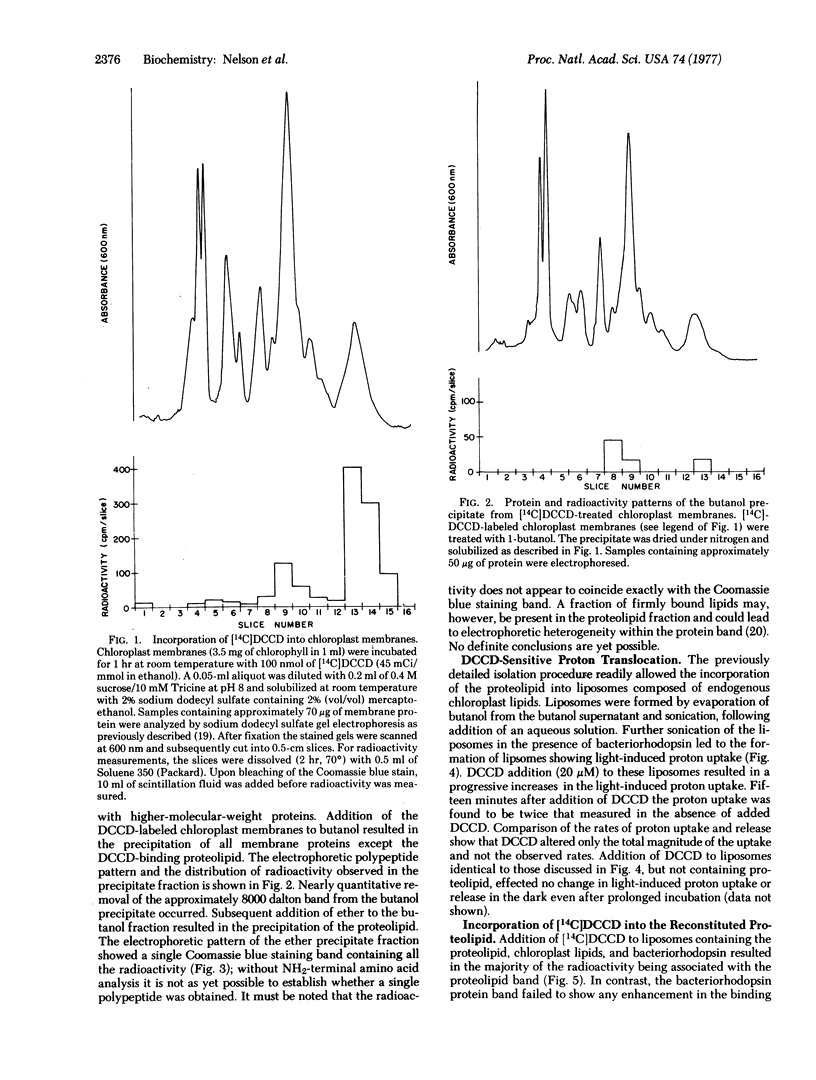
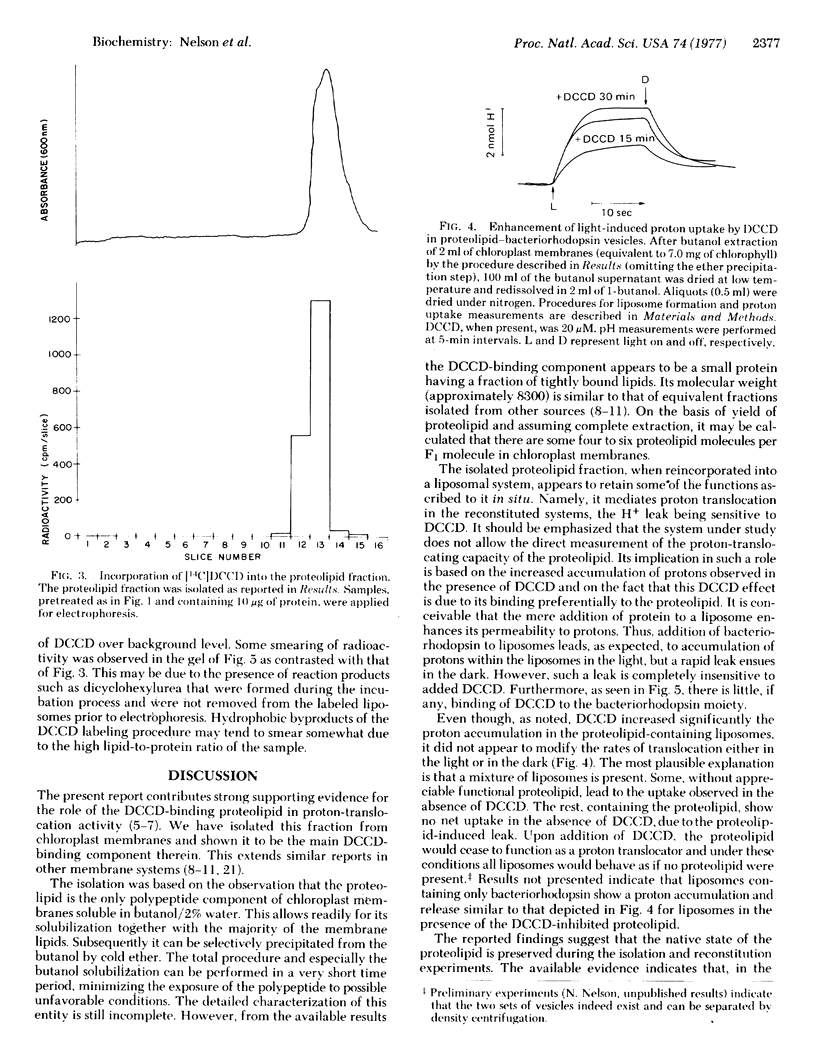
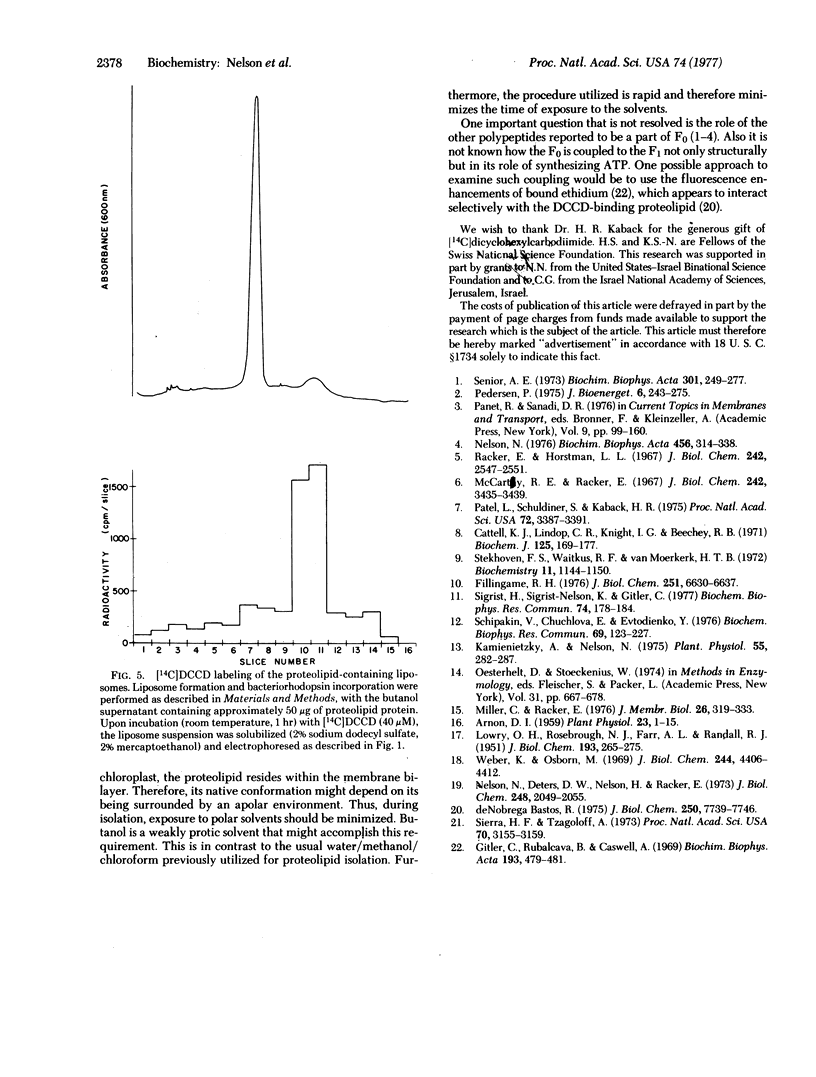
Abstract
The N,N'-dicyclohexylcarbodiimide-binding proteolipid from lettuce chloroplast membranes has been purified by a novel, rapid technique involving I-butanol extraction and ether precipitation. Reconstitution of this proteolipid into liposomes composed of chloroplast lipids and subsequent incorporation of bacteriorhodopsin resulted in the formation of liposomes exhibiting a light-dependent accumulation of protons. This accumulation was significantly enhanced upon addition of N,N'-dicyclohexylcarbodiimide at concentrations similar to those that inhibit chloroplast adenosinetriphosphatase activity. Radioactively labeled N,N'-dicyclohexylcarbodiimide was found to be incorporated essentially into the proteolipid of the reconstituted liposomes. These results suggest that the functional unit responsible for proton channeling in the chloroplast membrane has been isolated and reconstituted in the native state.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastos R. N. Use of diazido ethidium bromide as a specific probe for mitochondrial functions. J Biol Chem. 1975 Oct 10;250(19):7739–7746. [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Gitler C., Rubalcava B., Caswell A. Fluorescence changes of ethidium bromide on binding to erythrocyte and mitochondrial membranes. Biochim Biophys Acta. 1969;193(2):479–481. doi: 10.1016/0005-2736(69)90208-9. [DOI] [PubMed] [Google Scholar]
- Kamienietzky A., Nelson N. Preparation and properties of chloroplasts depleted of chloroplast coupling factor 1 by sodium bromide treatment. Plant Physiol. 1975 Feb;55(2):282–287. doi: 10.1104/pp.55.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller C., Racker E. Fusion of phospholipid vesicles reconstituted with cytochrome c oxidase and mitochondrial hydrophobic protein. J Membr Biol. 1976 May;26(4):319–333. doi: 10.1007/BF01868880. [DOI] [PubMed] [Google Scholar]
- Nelson N., Deters D. W., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 8. Properties of isolated subunits of coupling factor 1 from spinach chloroplasts. J Biol Chem. 1973 Mar 25;248(6):2049–2055. [PubMed] [Google Scholar]
- Nelson N. Structure and function of chloroplast ATPase. Biochim Biophys Acta. 1976 Nov 30;456(3-4):314–338. doi: 10.1016/0304-4173(76)90003-3. [DOI] [PubMed] [Google Scholar]
- Patel L., Schuldiner S., Kaback H. R. Reversible effects of chaotropic agents on the proton permeability of Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3387–3391. doi: 10.1073/pnas.72.9.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E., Horstman L. L. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 13. Structure and function of submitochondrial particles completely resolved with respect to coupling factor. J Biol Chem. 1967 May 25;242(10):2547–2551. [PubMed] [Google Scholar]
- Senior A. E. The structure of mitochondrial ATPase. Biochim Biophys Acta. 1973 Dec 31;301(3):249–277. doi: 10.1016/0304-4173(73)90006-2. [DOI] [PubMed] [Google Scholar]
- Shchipakin V., Chuchlova E., Evtodienko Y. Construction of mitochondrial H+ -transporting system in proteoliposomes. Biochem Biophys Res Commun. 1976 Mar 8;69(1):123–127. doi: 10.1016/s0006-291x(76)80281-1. [DOI] [PubMed] [Google Scholar]
- Sierra M. F., Tzagoloff A. Assembly of the mitochondrial system. Purification of a mitochondrial product of the ATPase. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3155–3159. doi: 10.1073/pnas.70.11.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist H., Sigrist-Nelson K., Gitler C. Single-phase butanol extraction: a new tool for proteolipid isolation. Biochem Biophys Res Commun. 1977 Jan 10;74(1):178–184. doi: 10.1016/0006-291x(77)91391-2. [DOI] [PubMed] [Google Scholar]
- Stekhovan F. S., Waitkus R. F., Van Moerkerk H. T. Identification of the dicyclohexylcarbodiimide-binding protein in the oligomycin-sensitive adenosine triphosphatase from bovine heart mitochondria. Biochemistry. 1972 Mar 28;11(7):1144–1150. doi: 10.1021/bi00757a005. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


