107:118–129. doi: 10.1037//0735-7044.107.1.118. [DOI] [PubMed] [Google Scholar]
- Parker LA, Carvell T. Orofacial and somatic responses elicited by lithium-, nicotine-, and amphetamine-paired sucrose solution. Pharmacology, Biochemistry, & Behavior. 1986;24:883–887. doi: 10.1016/0091-3057(86)90431-4. [DOI] [PubMed] [Google Scholar]
- Parker LA, Gillies T. THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behavioral Neuroscience. 1995;109:71–78. doi: 10.1037//0735-7044.109.1.71. [DOI] [PubMed] [Google Scholar]
- Puhl MD, Blum JS, Acosta-Torres S, Grigson PS. Environmental enrichment protects against the acquisition of cocaine self-administration but does not eliminate avoidance of a drug-associated saccharin cue. Behavioral Pharmacology. 2012;23:43–53. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reicher MA, Holman EW. Location preference and flavor aversion reinforced by amphetamine in rats. Animal Learning & Behavior. 1977;5:343–346. [Google Scholar]
- Riley AL. The paradox of drug taking: The role of the aversive effects of drugs. Physiology & Behavior. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions B. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Perrott MA, Hobel J. Effects of smoking opportunity on cue-elicited urge: a facial coding analysis. Experimental & Clinical Psychopharmacology. 2003;11:218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroy PL, Wheeler RA, Davidson C, Scalera G, Twining RC, Grigson PS. Role of gustatory thalamus in anticipation and comparison of rewards over time in rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2005;288:R966–R980. doi: 10.1152/ajpregu.00292.2004. [DOI] [PubMed] [Google Scholar]
- Spector AC, Breslin P, Grill HJ. Taste reactivity as a dependent measure of the rapid formation of conditioned taste aversion: a tool for the neural analysis of taste-visceral associations. Behavioral Neuroscience. 1988;102:942–952. doi: 10.1037//0735-7044.102.6.942. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. Journal of Neuroscience. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology. 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Travers JB, Norgren R. Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behavioral Neuroscience. 1986;100:544–555. doi: 10.1037//0735-7044.100.4.544. [DOI] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behavioral Neuroscience. 2009;123:913–925. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biological Psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:1–12. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- White N, Sklar L, Amit Z. The reinforcing action of morphine and its paradoxical side effect. Psychopharmacology Berlin. 1977;52:63–66. doi: 10.1007/BF00426601. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Delgado MR, Mckee SA, Grigson PS, MacLean RR, Nichols TT, Henry SL. Ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cognitive, Affective, & Behavioral Neuroscience. 2014 doi: 10.3758/s13415-014-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Effect of smoking opportunity on responses to monetary gain and loss in the caudate nucleus. Journal of Abnormal Psychology. 2008;117:428–434. doi: 10.1037/0021-843X.117.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Yokel RA, DeWit H. Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science. 1976;191:1273–1275. doi: 10.1126/science.1257748. [DOI] [PubMed] [Google Scholar]


