Abstract
The 5-HT4 receptor agonist tegaserod (TEG) has been reported to modulate visceral pain. However, the underlying mechanism remains unknown. The objective of the present study was to examine the analgesic mechanism and site of action of TEG. In male rats, visceral pain was assessed by measuring visceromotor response (VMR) to colorectal distension (CRD). Inflammation was induced by intracolonic injection of tri-nitrobenzene sulfonic acid (TNBS). The effect of TEG on the VMR was tested by injecting intraperitoneal (i.p.), intrathecal (i.t.), intracerebroventricular (i.c.v) or in the rostroventral medulla (RVM). The effect of the drug was also tested on responses of CRD-sensitive pelvic nerve afferents (PNA) and lumbo-sacral (LS) spinal neurons. Systemic injection of TEG attenuated VMR in naive and TNBS-treated rats. Similarly, supraspinal, but not spinal, injection of TEG attenuated the VMR. While GR113808, (selective 5-HT4 antagonist) blocked the effect, naloxone (NLX) an opioid receptor antagonist reversed the effect of TEG. Although i.t. NLX did not block the inhibitory effect of TEG in VMR study, i.t. injection of α2-adrenergic receptor antagonist yohimbine blocked the effect of TEG when given systemically. While TEG had no effect on the responses of CRD-sensitive PNA, it inhibited the responses of CRD-sensitive LS neurons in spinal intact condition. This inhibition was blocked by GR113808, NLX and β-funaltrexamine (β-FNA) when injected into the RVM. Results indicate that TEG produces analgesia via activation of supraspinal 5-HT4 receptors which triggers the release of opioids at supraspinal site, which activates descending noradrenergic pathways to the spinal cord to produce analgesia.
Keywords: 5-HT4 receptors, RVM, Visceral pain, Colon, Descending modulation
1. Introduction
In humans and mammalian vertebrates, 5-hydroxytryptamine (5-HT, serotonin) is primarily synthesized by enterochromaffin (EC) cells in the gastrointestinal (GI) tract. A small amount of 5-HT is also synthesized by the neurons in the central nervous system (CNS) (Gershon, 2000). While 5-HT produces a wide range of functions in the brain and periphery by interacting with several receptor subtypes (5-HT1–5-HT7), activation of 5-HT4 receptors has been mostly associated with GI peristalsis (i.e., prokinetic), intestinal chloride secretion and action on enteric neurons (Borman and Burleigh, 1993; Grider et al., 1998; Kellum et al., 1999). In addition, 5-HT4 receptor agonists have been shown to improve visceral and somatic sensitivity in IBS patients. For example, 5-HT4 receptor agonist tegaserod (TEG) significantly attenuates distension-induced rectal sensitivity in IBS patients and reduces somatic pain in patients with co-existing fibromyalgia and IBS (Lian et al., 2005; Reitblat et al., 2009; Sabaté et al., 2008). However, the exact mechanism for the anti-nociceptive effect of 5-HT4 receptor agonist is still not clear.
Although in two recent reports a peripheral site of action for TEG have been proposed (Schikowski et al., 2002; Van Greenwood-Van Meerveld et al., 2006) for visceral analgesia, it is well recognized that 5-HT4 receptors modulate neurotransmitter release in the CNS (Barnes and Sharp, 1999). Microdialysis in the brain has shown an increase in acetylcholine release following intra-cerebroventricular (i.c.v) injection of the 5-HT4 agonists and this effect can be reduced by co-administration of the 5-HT4 antagonists (Consolo et al., 1994). Studies have also documented that relatively high expression of 5-HT4 receptors in the region of the limbic system, hippocampus, basal ganglia and nigrostriatal pathways that play important roles in learning, memory, depression and anxiety (Grossman et al., 1993; Waeber et al., 1993; Jakeman et al., 1994; Doménech et al., 1994; Schiavi et al., 1994; Waeber et al., 1994; Patel et al., 1995; Mengod et al., 1996; Langlois and Fischmeister, 2003). The rostroventral medulla (RVM) forms part of a descending pathway that modulates nociceptive neurotransmission at the level of the dorsal horn. There are primarily two pathways (inhibitory and facilitatory) that may have opposing actions on nociceptive transmission. The RVM has been shown to contribute in major way in opioid related antinociception and opioid receptor subtypes (µ-, κ-, and δ-opioid receptors) exist on spinally projecting descending serotonergic neurons (Marinelli et al., 2002). Therefore, it is conceivable that TEG may exert its visceral analgesia via a supraspinal mechanism linked to descending opioidergic system.
Although TEG was approved for the treatment of constipation predominant irritable bowel syndrome (IBS-C), it was withdrawn from the market due to adverse cardiovascular effects including myocardial infarction, stroke, and unstable angina. However, a recent report found no association between TEG and adverse cardiovascular outcomes (Anderson et al., 2009). Regardless, understanding the mechanism of action of TEG and the role 5-HT4 receptors in visceral analgesia is important for the development of pharmacological targets aimed at treating pain associated with functional bowel disorders.
The objective of the present study was to investigate the site of action and the mechanism underlying the antinociceptive property of TEG in a rat model of visceral hypersensitivity by evaluating its effects on – 1) the viscero-motor response (VMR) in awake, naïve and TNBS-treated colitis (i.e., inflamed colon) rats, 2) the sensitivity of colorectal distension (CRD)-sensitive pelvic nerve afferent (PNA) fibers and, (3) responses of CRD-sensitive lumbosacral (LS) spinal neurons.
2. Methods
The study was carried out using male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) with an average weight of 400 g (range: 350–450 g). All experiments were performed according to the approved guidelines of the Institutional Animal Care and Use committee at the Medical College of Wisconsin (approval# AUA0000356) and the International Association for the Study of Pain (IASP).
2.1. General surgical procedure
All survival surgical procedures were performed in adult rats under deep anesthesia with sodium pentobarbital (Nembutal, 45–50 mg/kg, i.p., Ovation Pharmaceuticals Inc. Brown Deer, IL, USA). To record the electrical activity (EMG) from the lower abdominal muscle to colon distension, a pair of teflon coated electrodes were implanted into the external oblique muscle and externalized through the neck as previously described (Miranda et al., 2004). All rats received analgesic (carprofen, 5 mg/kg/day, i.m. for 3 days) and antibiotic (enrofloxacin, 2.5 mg/kg/day, i.m. for 3 days) post-operatively. Following the surgery rats were housed separately and allowed to recover for at least 3 days prior to further interventions.
The implantation of chronic drug delivery catheters varied in animals depending on the intended site for drug delivery: (1) for intravenous (i.v.) drug administration, a catheter (PE-50) was placed into the right femoral vein and externalized dorsally near the neck, (2) for intrathecal (i.t.) drug administration, a catheter (PE-10) was inserted through the dura overlying the atlanto-occipital junction into the spinal sub-arachnoid space and guided until the tip of catheter lay in the lumbosacral (LS) segment of the spinal cord, (3) for intra-cerebroventricular (i.cv) drug administration, a guide cannula (part # 8IC311 GS, PlasticOne, Roanoke, VA, USA) was implanted (A-P: –2 mm bregma, 0.8 mm lateral to sagittal suture, 5 mm down from the skull surface) and secured in place by applying tissue glue (3M Vetbond, MN, USA) and capped with a dummy cannula (part # 8IC311DC, PlasticOne, Roanoke, VA, USA) and (4) for rostroventral medulla (RVM) microinjections, a small craniotomy (0.25 cm × 0.25 cm) was made and the guide cannula (length 9.4 mm below the pedestal) was inserted (A-P: –2.5 mm lamda, 0 mm from midline, 9.3 mm dorso-ventral) into the RVM area.
2.2. Recording of viscero-motor response (VMR) to colon distension
Seventy-two hours post-surgery, rats were placed inside the plexiglass restraining tubes two times a day for two hours in order to acclimatize them to experimental conditions. The VMR to colorectal distension (CRD) was used as an objective measure of visceral sensation in all groups as previously described (Miranda et al., 2004). A latex balloon (6–7 cm in length) made from condom was inserted transanally into the hindgut. Electrodes were connected to bioamplifier (A-M System AC Differential Amplifier, model 1700). To record the VMR, graded isobaric (constant pressure) distention pressures (10, 20, 30, 40, and 60 mmHg) were applied for 30 s with a 180 s inter-stimulus interval. A stimulus-response function (SRF) to graded distension pressure was constructed for every rat prior to any pharmacological intervention and repeated following drug administration.
2.3. Induction of colonic inflammation
Following baseline SRF’s, rats were lightly anesthetized with pentobarbital sodium (10–20 mg/kg, i.p.) and 0.5 ml of 50% tri-nitrobenzene sulfonic acid (TNBS, dissolved in ethanol) was slowly injected into the descending colon using a 16 gauge gavage needle. Rats were then allowed to recover for 7 days prior to recording VMR to assess colonic hypersensitivity due to inflammation.
2.3.1. Electrophysiology
2.3.1.1. Recording from sacral S1 dorsal root
The surgical procedure and recordings from the S1 dorsal root were carried out as previously described (Sengupta and Gebhart, 1994). Briefly, following anesthesia the lower abdomen was exposed by a 3–4 cm long incision laterally at the left flank. The urinary bladder was emptied and catheterized (PE-100) through the apex of the bladder. The prostate lobe was reflected laterally to access the major pelvic ganglion (MPG) and pelvic nerve. A pair of teflon-coated stainless steel wires stripped (1–1.5 cm) at the tips was wrapped around the pelvic nerve. The exposed parts of the wires were covered with peritoneal fat tissue. The abdomen was closed in layers (muscle followed by skin) with 3–0 silk sutures.
The LS spinal cord was exposed by laminectomy (T13-S2) and the spinal cord was stabilized by clamping the thoracic and ischial vertebrae. The dorsal skin was reflected laterally and tied to spinal posts to make a pool for mineral oil. The dura was carefully removed and the spinal cord was covered with warm (37 °C) mineral oil. The pelvic nerve afferent fibers in the sacral S1 dorsal root were identified by recording the evoked response to electrical stimulation (5–10 mv, 0.5–1 ms square wave pulse) of the pelvic nerve. The identified fiber was then tested to CRD (40 mmHg) and once confirmed as a CRD-sensitive fiber, a stimulus-response function (SRF) to graded CRD (10–60 mmHg, 30 s) was recorded. At the end of the experimental protocol, rats were euthanized by injecting a lethal dose (0.6 ml/ kg, i.v.) of Beuthanasia-D (390 mg pentobarbital, 50 mg phenytoin sodium, 2% benzyl alcohol; Schering-Plough Animal Health, USA).
2.3.1.2. Recording from lumbo-sacral (LS) spinal neurons
The surgical procedure for spinal recordings was the same as described in the previous section. Stainless steel microelectrodes (8–10 M,FHC, Bowdoinham, ME) were used to record neurons 0.1– 0.5 mm lateral from the spinal midline and 0.6–1.3 mm ventral from the dorsal surface. The action potentials were amplified through a low-noise AC differential amplifier (model 3000; A-M Systems) and continuously monitored and displayed on an oscilloscope. Once a CRD-sensitive neuron was identified, an SRF to graded CRD (10–60 mmHg, 30 s) was constructed.
2.3.2. Experimental protocols
2.3.2.1. Visceromotor response (VMR)
In naïve, non-inflamed rats, the VMR to graded CRD (10–60 mmHg, 30 s) was recorded before and 20 min after drug injection. In TNBS-treated rats, the VMR to graded CRD was measured before injecting TNBS into the colon and repeated seven days after injection. The test drug was given either (1) intra-peritoneally (i.p.) to test the systemic effect, (2) intrathecally to test the effect in the spinal cord, (3) in the lateral ventricle (i.c.v.) to test the supraspinal effect or (4) in the rostroventral medulla (RVM) to test the influence on the descending modulatory system. In all experiments, the SRF to graded CRD (10– 60 mmHg) was recorded before injecting TEG and 20 min following TEG. Similar experiments were performed with 10% propyleneglycol (PGL), the vehicle for TEG. To test the receptor specific antinociceptive effect of TEG, the drug was tested in the presence of several antagonists; (1) a non-selective 5-HT1, 5-HT2, and 5-HT7 antagonist methysergide (1 mg/kg, i.p.), (2) a selective 5-HT1A receptor antagonist WAY-1000135 (5 mg/kg, s.c.), or 3) a selective 5-HT4 receptor antagonist GR113808 (5 mg/kg, sc). Antagonists were injected 10 min prior to injection of TEG. In a different set of experiments, either atropine (20 mg/kg, i.v.) or naloxone (2 mg/kg, i.v.) was injected 10 min prior to TEG injection.
2.3.1.1. Electrophysiology recordings
A baseline SRF to graded CRD was recorded from PNA fibers in TNBS-treated rats and repeated 20 min after injection of TEG (1 mg/kg, i.p.). In a different set of rats, CRD-sensitive LS spinal neurons from TNBS-treated rats were identified and an SRF to graded CRD was constructed before and 20 min after microinjection of TEG through the implanted guide cannula (60 nmol in i.c.v. or 10 nmol in RVM). The volume of injections into the lateral ventricle and RVM was restricted to 5.0 µl and 0.2 µl, respectively. In some experiments, 0.2 µl of 1% alcian blue dye was injected into the RVM at the end of the experiment to identify the site of injection. In these rats, after euthanizing the whole brain was immediately removed and fixed in 4% paraformaldedyde. Serial sections (30–40 µm) were made rostro-caudally from midcollicular area to cervical (C3) spinal cord to locate the dye-injected area. To investigate a potential link between TEG and the opioidergic pathway, the effect of TEG injection into the lateral ventricle was tested in the presence of systemic NLX (2 mg/kg, i.v.). In additional experiments, we recorded responses of LS neurons to microinjection of TEG into the RVM 10 min after the microinjection of NLX (10 nmol) or beta-funaltrexamine (β-FNA) into the RVM.
2.4. Drugs
The 5-HT4 agonist, tegaserod maleate (TEG), was obtained from Procter & Gamble Pharmaceuticals, Inc. and was dissolved in 10% propyleneglycol (PGL). GR113808 (Tocris Bioscience Ellisville, MO, USA), was either dissolved in ethanol for subcutaneous (s.c.) injections or in saline for microinjections into i.c.v or in RVM. Naloxone hydrochloride, 8-hydroxy-2-(di-n-propylamino)-tetralin (DPAT) and methysergide maleate (Sigma Aldrich, Louis, MO, USA) were dissolved in saline. Atropine sulfate (Atrop, Sigma Aldrich, MO, USA) was dissolved in distilled water and was injected either i.p. or s.c. WAY-1000135 (Tocris Bioscience Ellisville, MO, USA) was dissolved in water and injected s.c. β-funaltrexamine hydrochloride (β-FNA; Tocris Bioscience Ellisville, MO, USA) was dissolved in saline for intra-RVM injections.
2.4.1. Data analysis
2.4.1.1. Behavioral studies (VMR)
In naïve rats, the EMG activity following drug administration for each distending pressure was expressed as percentage of maximum response exhibited by the rat at 60 mmHg prior to drug injection. Following TNBS, the EMG activity for each distending pressure was expressed as percentage of maximum response exhibited at 60 mmHg prior to TNBS instillation (i.e., baseline). Each rat served its own control. Statistical analysis was performed using SigmaStat (V2.03, SPSS Inc, Chicago, IL). All results are expressed as mean ± SEM. Values with p < 0.05 were considered to be significant. EMG response at distending pressure was calculated as area under the curve. The effect of the drug was statistically compared and represented by applying Student ‘t’-test.
2.4.1.2. Electrophysiology studies
The total number of action potentials before (60 s) and during the distension period (30 s) were counted and represented as impulses/ sec (imp/s). The firing frequency during the resting period was subtracted from the firing frequency during distension to determine actual response during the distension. In each experiment, a baseline stimulus-response function (SRF) was constructed for each neuron prior to drug injection and repeated following drug injection. Since each spinal neuron had different spontaneous firing and response to CRD, we normalized the value against its maximum response to distending pressure of 60 mmHg. The data were analyzed at each distending pressure and the effect of drug was statistically represented using Student ‘t’-test.
3. Results
3.1. Behavioral studies
3.1.1. Effect of TEG on freely moving rats
TEG was injected intraperitoneally (i.p.) to test the effect on behavior in naïve, freely moving rats (n = 10). At doses of 5 and 10 mg/kg, TEG produced severe CNS effects including tremor, ataxia and loss of explorative behavior. These effects were evident within a minute after injection and lasted for more than an hour. Pre-treatment with atropine sulfate (Atrop, 20 mg/kg, i.p.) was found to delay the above mentioned CNS effects by 20–30 min. However, at a dose ≤ 1 mg/kg or less, TEG did not produce any noticeable CNS effect. Therefore, in subsequent experiments a dose of 1 mg/kg, i.p. of TEG was used to test the visceral analgesic effects.
3.1.2. Effect of TEG on VMR
The majority of rats exhibited an intensity-dependent increase in the VMR to graded CRD (10–60 mmHg). The effect of TEG on the VMR was tested in naïve and TNBS-treated rats. Following intra-colonic TNBS, rats exhibited a significant increase in the VMR at all intensities of distension >10 mmHg. The rationale for using non-inflamed naïve and TNBS-induced inflamed rats was to compare the efficacy of the drug in producing visceral analgesia under normal and pathological condition.
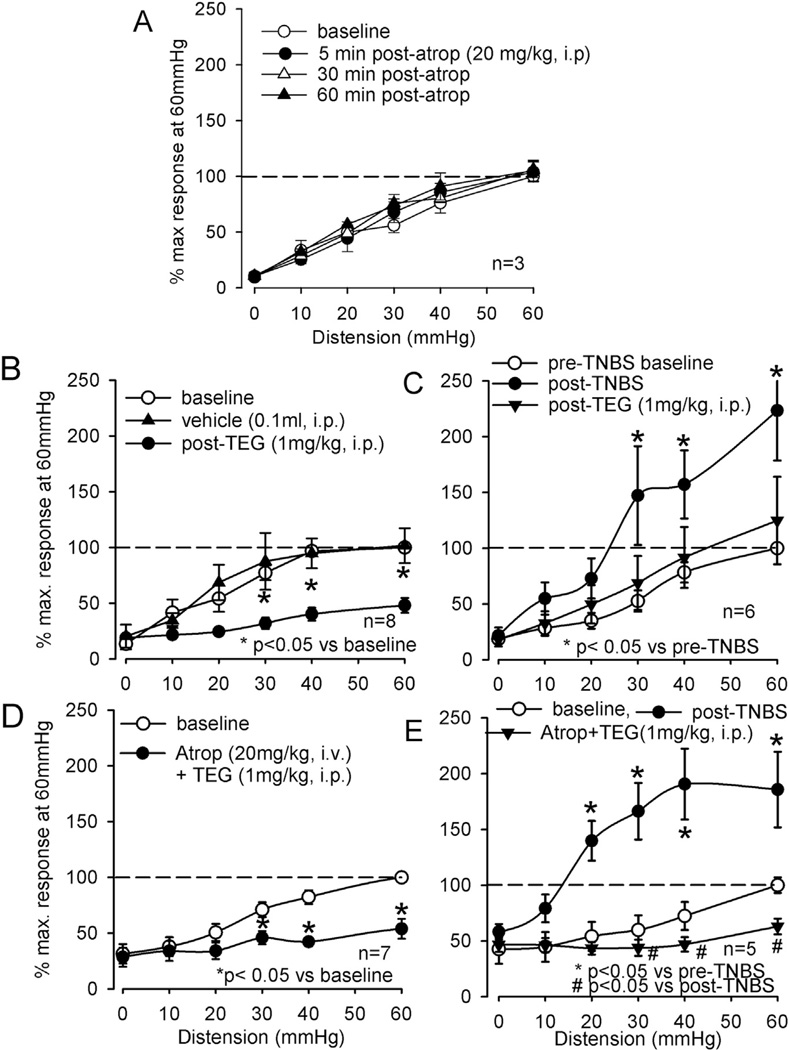
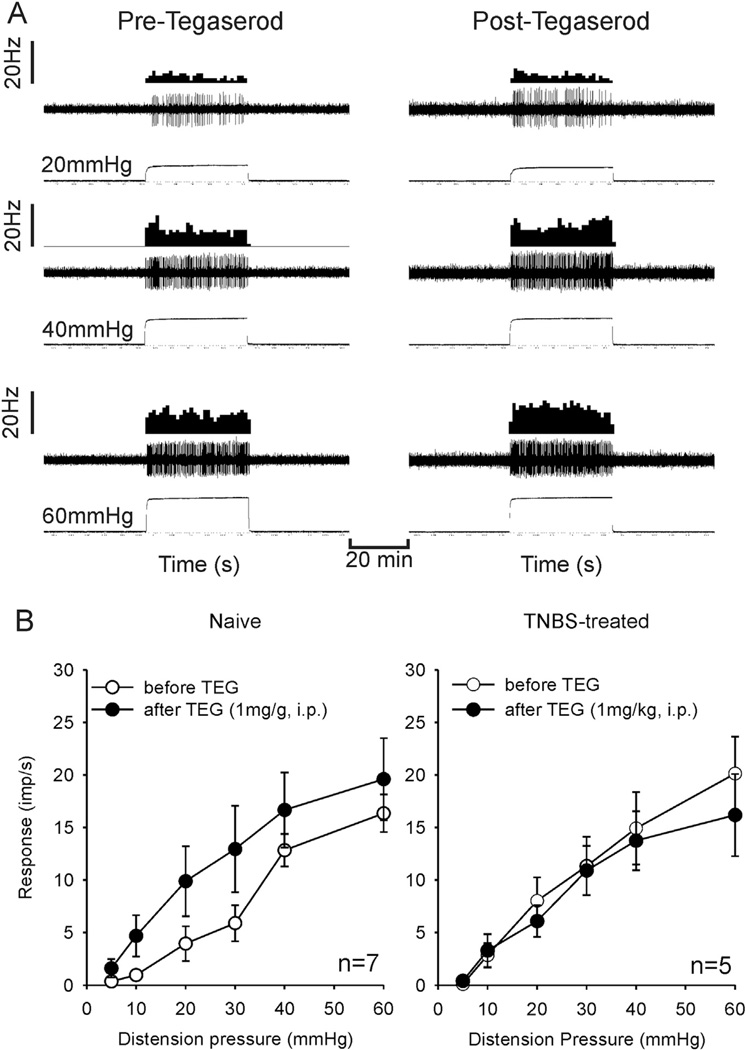
The EMG response to graded CRD was inhibited at all intensities of distending pressures following injection of TEG (1 mg/kg, i.p.) (Fig. 1). The analytical data from both naïve (n = 8) and TNBS-treated rats (n = 6) indicate that TEG (1 mg/kg, i.p.) significantly inhibited the VMR to CRD (Fig. 2B and 2C), whereas 10% propyleneglycol (PGL, 0.1 ml, i.p.), the vehicle for TEG, had no effect on VMRs of these rats (Fig. 2B). Since in freely moving rats, atropine prevented the CNS effects of high dose of TEG, we tested the effect of atropine alone on VMR and whether atropine could block the visceral analgesic effect of TEG in naïve or TNBS treated rats. In naïve rats (n = 3), atropine (20 mg/kg, i.p.) did not facilitate VMR response (Fig. 2A). Additionally, in both naïve (n = 5) and TNBS-treated (n = 5) rats, atropine (20 mg/kg, i.v.) pretreatment did not block the effect of TEG, suggesting that the VMR inhibitory effect of TEG is not mediated via the cholinergic mechanism (Fig. 2C and D).
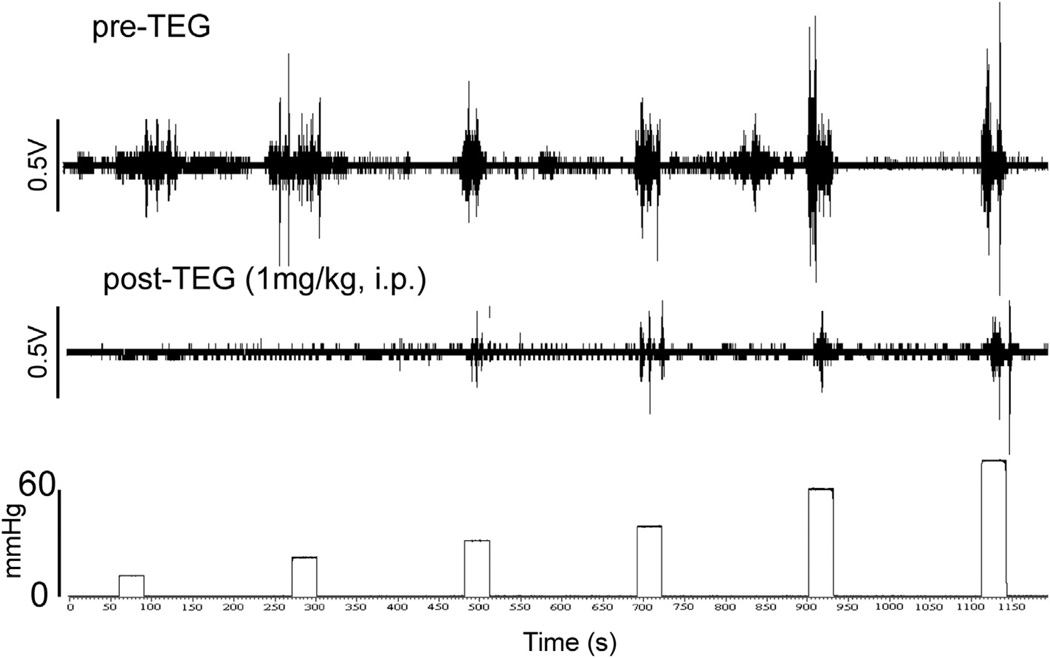
Fig. 1.
Viscero-motor response (VMR) represented as electromyographic (EMG) activities to contraction of external oblique abdominal muscle to graded (10–60 mmHg) colorectal distension (CRD). The top trace represents progressive increase in EMG to graded CRD before injection of TEG. The bottom trace illustrates marked reduction in EMG following intraperitoneal (i.p.) injection of TEG (1 mg/kg).
Fig. 2.
Summary data of effects of TEG (1 mg/kg, i.p.) on VMR to CRD in naïve and TNBS-treated rats and the effect of cholinergic antagonist atropine sulfate (Atrop). A: VMRs of naïve rats before and after injection of Atrop in naïve rats. Atrop did not change the VMRs of these rats. B: TEG significantly attenuated the VMR to graded CRD in naïve rats at distending pressures ≥ 30 mmHg. The vehicle propyleneglycol (0.1 ml, i.p.) did not attenuate the VMR. C: TNBS-treated rats exhibited significantly greater VMR that was attenuated by TEG. D and E: in naïve and TNBS-treated rats, pretreatment with Atrop (20 mg/kg, iv) did not block the inhibitory effect of TEG.
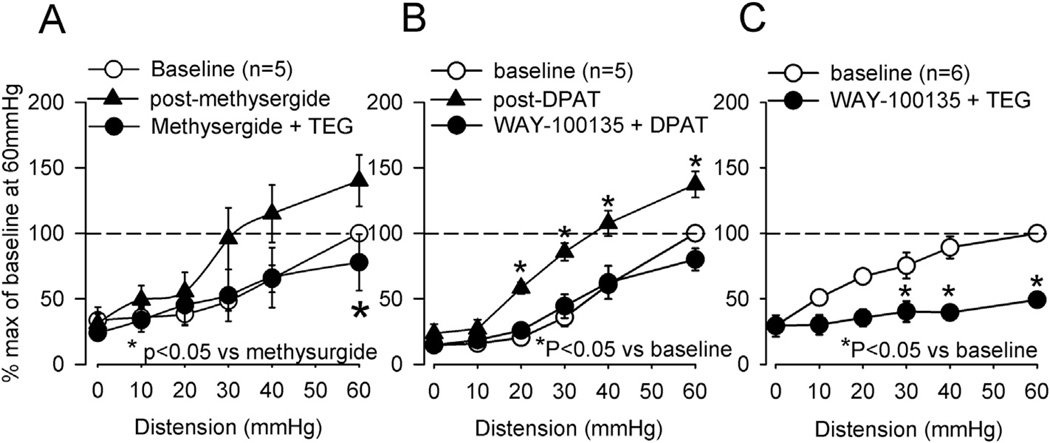
The effect of TEG was tested in rats (n = 6) preemptively treated with the non-selective 5-HT (5-HT1, 5-HT2, 5-HT7) receptor antagonist, methysergide (1 mg/kg, i.p.). Methysergide injection significantly increased the VMR, but it did not block the inhibitory effect of TEG (Fig. 3A). While TEG is preferentially a 5-HT4 receptor agonist, the drug also has affinity for 5-HT1A receptors and this may contribute to the analgesic effects (Doménech et al., 1994). To test the possibility of 5-HT1A receptors mediated analgesia, TEG was given to naïve rats that were pretreated with the selective 5-HT1A antagonist WAY-100135 (5 mg/kg, s.c.). Prior to testing the possible blocking effect of WAY-100135, we determined the effective blocking dose of WAY-100135 by testing it against a selective 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)-tetralin (DPAT). DPAT (50 µg/kg, i.p., n = 5) injection produced significant increase in VMR, indicating a pro-nociceptive effect of the drug. This effect of DPAT was completely blocked when rats were pre-treated with WAY-100135 (5 mg/kg, s.c. Fig. 3B, n = 5). However, pretreatment with the same dose of WAY-100135 failed to block the inhibitory effect of TEG (Fig. 3C, n = 6).
Fig. 3.
Summary data of effects of TEG (1 mg/kg, i.p.) on VMR to CRD in naïve rats and the effect of selective and non-selective 5-HT receptor antagonists. A: non-selective 5-HT1, 5-HT2 and 5-HT7 antagonist methysergide (1 mg/kg, i.p.) produced increase in the VMR. However, the antagonist did not block the inhibitory effect of TEG. B: the selective 5-HT1A receptor agonist 8-OH-DPAT (DPAT, 50 µg/kg, i.p., n = 5) produced a significant increase in the VMR. DPAT failed to increase VMR in rats (n = 5) pre-treated with the selective 5-HT1A antagonist WAY-100135 (5 mg/kg, sc). C: pre-treating rats (n = 6) with the same dose of WAY-100135 (5 mg/kg, sc) did not block the TEG-induced inhibition of the VMR, suggesting that the inhibitory effect of TEG was not via 5-HT1A receptors.
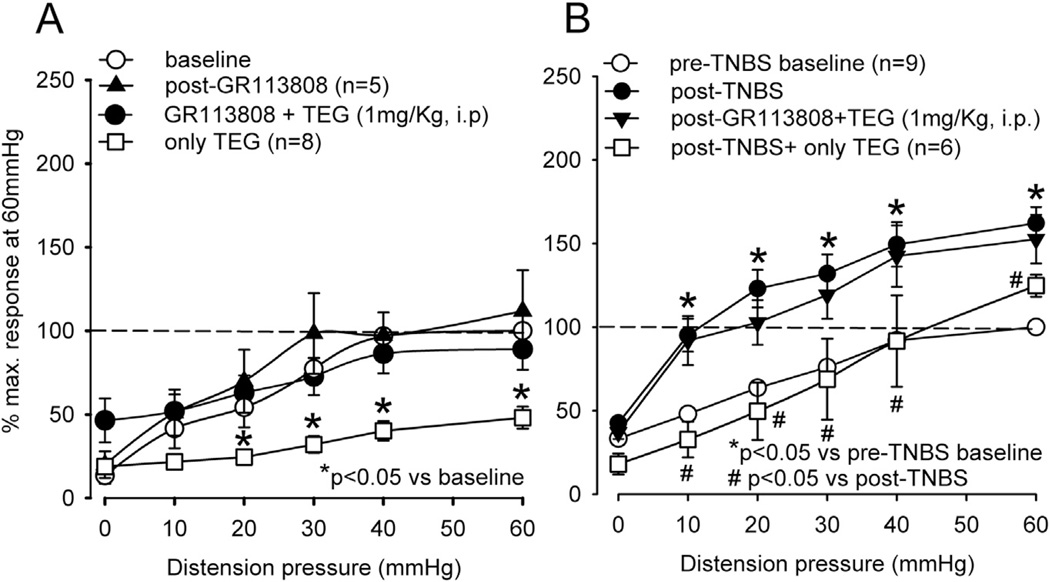
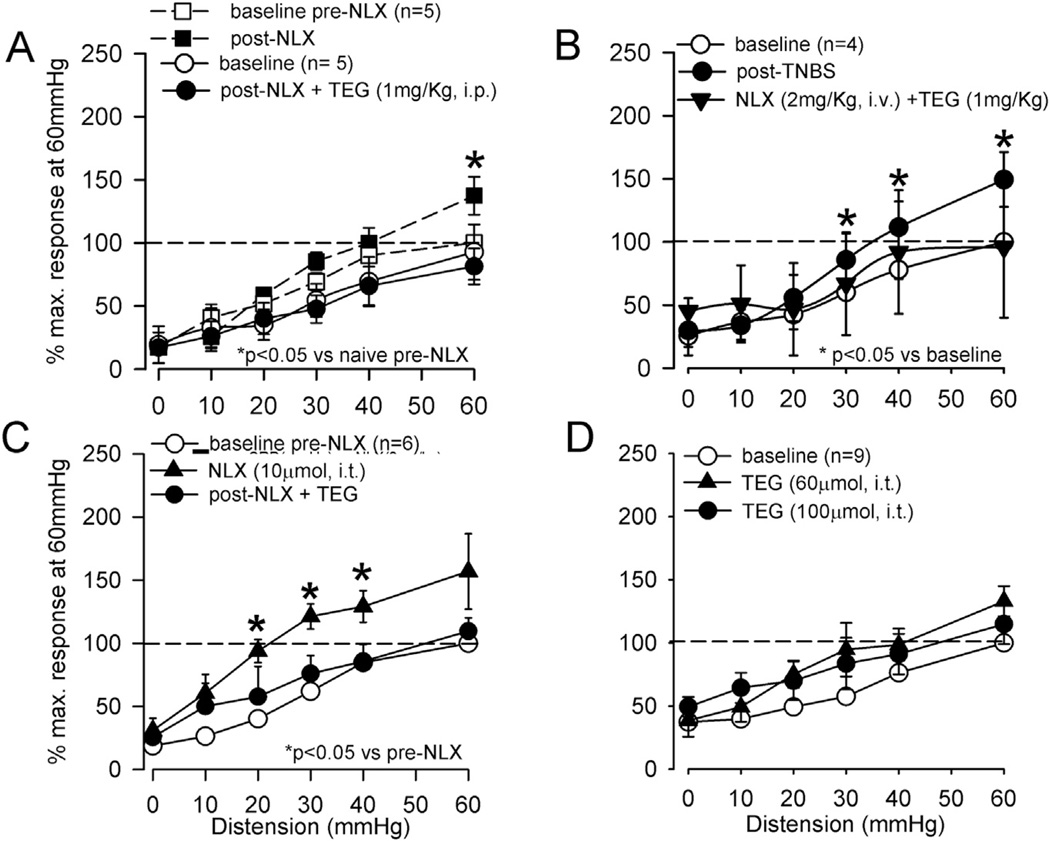
In another groups of rats (5 naive and 9 TNBS-treated), the inhibitory effect of TEG was significantly blocked by preemptive administration of the selective 5-HT4 receptor antagonist GR113808 (5 mg/kg, s.c.), suggesting that the inhibitory effect of TEG is primarily via the activation of 5-HT4 receptors (Fig. 4A and B). GR113808 (5 mg/kg, s.c.) itself did not affect the VMR in naïve rats (Fig. 4A). To further examine a potential link between 5-HT4 receptor mediated visceral analgesia and the opioidergic system; we tested the effect of TEG in presence of the opioid receptor antagonist naloxone (NLX, 2 mg/kg, i.v.). The systemic injection of NLX produced a significant increase in the VMR at distending pressures only at 60 mmHg (Fig. 5A, n = 5). In another set of naïve rats (n = 5), the VMR was tested following injection of NLX and TEG. NLX (i.v.) in the presence of TEG (i.p.) prevented inhibition of the VMR (Fig. 5A). A similar result was also observed in TNBS-treated rats (Fig. 5B).
Fig. 4.
The summary data of effects of selective 5-HT4 antagonist GR113808 on TEG-induced inhibition of the VMR. A: GR113808 (5 mg/kg, s.c.) did not affect the VMR of naïve rats (n = 5), but completely blocked the TEG-induced inhibition. B: TNBS-treated rats (n = 9) exhibited significantly (p < 0.05) greater VMR compared to pre-TNBS. Similar to naïve rats GR113808 pretreatment completely blocked the inhibitory effect of TEG.
Fig. 5.
The summary data of TEG-induced inhibition of VMR and the effect of opioid receptor antagonist naloxone (NLX) in naïve rats. A: in two sets of experiments (5 rats in each set) effect of NLX (n = 5) and NLX + TEG (n = 5) were tested on the VMR. In the first set of experiment (indicated in dashed lines), systemic injection of NLX (2 mg/kg, i.v.) significantly enhanced the VMR to distention pressures ≥ 30 mmHg. In the second set of experiment (indicated in solid lines), TEG (1 mg/kg, i.p.) did not produce inhibition of VMR in rats pre-treated with NLX. B: Similarly, in TNBS-treated rats NLX pre-treatment prevented TEG-induced inhibition. C: However, systemic injection of TEG (1 mg/kg, i.p.) produced significant inhibition of the VMR in rats (n = 6) spinally pretreated with NLX (10 µmol, i.t), suggesting that the spinal opioidergic system is not involved in TEG-induced inhibition. D: Spinal injection of two different doses (10 and 60 µmol, i.t.) of TEG did not attenuate the VMR, confirming the supra-spinal effect of the drug.
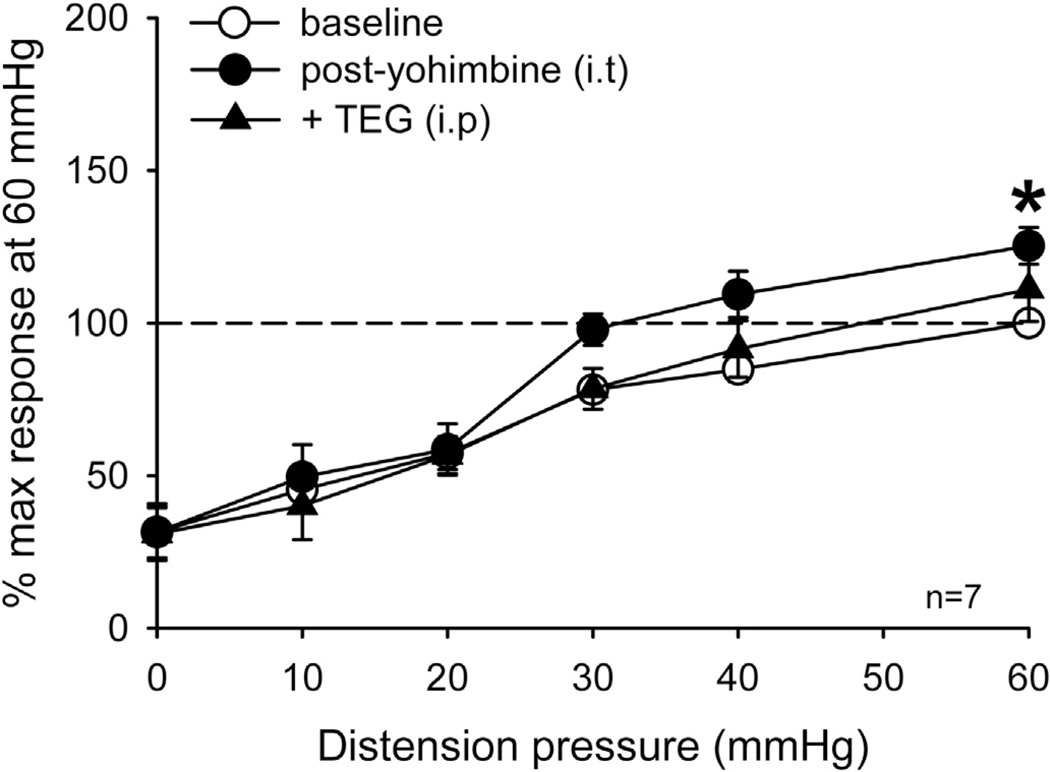
To further investigate the site of action of TEG, in a separate set of experiments (n = 6), NLX (10 µmol in 10 µl, i.t.) was injected into the lumbo-sacral (LS) spinal cord prior to systemic injection of TEG (1 mg/kg, i.p.). Intrathecal injection of NLX produced a significant increase in VMR in these rats, but failed to block the inhibitory effect of systemically injected (1 mg/kg, i.p.) TEG (Fig. 5C). This result suggests that the TEG-induced inhibition is not via the spinal opioidergic pathway. Subsequently, to confirm that TEG effect is not directly at the spinal level we injected the drug directly into the LS spinal cord (n = 7) and tested its effect on VMR. Both low (10 µmol) and high (60 µmol) doses of TEG were ineffective to attenuate VMR (Fig. 5D). In order to investigate whether supraspinal effect of TEG influences the descending noradrenergic pathways in the spinal cord to produce inhibition, we injected selective α2-adrenergic receptor blocker yohimbine (10 µmol) into the LS spinal cord prior to systemic injection of TEG. Yohimbine did not produce significant increase in VMR, but it blocked the inhibitory effect of TEG, suggesting that TEG in someway activates the descending noradrenergic pathway to attenuate VMR (Fig. 6).
Fig. 6.
The summary data of effect of α2 – adrenergic receptor antagonist yohimbine on inhibitory effect of TEG. Intrathecal injection of yohimbine (10 µmol) into the lumbosacral segment of the spinal cord produced slight, but not significant, increase in VMR in these rats. However, this dose of yohimbine completely blocked the inhibitory effect of TEG (1 mg/kg, i.p.).
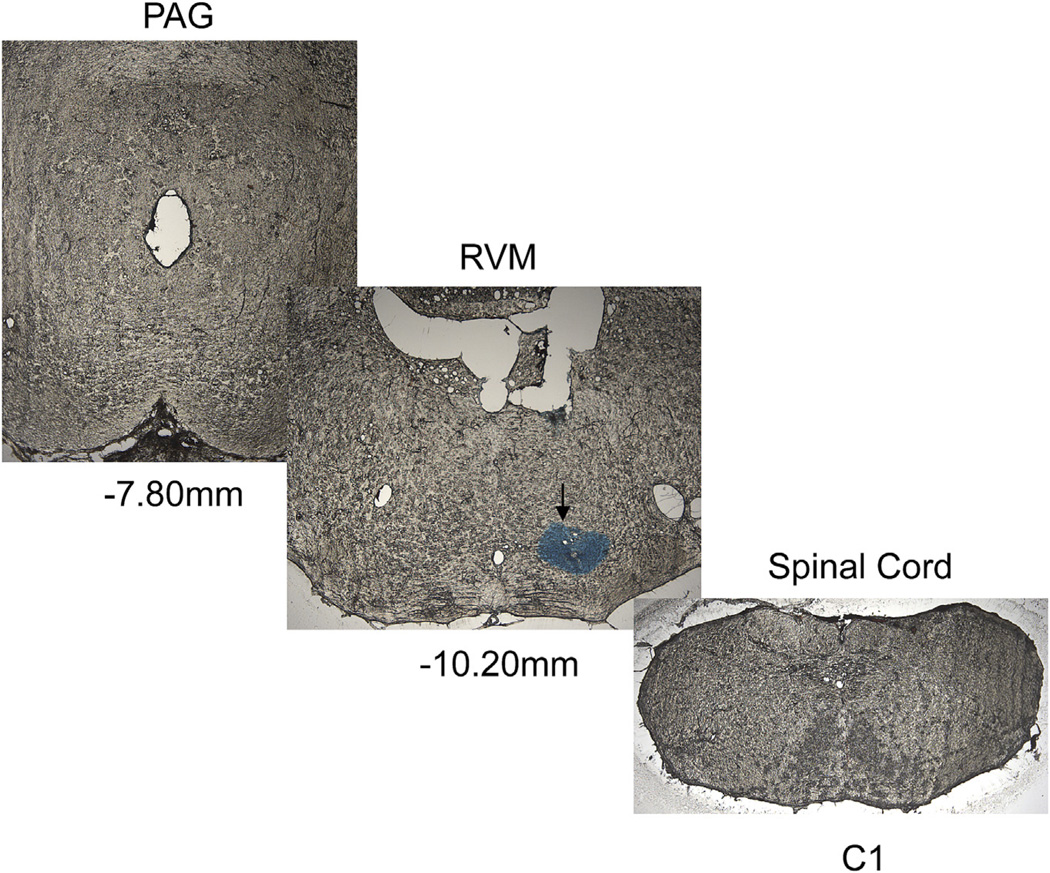
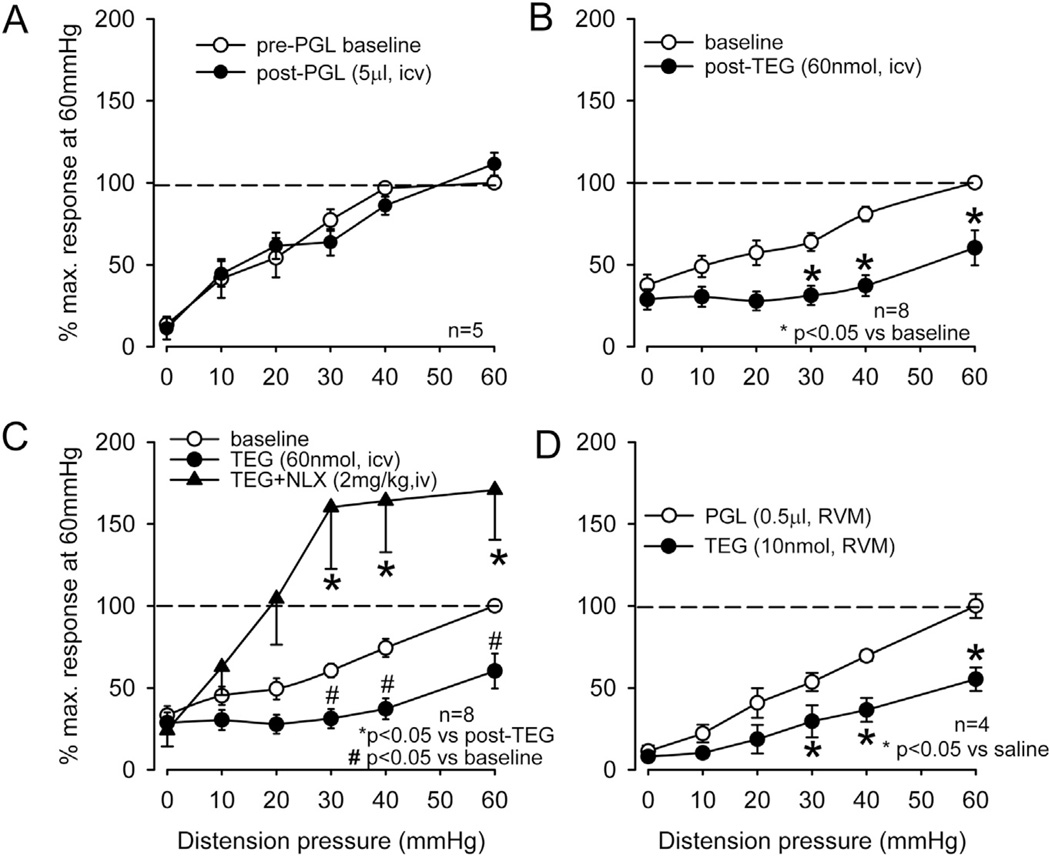
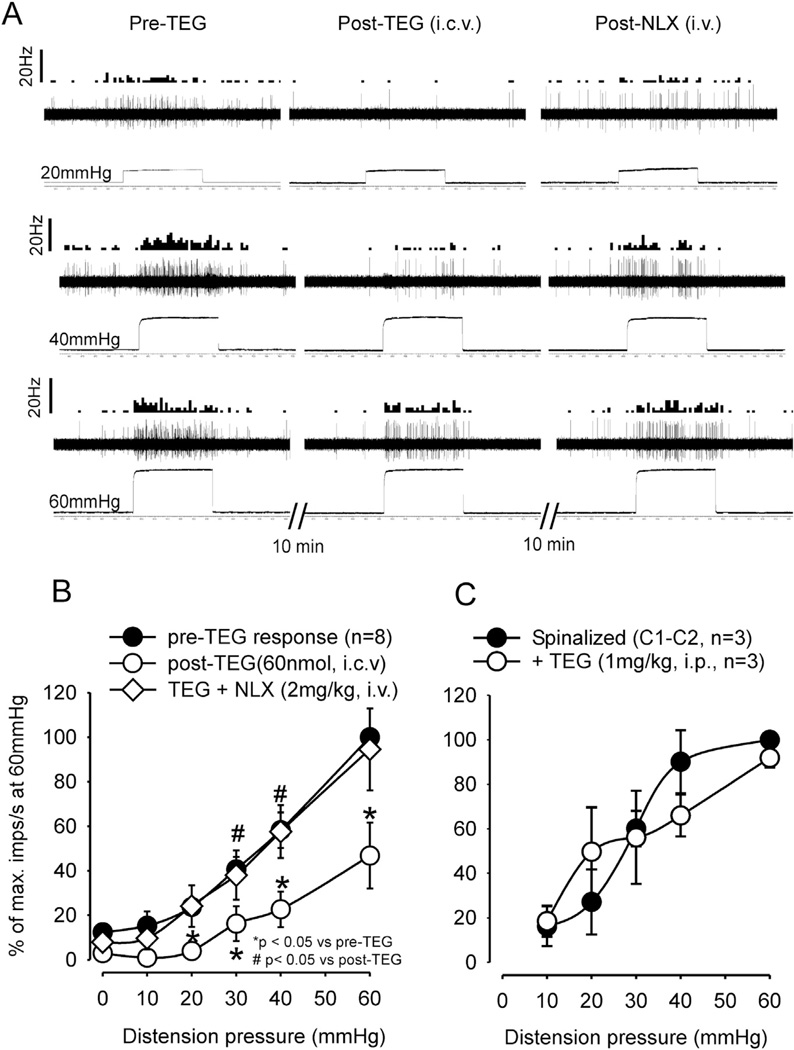
To confirm the effect of TEG at the supraspinal level, the drug was injected into the lateral ventricle (i.c.v) and to further investigate the site of action of the drug in the brain we also micro-injected (0.2 µl) the drug into the RVM. Fig. 7 illustrates an example of microinjection of TEG + 1% alcian blue to identify the exact site of injection. The serial sections clearly indicate that the drug was restricted to RVM and did not spread rostro-caudally in other areas of the brain and spinal cord. To exclude a possible influence of the vehicle propyleneglycol (PGL) for TEG in the brain, we tested the VMR before and after i.c.v. injection of 5 µl of 10% PGL into the lateral ventricle, which had no effect on the VMR (Fig. 8A, n = 5). However, the injection of TEG (60 nmol in 5 µl, i.c.v., n = 8) significantly inhibited the VMR (p < 0.05 vs pre-injection baseline, Fig. 8B). This inhibitory effect of TEG was reversed by systemic injection of NLX (2 mg/kg, i.v., n = 8) (Fig. 8C). To examine whether TEG influences the RVM descending pathway, TEG was injected directly into the RVM (10 nmol in 0.2 µl). This dose of the drug produced a significant (p < 0.05 vs PGL) attenuation of the VMR (Fig. 8D).
Fig. 7.
Illustrates microinjection of 1% alcian blue dye into the RVM area. Serial sections from the periaqueductal grey (PAG) to cervical spinal cord indicate that the dye was restricted at RVM area, but did to spread rostro-caudally to other brain structures. The coordinate reading for RVM injection was A–P: −2.5 mm lamda, 0 mm from midline, 9.4 mm dorso-ventral.
Fig. 8.
The summary data of intra-cerebroventricular (i.c.v) and intra-rostroventral medulla (RVM) injection of TEG on VMR. A: injection of TEG (60 nmol in 5 µl, i.c.v.) into the lateral ventricle significantly (p < 0.05) attenuated VMR. The vehicle propyleneglycol (10 µl i.c.v.) did not attenuate the VMR B: systemic injection of NLX (2 mg/kg, i.v.) not only recovered the inhibitory effect of TEG, but also significantly enhanced the VMR response at distending pressures ≥30 mmHg. C: injection of TEG (10 nmol) into the RVM significantly attenuated VMR in naïve rats.
3.2. Electrophysiology studies
3.2.1. Effect of TEG on colonic pelvic nerve afferent fibers in S1 dorsal root
To test the peripheral action of TEG on visceral sensory neurons, we recorded the responses of CRD-sensitive PNA fibers to graded CRD (5–60 mmHg, 30 s) before and after injection of TEG (1 mg/kg, i.p.). TEG did not attenuate mechanotransduction properties of these PNA fibers from naïve (n = 7) and TNBS-treated (n = 5) rats (Fig. 9A and B).
Fig. 9.
Effect of TEG (1 mg/kg, i.p.) on the mechanotransduction of CRD-sensitive pelvic nerve afferent fibers. A: illustrates responses of a fiber from a naive rat to CRD before and after injection of TEG. In each panel, the top trace represents the firing frequency histogram (1 s binwidth) of the neuron, middle trace represents nerve action potentials and the bottom trace represents distension pressure. The fiber exhibited intensity-dependent increase in firing frequency to CRD (5–60 mmHg), which was unaffected by TEG. B: mean SRFs of 12 neurons (7 from naïve and 5 from TNBS-treated rats) before and after TEG injection. TEG had no effect on the mean SRFs of pelvic nerve afferent fibers to CRD.
3.2.2. Effect of TEG on CRD-sensitive LS spinal neurons
Experiments were conducted to test the effect of TEG on CRD-sensitive, LS spinal neurons and to explore the influence on supraspinal site. We designed three different types of experiments: (1) effect of systemic injection of TEG (1 mg/kg, i.p.) on spinal neurons in cervical (C1–C2) spinal transected rats, (2) effect of i.c.v. injection of TEG (60 nmol) on spinal neurons in rats with intact spinal cord, and (3) effect of TEG microinjection in RVM on responses of LS spinal neurons in the presence of NLX in the RVM or i.v. The mean depth of recording of LS neurons was 905.93 ± 94.18 µm (range: 760–1350 µm) from the dorsal surface. In spinal transected rats (n = 3), i.p. injection of TEG had no effect on responses of CRD-sensitive neurons to graded CRD (Fig. 10C). This result is in agreement with our behavioral results showing that TEG did not produce inhibition of the VMR by acting at the spinal level. In contrast, i.c.v. injection of TEG inhibited the spontaneous firing and response to CRD (Fig. 10). Fig. 10A illustrates responses of one neuron to three intensities (20, 40 and 60 mmHg) of CRD before and after TEG (60 nmol, i.c.v.) injection and the recovery of response following injection of NLX (2 mg/kg, i.v.). The firing of these LS neurons to distention pressures ≥ 20 mmHg significantly decreased after TEG injection (p < 0.05 vs pre-TEG, n = 8). The responses to CRD were significantly recovered following NLX (p < 0.05 vs post-TEG) (Fig. 10B).
Fig. 10.
Illustrates responses of CRD-sensitive lumbo-sacral (LS) spinal neurons before and after injection of TEG into the lateral ventricle and the recovery of response after systemic injection of NLX (2 mg/kg, i.v.). A: responses of a CRD-sensitive LS neuron (recording depth of 1113.5 mm) before and after TEG (60 nmol in 10 µl, i.c.v.) injection and recovery of response following NLX (2 mg/kg, iv). In each panel, the top trace represents the firing frequency histogram (1 s binwidth) of the neuron, middle trace represents nerve action potentials and the bottom trace represents distension pressure. Ten minutes following i.c.v. injection of TEG, there was marked reduction of spontaneous firing and inhibition of response to CRD (middle column). The reduction of spontaneous firing and inhibition of response to CRD recovered after i.v. injection of NLX (last column). B: illustrates the mean stimulus-response functions (SRFs) of eight CRD-sensitive LS neurons before and after TEG (60 nmol, i.c.v.) injection and significant recovery of response following NLX (2 mg/kg, i.v.) injection. C: systemic injection of TEG (1 mg/kg, i.p.) did not attenuate responses of LS neurons to CRD in cervical (C1–C2) spinal transected rats, confirming no effect of i.t. injection of TEG on VMR (see Fig. 5D).
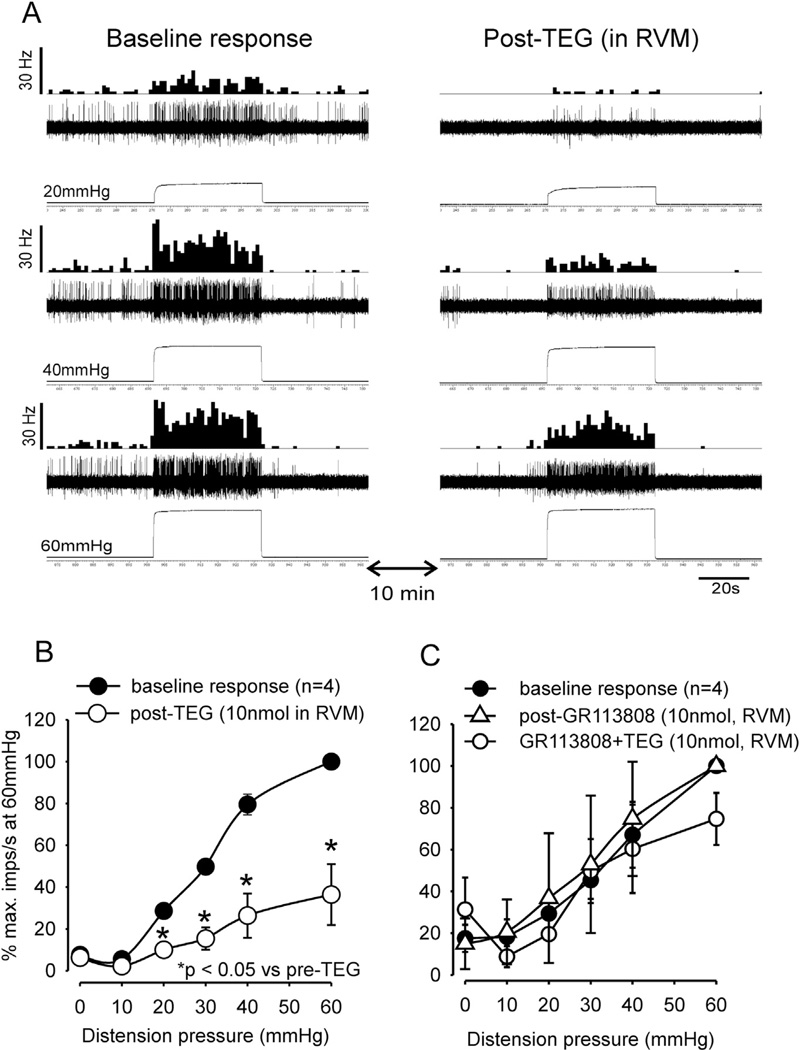
To test the involvement of RVM in TEG-induced inhibition of CRD-sensitive LS neurons, in another set of experiments, TEG (10 nmol in 0.2 µl) was injected into the RVM. In some neurons the drug reduced the spontaneous firing (Fig. 11A). The mean spontaneous firing of four neurons post-drug did show significant difference (mean: 0.86 ± 0.55 imps/s post-TEG vs 0.85 ± 0.17 imp/s, p > 0.05). Responses of all these neurons to colon distending pressure ≥ 20 mmHg were significantly (p < 0.05 vs pre-TEG) attenuated (Fig. 11B). To further investigate whether the inhibitory effect of TEG is mediated via the activation of 5-HT4 receptors, GR113808 (10 nmol in 0.2 µl, n = 4) was microinjected 10 min prior to TEG microinjection into the RVM. GR113808 injection did not alter the mean spontaneous firing (26.24 ± 6.34 imps/s) post-GR113808 vs 14.96 ± 12.12 imps/s post-GR113808, (p > 0.05, n = 4) or response to CRD, but was able to completely block the inhibitory effect of TEG (Fig. 11C).
Fig. 11.
Illustrates responses of CRD-sensitive LS spinal neuron before and after microinjection of TEG into the RVM and blocking of TEG effect by the selective 5-HT4 receptor antagonist GR113808. A: responses of a CRD-sensitive LS neuron (recording depth of 779 µm) before and after injection of TEG (10 nmol) into the RVM. In each panel, the top trace represents the firing frequency histogram (1 s binwidth) of the neuron, middle trace represents nerve action potentials and the bottom trace represents distension pressure. Ten minutes following microinjection of TEG there was marked reduction of spontaneous firing and inhibition of response to CRD (right column). B: illustrates the mean stimulus-response functions (SRFs) of four CRD-sensitive LS neurons before and after TEG microinjection into the RVM. TEG significantly (p < 0.05) attenuated responses of these neurons to graded CRD. C: shows mean SRFs following microinjection of GR113808 (10 nmol) into the RVM followed by microinjection of TEG. GR113808 did not influence responses of these neurons to graded CRD, but completely blocked the inhibitory effect of TEG.
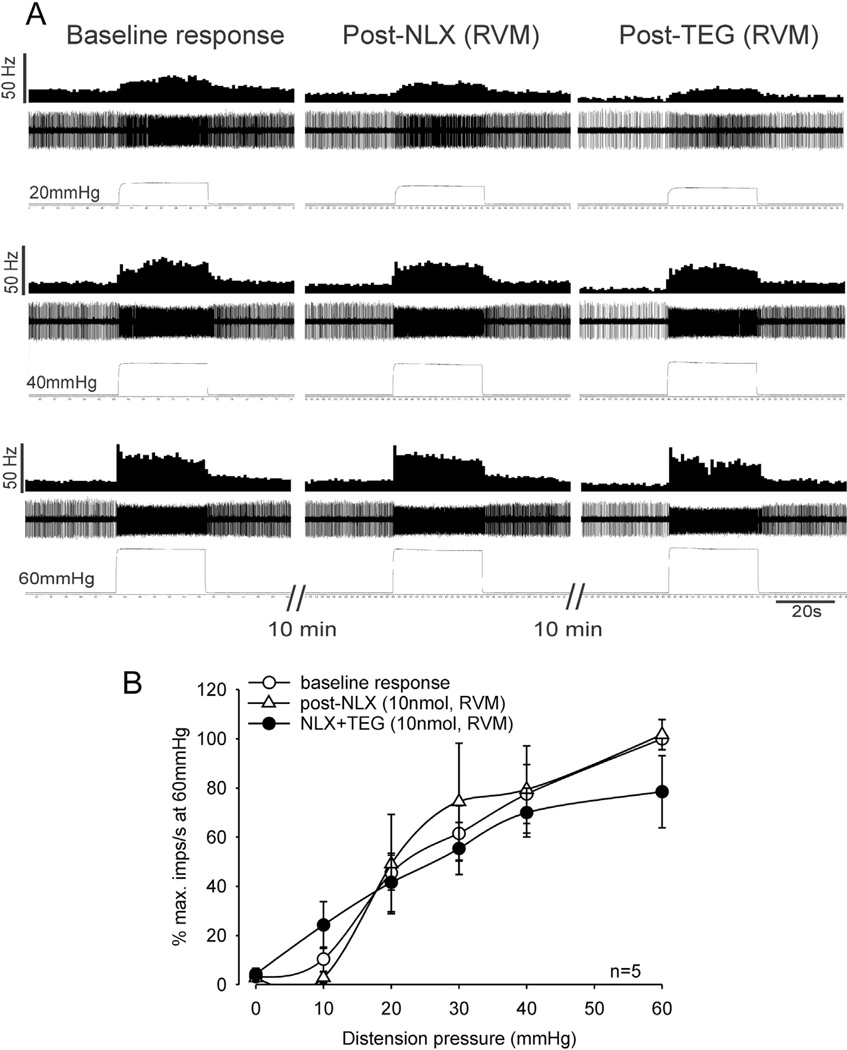
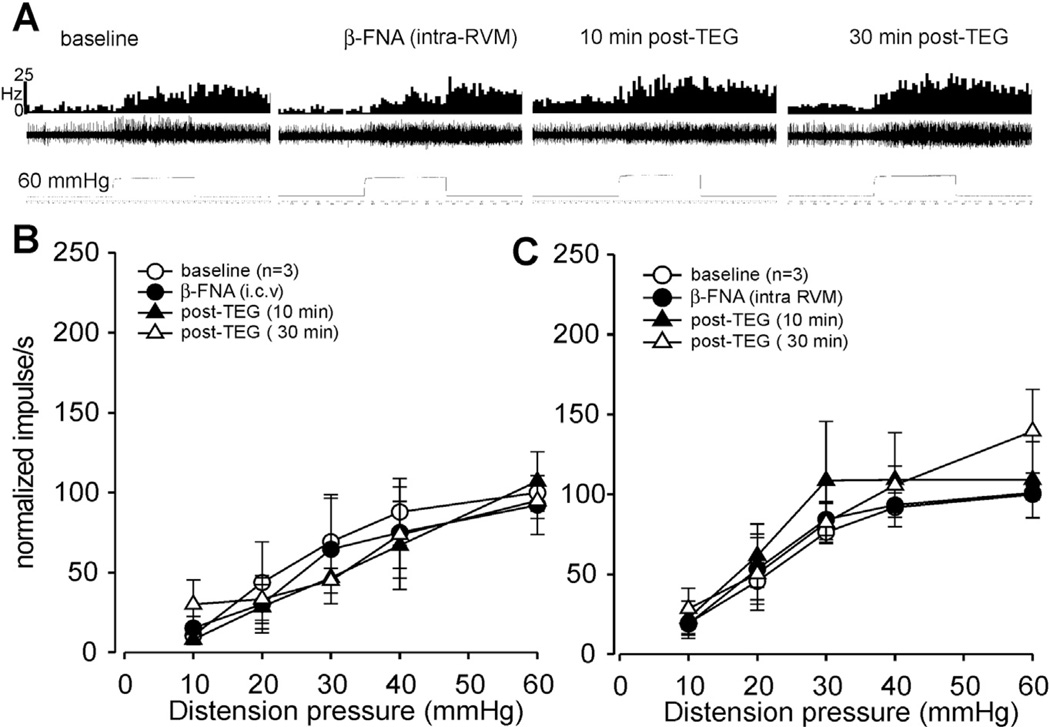
To test whether the inhibitory effect of TEG is associated with the RVM opioidergic system, TEG was microinjected into the RVM 10 min following microinjection of NLX (10 nmol in 0.2 µ, n = 5 in 5 rats) into the RVM. This dose of NLX did not change the mean spontaneous firing of these neurons (3.27 ± 1.74 imps/s post-NLX vs 2.80 ± 1.34 imps/s pre-NLX, n = 5) nor did it influence the neuronal responses to graded CRD. However, NLX microinjection into the RVM completely blocked the inhibitory effect of TEG (Fig. 12A and B). Since NLX is reported to be a non-selective, short-acting opioid receptor antagonist with a very short half-life (10– 15 min), we performed additional experiments with β-Funaltrex-amine hydrochloride (β-FNA) which is a highly selective long acting µ-opioid receptor antagonist. Fig. 13A illustrates responses of one neuron to 60 mmHg of CRD before and after β-FNA injection into the RVM and the response following injection of TEG (1 mg/kg, i.p.). Results showed that i.c.v microinjection (Fig. 13B) or intra-RVM injection (Fig. 13B) of β-FNA completely blocked the inhibitory effect of TEG.
Fig. 12.
Illustrates responses of CRD-sensitive LS spinal neurons before and after microinjection of NLX (10 nmol) and TEG (10 nmol) into the RVM. A: responses of a CRD-sensitive LS neuron (recording depth of 1020.5 µm) before and after injection of NLX into the RVM followed by microinjection of TEG. In each panel, the top trace represents the firing frequency histogram (1 s binwidth) of the neuron, middle trace represents nerve action potentials and the bottom trace represents distension pressure. Microinjection of NLX into the RVM did not markedly change the spontaneous firing and responses of the neuron to CRD (middle column). However, NLX injection into the RVM completely blocked the inhibitory effect of TEG (right column). B: illustrates the mean stimulus-response functions (SRFs) of five CRD-sensitive LS neurons before and after microinjection of NLX and TEG into the RVM. SRFs indicate that NLX blocked the inhibitory effect of TEG on responses of CRD-sensitive LS spinal neurons to CRD.
Fig. 13.
Illustrates responses of CRD-sensitive lumbo-sacral (LS) spinal neurons before and after injection of β-FNA into the RVM and the response after systemic injection of TEG (1 mg/kg, i.p.). A: responses of a CRD-sensitive LS neuron before and after β-FNA (1 nmol in 0.2 µl, intra-RVM.) injection and the response following TEG (1 mg/kg, i.p). In each panel, the top trace represents the firing frequency histogram (1 s binwidth) of the neuron, middle trace represents nerve action potentials and the bottom trace represents distension pressure. Microinjection of β-FNA into the RVM did not markedly change the spontaneous firing and responses of the neuron to CRD (middle column). However, inhibitory effect of TEG (i.p) was completely blocked by prior beta-FNA injection. B: illustrates the mean stimulus-response functions (SRFs) of three CRD-sensitive LS neurons before and after β-FNA (10 nmol, i.c.v.) injection and the response following TEG (1 mg/kg, i.p.) injection (filled and open triangles). C: illustrates the mean stimulus-response functions (SRFs) of three CRD-sensitive LS neurons before and after β-FNA (1 nmol, intra-RVM) injection and the response following TEG (1 mg/kg, i.p.) injection (filled and open triangles).
4. Discussion
The study involved behavioral and electrophysiology experiments to document the antinociceptive effect of the 5-HT4 receptor agonist, TEG and the possible sites of action of the drug in the neuraxis. Our results indicate that the antinociceptive effect of TEG is primarily mediated via the activation of 5-HT4 receptors linked to supraspinal opioidergic system, but not via cholinergic pathways. Support for this statement comes from two major findings; 1) methysergide (a non-selective 5-HT1, 5-HT2 and 5-HT7 antagonist) or WAY 100135 (a selective 5-HT1A receptors antagonist) did not block the effect of TEG, whereas the 5-HT4 receptor antagonist GR113808 significantly blocked the effect, (2) pre-treatment with NLX, but not atropine, significantly attenuated the anti-nociceptive effect. Additional findings confirm the exclusive supraspinal site of action of TEG, which is linked to central opioidergic systems. Further results also indicate that the supraspinal action of TEG in someway activates the descending noradrenergic pathways to produce visceral analgesia. These findings are drawn from following results: 1) TEG did not attenuate VMR when injected directly into the LS spinal cord and it did not inhibit responses of CRD-sensitive LS spinal neurons in upper cervical spinal transected rats, 2) TEG significantly attenuated VMR after ventricular (i.c.v.) injection and microinjection into the RVM, 3) TEG inhibited responses of CRD-sensitive LS neurons following i.c.v. injection or microinjection into the RVM, 4) there was no effect of TEG on CRD-sensitive PNA fibers and finally 5) although TEG does not act directly at the spinal level, its supraspinal influence activates descending noradrenergic pathways as i.t. injection of α2-adrenergic receptor antagonist yohimbine completely blocks the inhibitory effect of TEG.
The finding that the effect of TEG is associated with the opioidergic system in the RVM was confirmed by administering naloxone prior to TEG injection in the RVM, which antagonized the effect of TEG. Since systemic and focal injection of NLX and β-FNA into the RVM blocked the inhibitory effect of TEG on VMR and CRD-sensitive lumbosacral neurons, it is likely that the action of TEG is in someway linked with opioids. It is also to be noted that i.t. injection of NLX did not block the effect of TEG suggesting that the effect of the drug possibly does not influence spinal opioidergic system. However, it is very unlikely that the visceral antinociceptive effect of TEG occurs exclusively in the RVM since 5-HT4 receptors are also expressed in other brain areas. In retrograde fluorogold labeling of dorsal column of lumbosacral spinal cord, we have observed a large proportion of labeled soma in the raphe area. A majority of these labeled cells express 5-HT4 receptors (unpublished data). In addition, many of these 5-HT4-positive neurons are m-enkephalin and/or β-endorhin positive (unpublished data). In a recent report, it has been documented that RVM ‘ON’ cells are 5-HT3 expressing neurons that exert visceral pronociceptive effects in rats (Sikandar et al., 2012). Intrathecal injection of 5-HT3 antagonist ondansetron attenuates visceral hyperalgesia induced by intracolonic mustard oil. We have documented similar result where alosetron, a 5-HT3 antagonist, prevented somatic hypersensitivity and visceral hyperalgesia when injected either systemically or intrathecally (Miranda et al., 2006).
Nociceptive transmission from the periphery via the ascending spinal pathway is generally under the influence of the descending inhibitory and facilitatory pathways (Cervero and Wolstencroft, 1984; Zhuo and Gebhart, 1997, 2002 Porreca et al., 2002; Zhuo et al., 2002; Vanegas and Schaible, 2004; Vera-Portocarrero et al., 2006). Descending control of pain arises from several supraspinal areas including the PAG-RVM-spinal pathway (Reynolds, 1969; Pertovaara, 2006; Tavares and Lima, 2002; Heinricher et al., 2009). This pathway has been recognized as the dominant pathway involved in the modulation of visceral pain (Fields and Heinricher, 1985, 1989; Reynolds, 1969). Further, it has been proposed that RVM neurons may exert bi-directional control of nociception through descending serotonergic pathways (Fields and Heinricher, 1991; Roychowdhury and Heinricher, 1997; Vanegas and Schaible, 2004). While several studies have suggested an aberrant opioidergic descending inhibitory system in animal models of visceral hyperalgesia and in patients with IBS, a link to serotonin and particularly 5-HT4 receptors has not been systematically investigated (Coutinho et al., 2002; Lembo et al., 2000; DeBerry et al., 2007). This study provides convincing evidence that the analgesic effect of TEG is likely mediated via an opioidergic system linked to 5-HT4 receptor, since both the 5-HT4 receptor antagonist GR113808 and opioid receptor antagonist NLX significantly blocked the effect of TEG in behavioral and electrophysiological studies. Interestingly, in naïve rats, NLX was pro-nociceptive when given i.p. and led to an increase the VMR to CRD. NLX (i.v.) in the presence of TEG (i.p.) prevented inhibition of the VMR in both naïve and TNBS-treated rats. However, this result can be interpreted in two ways: (1) the blockade of opioid action released by TEG or (2) a simple counterbalance of TEG-induced inhibition unrelated to opioid release and opioidergic pathway. Since we could not rule out the possibility that NLX was nonspecifically masking the inhibitory effect of TEG, we carried out additional experiments to confirm a specific site of action. Electrophysiology experiments showed that NLX, administered in the RVM, does not alter the response of CRD-sensitive spinal neurons and more importantly, it was able to block the effect of TEG administered in the RVM. Since NLX is nonspecific, short-acting and rapidly crosses the blood-brain barrier its antagonistic property can be questioned. Our electrophysiology experiments using β-FNA validate the involvement of opioid, since this selective long acting antagonist completely blocked the inhibitory effect of TEG when injected into the RVM. These results confirm that the antinociceptive effect of TEG is in the RVM involves opioidergic system.
Human and animal studies suggest that 5-HT3 and 5-HT4 receptors play a critical role in visceral hypersensitivity in IBS, but the exact mechanisms remain unknown (Gershon and Liu, 2007; Greenwood-van Meerveld, 2007; Spiller, 2007). Recent reports have documented that both 5-HT3 antagonists and 5-HT4 agonists have antinociceptive effects to noxious colon distension (Kozlowski et al., 2000; Greenwood-Van Meerveld et al., 2006). Interestingly, some studies have suggested that TEG has a peripheral analgesic effect. However, in the present study, we have shown that the dose of TEG that attenuates VMR in awake animals does not modulate responses of mechanosensitive CRD-sensitive pelvic nerve afferents, suggesting that TEG-induced visceral analgesia is not peripherally mediated. The present result also shows that unlike other compounds that work only in hyperalgesic conditions, TEG is also efficacious in altering visceral nociception in naïve conditions.
4.1. Functional role of central 5-HT4 receptors and possible mechanisms
It is apparent from the present study that the CNS effect of TEG has two dose-related components. At higher doses (≥ 5 mg/kg, i.p.), it produces CNS effects via the release of acetylcholine, since atropine delayed these effects. However, at lower doses (≤1 mg/kg), it produces visceral antinociception via opioids and via muscarinic pathway (see Fig. 2). In addition to their role in cognitive behavior, 5-HT4 receptors are also involved in somatic pain, possibly through a different mechanism. In mice and rats, microinjection of 5-HT4 agonists (BIMU1 and BIMU8) into the ventricular space produced significant antinociception to hot plate and paw-pressure tests, which was prevented by the selective 5-HT4 antagonists (SDZ 205–557, GR 125487) and by atropine (Ghelardini et al., 1996). Based on these results, it was concluded that 5-HT4 receptor agonists produced somatic antinociception by amplifying central cholinergic transmission. The effect of TEG on cutaneous or somatic hypersensitivity was not explored in the present study and requires further investigation.
The present data provide convincing evidence that the anti-nociceptive effect of TEG in naïve and TNBS-treated rats occurs via 5-HT4 receptors linked to central opioidergic system. Although additional brain areas need to be examined in further studies, we have shown that the drug produces its analgesic effects by acting in medullary sites, specifically by activation of opioidergic neurons in the RVM. Since the neural circuitry in RVM is quite complicated, it is difficult to have a straightforward explanation on how exactly the 5-HT4 receptor agonist produces descending spinal inhibition. NLX or β-FNA microinjected into the RVM area does block the anti-nociceptive effect of TEG, thus showing that local terminals of these opioidergic neurons are involved, but these neurons may not be the same spinally projecting neurons as NLX fails to block the effect of TEG at the spinal level. 5-HT4 is a positive G-protein-coupled receptor; activation of which leads to an increase in adenyl cyclase activity and thus facilitates the release of opioid by activating presynaptic terminals of opioidergic neurons that are expressing 5-HT4 receptors. In RVM, ‘On’-cells are the only neurons directly inhibited by opioids and since these cells serve descending facilitation (Fields and Heinricher, 1991; Roychowdhury and Heinricher, 1997; Vanegas and Schaible, 2004), their inhibition by locally released opioids from 5-HT4 receptor expressing opioidergic neurons would result in net spinal anti-nociception. Another possibility can be offered by the fact that RVM ‘Off’-cells, which serve descending inhibition in somatic pain, are inhibited by GABAergic neurons, and GABA release is decreased by opioids (Heinricher, 1994). Therefore, activation of 5-HT4 receptors expressing opioidergic neurons might lead to opioid release and inhibition of synaptic GABA release and disinhibition of ‘Off’-cells in charge of descending inhibition. However, it needs to be acknowledged that the current literature regarding the roles of ‘ON and ‘OFF’ cells are debatable.
It is very clear from our study that TEG has no direct action at the spinal level, it definitely activates descending noradrenergic pathway to produce inhibition via the activation of α2 adrenergic receptors. There could be several possible mechanisms by which systemic injection of TEG can influence the descending noradrenergic/adrenergic pathways to produce antinociception; (1) an opioid independent direct activation of supraspinal adrenergic cell clusters in medullary C1 and C2 nuclei, noradrenergic nuclei in A5, A6 (locus coeruleus) and pontine A7 (subcoeruleus) regions (Tucker et al., 1987; Kwiat and Basbaum, 1992; Westlund, 1992; Tavares et al., 1996; Millan, 1997; Schreihofer and Guyenet, 1997; Simpson et al., 1997; Hökfelt et al., 2000), (2) inhibition of RVM GABAergic neurons by opioids can disinhibit descending noradrenergic pathways (Nuseir and Proudfit, 2000) and (3) by activation of RVM opioidergic neurons projecting to A7 subcoeruleus nuclei (i.e., RVM-A7 opioidergic link) to excite descending noradrenergic pathway (Brodie and Proudfit, 1986; Gebhart and Randich, 1992; Grabow et al., 1992; Holden and Proudfit, 1998; Holden et al., 1999; Kellum et al., 1999). Since our results show that the TEG effect is opioid dependent, we believe that the last two possibilities involving RVM are the most likely mechanisms of TEG-induced analgesia. However, more systematic studies are warranted in the future to delineate the effect of 5-HT4 receptor agonists in other brain areas.
Acknowledgment
The study has been supported by Procter & Gamble Pharmaceutical, Inc., unrestricted research fund and NIH 1R56DK089493-01 awarded to Jyoti N. Sengupta. Authors have no conflict of interest.
Footnotes
Ethics approval
All experiments were performed according to the approved guidelines of the Institutional Animal care and Use committee at the Medical college of Wisconsin (approval # AUA356) and International Association for the Study of Pain (IASP) for humane use of laboratory animals.
References
- Anderson JL, May HT, Bair TL, Muhlestein JB, Horne BD, Carlquist JF. Lack of association of tegaserod with adverse cardiovascular outcomes in a matched case-control study. J. Cardiovasc. Pharmacol. Ther. 2009;14:170–175. doi: 10.1177/1074248409340158. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Borman RA, Burleigh DE. Evidence for the involvement of a 5-HT4 receptor in the secretory response of human small intestine to 5-HT. Br. J. Pharmacol. 1993;110:927–928. doi: 10.1111/j.1476-5381.1993.tb13901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Proudfit HK. Antinociception induced by local injection of carbachol into the nucleus raphe magnus: alteration by intrathecal injection of noradrenergic antagonists. Brain Res. 1986;371:70–79. doi: 10.1016/0006-8993(86)90811-5. [DOI] [PubMed] [Google Scholar]
- Cervero F, Wolstencroft JH. A positive feedback loop between spinal cord nociceptive pathways and antinociceptive areas of the cat’s brain stem. Pain. 1984;20:125–138. doi: 10.1016/0304-3959(84)90094-0. [DOI] [PubMed] [Google Scholar]
- Consolo S, Arnaboldi S, Giorgi S, Russi G, Ladinsky H. 5-HT4 receptor stimulation facilitates acetylcholine release in rat frontal cortex. Neuroreport. 1994;5:1230–1232. doi: 10.1097/00001756-199406020-00018. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- DeBerry J, Ness TJ, Robbins MT, Randich A. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distention: modulation by endogenous opioids and the effects of early-in-life experience with bladder inflammation. J. Pain. 2007;8:914–923. doi: 10.1016/j.jpain.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doménech T, Beleta J, Fernández AG, Gristwood RW, Cruz Sánchez F, Tolosa E, Palacios JM. Identification and characterization of serotonin 5-HT4 receptor binding sites in human brain: comparison with other mammalian species. Mol. Brain. Res. 1994;21:176–180. doi: 10.1016/0169-328x(94)90392-1. [DOI] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM. Brainstem modulation of nociceptor-driven withdrawal reflexes. Ann. N. Y. Acad. Sci. 1989;563:34–44. doi: 10.1111/j.1749-6632.1989.tb42188.x. [DOI] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu. Rev. Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Gebhart GF, Randich A. Vagal modulation of nociception. Am. Pain. Soc. J. 1992;1:26–32. doi: 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Liu MT. Serotonin and neuroprotection in functional bowel disorders. Neurogastroenterol. Motil. 2007;9:19–24. doi: 10.1111/j.1365-2982.2007.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelardini C, Galeotti N, Casamenti F, Malmberg-Aiello P, Pepeu G, Gualtieri F, Bartolini A. Central cholinergic antinociception induced by 5HT4 agonists: BIMU 1 and BIMU 8. Life. Sci. 1996;58:2297–2309. doi: 10.1016/0024-3205(96)00230-5. [DOI] [PubMed] [Google Scholar]
- Gershon MD. 5-HT (serotonin) physiology and related drugs. Curr. Opin. Gastroenterol. 2000;16:113–120. doi: 10.1097/00001574-200003000-00004. [DOI] [PubMed] [Google Scholar]
- Grabow TS, Hurley RW, Banfor PN, Hammond DL. Supraspinal and spinal delta2 opioid receptor-mediated antinociceptive synergy is mediated by spinal alpha2-adrenoceptors. Pain. 1992;83:47–55. doi: 10.1016/s0304-3959(99)00084-6. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Venkova K, Hicks G, Dennis E, Crowell MD. Activation of peripheral 5-HT receptors attenuates colonic sensitivity to intraluminal distension. Neurogastroenterol. Motil. 2006;18:76–86. doi: 10.1111/j.1365-2982.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- Greenwood-van Meerveld B. Importance of 5-hydroxytryptamine receptors on intestinal afferents in the regulation of visceral sensitivity. Neurogastroenterol. Motil. 2007;19:13–18. doi: 10.1111/j.1365-2982.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115:370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- Grossman CJ, Kilpatrick GJ, Bunce KT. Development of radioligand binding assay for 5-HT4 receptors in guinea-pig and rat brain. Br. J. Pharmacol. 1993;109:618–624. doi: 10.1111/j.1476-5381.1993.tb13617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain. Res. Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience. 1994;63:533–546. doi: 10.1016/0306-4522(94)90548-7. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Arvidsson U, Cullheim S, Millhorn D, Nicholas AP, Pieribone V, Seroogy K, Ulfhake B. Multiple messengers in descending serotonin neurons: localization and functional implications. J. Chem. Neuroanat. 2000;18:75–86. doi: 10.1016/s0891-0618(99)00037-x. [DOI] [PubMed] [Google Scholar]
- Holden JE, Proudfit HK. Enkephalin neurons that project to the A7 catecholamine cell group are located in brainstem nuclei that modulate nociception: ventromedial medulla. Neuroscience. 1998;83:929–947. doi: 10.1016/s0306-4522(97)00437-5. [DOI] [PubMed] [Google Scholar]
- Holden JE, Schwartz EJ, Proudfit HK. Microinjection of morphine in the A7 catecholamine cell group produces opposing effects on nociception that are mediated by α 1- and α 2-adrenoceptors. Neuroscience. 1999;91:979–990. doi: 10.1016/s0306-4522(98)00673-3. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, To ZP, Eglen RM, Wong EH, Bonhaus DW. Quantitative autoradiography of 5-HT4 receptors in brains of three species using two structurally distinct radioligands, [3H]GR113808 and [3H]BIMU-1. Neuropharmacology. 1994;33:1027–1038. doi: 10.1016/0028-3908(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl secretion via afferent neurons and 5-HT4 receptors. Am. J. Physiol. 1999;277:G515–G520. doi: 10.1152/ajpgi.1999.277.3.G515. [DOI] [PubMed] [Google Scholar]
- Kozlowski CM, Green A, Grundy D, Boissonade FM, Bountra C. The 5-HT(3) receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetized. rat. Gut. 2000;46:474–480. doi: 10.1136/gut.46.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiat GC, Basbaum AI. The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn of the rat. Somatosens. Mot. Res. 1992;9:157–173. doi: 10.3109/08990229209144768. [DOI] [PubMed] [Google Scholar]
- Langlois M, Fischmeister R. 5-HT4 receptor ligands: applications and new prospects. J. Med. Chem. 2003;46:319–344. doi: 10.1021/jm020099f. [DOI] [PubMed] [Google Scholar]
- Lembo T, Naliboff BD, Matin K, Munakata J, Parker RA, Gracely RH, Mayer EA. Irritable bowel syndrome patients’ show altered sensitivity to exogenous opioids. Pain. 2000;87:137–147. doi: 10.1016/S0304-3959(00)00282-7. [DOI] [PubMed] [Google Scholar]
- Liang LX, Zhang Q, Qian W, Hou XH. Antinociceptive property of tegaserod in a rat model of chronic visceral hypersensitivity. Chin. J. Dig. Dis. 2005;6:21–25. doi: 10.1111/j.1443-9573.2005.00182.x. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Vaughan CW, Schnell SA, Wessendorf MW, Christie MJ. Rostral ventromedial medulla neurons that project to the spinal cord express multiple opioid receptor phenotypes. J. Neurosci. 2002;22:10847–10855. doi: 10.1523/JNEUROSCI.22-24-10847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengod G, Vilaró MT, Raurich A, López-Giménez JF, Cortés R, Palacios JM. 5-HT receptors in mammalian brain: receptor autoradiography and in situ hybridization studies of new ligands and newly identified receptors. Histochem. J. 1996;28:747–758. doi: 10.1007/BF02272148. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The role of descending noradrenergic and serotoninergic pathways in the modulation of nociception: focus on receptor multiplicity. In: Dickenson A, Besson JM, editors. The Pharmacology of Pain, Handbook of Experimental Pharmacology. Vol. 130. Berlin: Springer; 1997. pp. 385–446. [Google Scholar]
- Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Miranda A, Peles S, McLean PG, Sengupta JN. Effects of the 5-HT3 receptor antagonist, alosetron, in a rat model of somatic and visceral hyperalgesia. Pain. 2006;126:54–63. doi: 10.1016/j.pain.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Nuseir K, Proudfit HK. Bidirectional modulation of nociception by GABA neurons in the dorsolateral pontine tegmentum that tonically inhibit spinally projecting noradrenergic A7 neurons. Neuroscience. 2000;96:773–783. doi: 10.1016/s0306-4522(99)00603-x. [DOI] [PubMed] [Google Scholar]
- Patel S, Roberts J, Moorman J, Reavill C. Localization of serotonin-4 receptors in the striatonigral pathway in rat brain. Neuroscience. 1995;69:1159–1167. doi: 10.1016/0306-4522(95)00314-9. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. Noradrenergic pain modulation. Prog. Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends. Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Reitblat T, Zamir D, Polishchuck I, Novochatko G, Malnick S, Kalichman L. Patients treated by tegaserod for irritable bowel syndrome with constipation showed significant improvement in fibromyalgia symptoms. A pilot study. Clin. Rheumatol. 2009;28:1079–1082. doi: 10.1007/s10067-009-1194-z. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Roychowdhury SM, Heinricher MM. Effects of iontophoretically applied serotonin on three classes of physiologically characterized putative pain modulating neurons in the rostral ventromedial medulla of lightly anesthetized rats. Neurosci. Lett. 1997;226:136–138. doi: 10.1016/s0304-3940(97)00270-x. [DOI] [PubMed] [Google Scholar]
- Sabaté JM, Bouhassira D, Poupardin C, Wagner A, Loria Y, Coffin B. Sensory signalling effects of tegaserod in patients with irritable bowel syndrome with constipation. Neurogastroenterol. Motil. 2008;20:134–141. doi: 10.1111/j.1365-2982.2007.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikowski A, Thewissen M, Mathis C, Ross HG, Enck P. Serotonin type-4 receptors modulate the sensitivity of intramural mechanoreceptive afferents of the cat rectum. Neurogastroenterol. Motil. 2002;14:221–227. doi: 10.1046/j.1365-2982.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- Schiavi GB, Brunet S, Rizzi CA, Ladinsky H. Identification of serotonin 5-HT4 recognition sites in the porcine caudate nucleus by radioligand binding. Neuropharmacology. 1994;33:543–549. doi: 10.1016/0028-3908(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J. Comp. Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J. Neurophysiol. 1994;71:2046–2060. doi: 10.1152/jn.1994.71.6.2046. [DOI] [PubMed] [Google Scholar]
- Sikandar S, Bannister K, Dickenson AH. Brainstem facilitations and descending serotonergic controls contribute to visceral nociception but not pregabalin analgesia in rats. Neurosci. Lett. 2012;519:31–36. doi: 10.1016/j.neulet.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KL, Altman DW, Wang L, Kirifides ML, Lin RCS, Waterhouse BD. Lateralization and functional organization of the locus coeruleus projection to the trigeminal somatosensory pathway in rat. J. Comp. Neurol. 1997;385:135–147. [PubMed] [Google Scholar]
- Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol. Motil. 2007;2:25–31. doi: 10.1111/j.1365-2982.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- Tavares I, Lima D. The caudal ventrolateral medulla as an important inhibitory modulator of pain transmission in the spinal cord. J. Pain. 2002;3:337–346. doi: 10.1054/jpai.2002.127775. [DOI] [PubMed] [Google Scholar]
- Tavares I, Lima D, Coimbra A. The ventrolateral medulla of the rat is connected with the spinal cord dorsal horn by an indirect descending pathway relayed in the A5 noradrenergic cell group. J. Comp. Neurol. 1996;374:84–95. doi: 10.1002/(SICI)1096-9861(19961007)374:1<84::AID-CNE6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Tucker DC, Saper CB, Ruggiero DA, Reis DJ. Organization of central adrenergic pathways. Part I. Relationships of ventrolateral medullary projection to the hypothalamus and spinal cord. J. Comp. Neurol. 1987;259:591–603. doi: 10.1002/cne.902590408. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain. Res. Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Nieoullon A, Bockaert J, Dumuis A. Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology. 1994;33:527–541. doi: 10.1016/0028-3908(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Grossman C, Javoy-Agid F, Bockaert J, Dumuis A. [3H]-GR113808 labels 5-HT4 receptors in the human and guinea-pig brain. Neuroreport. 1993;4:1239–1242. doi: 10.1097/00001756-199309000-00007. [DOI] [PubMed] [Google Scholar]
- Westlund KN. Anatomy of noradrenergic pathways modulating pain. In: Besson JM, Guilbaud G, editors. Towards the Use of Noradrenergic Agonists for the Treatment of Pain. Excerpta Medica: Elsevier, Amsterdam; 1992. pp. 91–118. [Google Scholar]
- Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J. Neurophysiol. 1997;78:746–758. doi: 10.1152/jn.1997.78.2.746. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Facilitation and attenuationofavisceral nociceptive reflex from the rostroventral medulla in the rat. Gastroenterology. 2002;122:1007–1019. doi: 10.1053/gast.2002.32389. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Sengupta JN, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J. Neurophysiol. 2002;87:2225–2236. doi: 10.1152/jn.2002.87.5.2225. [DOI] [PubMed] [Google Scholar]