Abstract
The presence of ribosomal protein S1 in 30S ribosomes is indispensable for the formation of 30S initiation complexes with natural mRNA. The 30S subunits lacking S1 retain activity with AUG as mRNA and are also active in poly(rU)-directed binding of Phe-tRNA. Isolated protein S1 stoichiometrically disrupts the secondary structure of helical and stacked single-stranded polynucleotides and converts them into their fully or partially denatured forms. A mono-N-ethylmaleimide derivatives of S1 is nearly devoid of any RNA helix-unwinding properties but is readily incorporated into 30S subunits deficient in S1. The resulting N-ethylmaleimide-S1-containing 30S subunits are completely inactive in the binding of MS2 [3H]RNA and in the formation of an initiation complex with MS2 RNA as mRNA. They retain activity in the binding of the initiator fMet-tRNA in response to the trinucleotide AUG and in the binding of Phe-tRNA in response to poly(U). They also retain the capacity to bind 50S subunits and to form 70S couples. These results suggest that a correlation exists between the RNA helix-unwinding capacity of isolated S1 and the function of S1 in the ribosomal binding of natural mRNA when the protein becomes part of the 30S subunit.
Full text
PDF
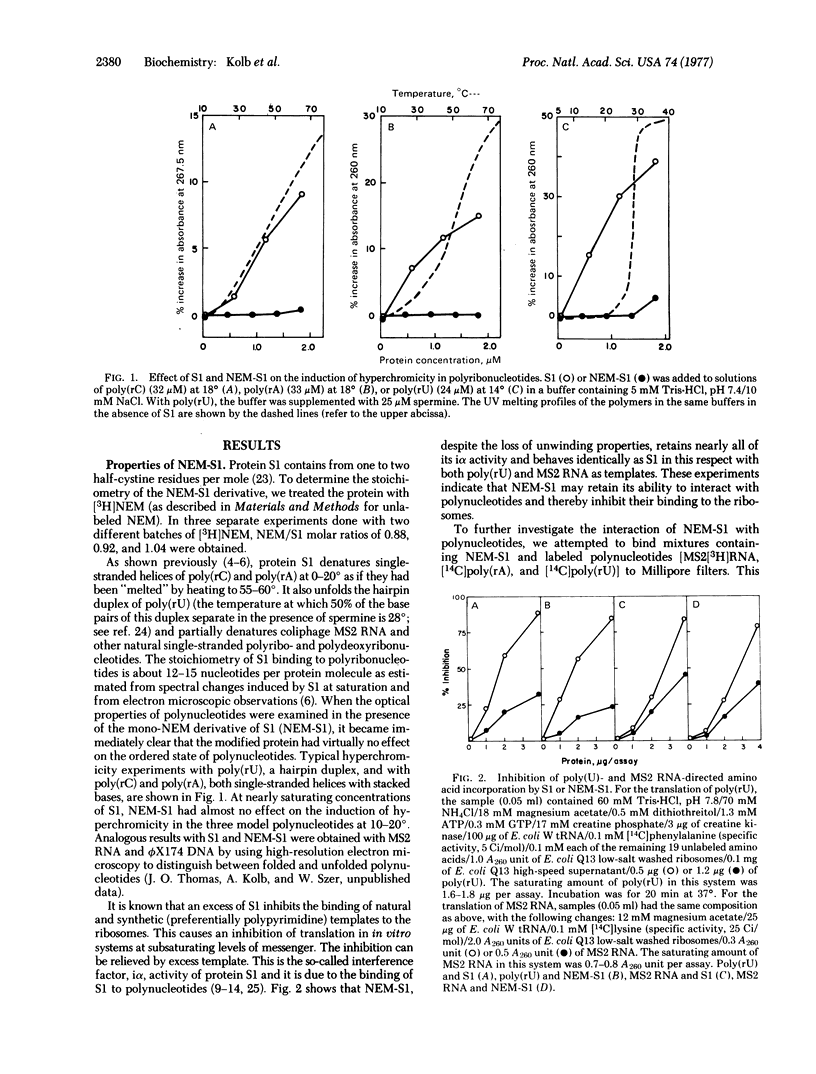
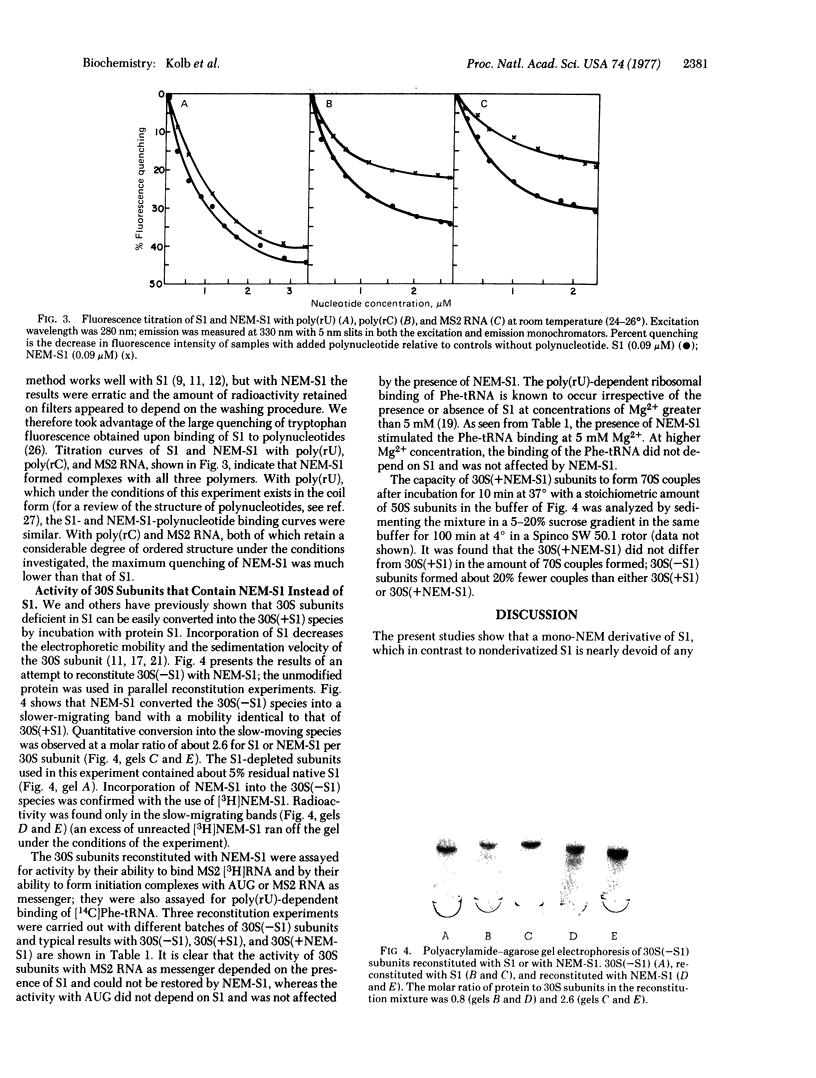


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear D. G., Ng R., Van Derveer D., Johnson N. P., Thomas G., Schleich T., Noller H. F. Alteration of polynucleotide secondary structure by ribosomal protein S1. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1824–1828. doi: 10.1073/pnas.73.6.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E. Binding of ribosomal protein S1 of Escherichia coli to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2940–2944. doi: 10.1073/pnas.72.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg A. E. Two forms of the 30 S ribosomal subunit of Escherichia coli. J Biol Chem. 1974 Dec 10;249(23):7673–7678. [PubMed] [Google Scholar]
- Groner Y., Scheps R., Kamen R., Kolakofsky D., Revel M. Host subunit of Q replicase is translation control factor i. Nat New Biol. 1972 Sep 6;239(88):19–20. doi: 10.1038/newbio239019a0. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Boublik M., Szer W. Conformation and activity of subspecies of 30 S ribosomes from Escherichia coli. Arch Biochem Biophys. 1976 Jul;175(1):181–184. doi: 10.1016/0003-9861(76)90497-5. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Szer W. Replacement of ribosomal protein S1 by interference factor ialpha in ribosomal binding of phage Ms2 RNA. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4708–4712. doi: 10.1073/pnas.71.12.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick G., Alberts B. Nucleic acid helix-coil transitions mediated by helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2133–2141. [PubMed] [Google Scholar]
- Herrick G., Alberts B. Purification and physical characterization of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2124–2132. [PubMed] [Google Scholar]
- Herrick G., Delius H., Alberts B. Single-stranded DNA structure and DNA polymerase activity in the presence of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2142–2146. [PubMed] [Google Scholar]
- Inouye H., Pollack Y., Petre J. Physical and functional homology between ribosomal protein S1 and interference factor i. Eur J Biochem. 1974 Jun 1;45(1):109–117. doi: 10.1111/j.1432-1033.1974.tb03535.x. [DOI] [PubMed] [Google Scholar]
- Jay G., Kaempfer R. Translational repression of a viral messenger RNA by a host protein. J Biol Chem. 1975 Aug 10;250(15):5749–5755. [PubMed] [Google Scholar]
- Kamen R., Kondo M., Römer W., Weissmann C. Reconstitution of Q replicase lacking subunit with protein-synthesis-interference factor i. Eur J Biochem. 1972 Nov 21;31(1):44–51. doi: 10.1111/j.1432-1033.1972.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Kenner R. A. A protein-nucleic acid crosslink in 30S ribosomes. Biochem Biophys Res Commun. 1973 Apr 16;51(4):932–938. doi: 10.1016/0006-291x(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Mazumder R. Initiation factor 2-dependent ribosomal binding of N-formylmethionyl-transfer RNA without added guanosine triphosphate. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2770–2773. doi: 10.1073/pnas.69.10.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Wahba A. J. Inhibition of synthetic and natural messenger translation. II. Specificity and mechanism of action of a protein isolated from Escherichia coli MRE 600 ribosomes. J Biol Chem. 1974 Jun 25;249(12):3808–3813. [PubMed] [Google Scholar]
- Moore P. B. The distribution of half-cystine in the ribosomal proteins of Escherichia coli. J Mol Biol. 1975 Oct 25;98(2):439–444. doi: 10.1016/s0022-2836(75)80129-x. [DOI] [PubMed] [Google Scholar]
- Senear A. W., Steitz J. A. Site-specific interaction of Qbeta host factor and ribosomal protein S1 with Qbeta and R17 bacteriophage RNAs. J Biol Chem. 1976 Apr 10;251(7):1902–1912. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Boublik M. Destabilization of the secondary structure of RNA by ribosomal protein S1 from Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):957–964. doi: 10.1016/0006-291x(76)90685-9. [DOI] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Leffler S. Ribosomal protein S1 and polypeptide chain initiation in bacteria. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2325–2329. doi: 10.1073/pnas.72.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Leffler S. Interaction of Escherichia coli 30S ribosomal subunits with MS2 phage RNA in the absence of initiation factors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3611–3615. doi: 10.1073/pnas.71.9.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W. Ordered state of poly-uridylic acid above room temperature. J Mol Biol. 1966 Apr;16(2):585–587. doi: 10.1016/s0022-2836(66)80200-0. [DOI] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]
- Wahba A. J., Miller M. J., Niveleau A., Landers T. A., Carmichael G. G., Weber K., Hawley D. A., Slobin L. I. Subunit I of G beta replicase and 30 S ribosomal protein S1 of Escherichia coli. Evidence for the identity of the two proteins. J Biol Chem. 1974 May 25;249(10):3314–3316. [PubMed] [Google Scholar]
- Zamir A., Miskin R., Elson D. Inactivation and reactivation of ribosomal subunits: amino acyl-transfer RNA binding activity of the 30 s subunit of Escherichia coli. J Mol Biol. 1971 Sep 14;60(2):347–364. doi: 10.1016/0022-2836(71)90299-3. [DOI] [PubMed] [Google Scholar]
- van Dieijen G., van Knippenberg P. H., van Duin J. The specific role of ribosomal protein S1 in the recognition of native phage RNA. Eur J Biochem. 1976 May 1;64(2):511–518. doi: 10.1111/j.1432-1033.1976.tb10330.x. [DOI] [PubMed] [Google Scholar]