Abstract
There is limited knowledge on the identity of primary CD4+ T cell subsets selectively targeted by HIV-1 in vivo. In this study, we established a link between HIV permissiveness, phenotype/homing potential, and lineage commitment in primary CD4+ T cells. CCR4+CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cells expressed cytokines and transcription factors specific for Th17, Th2, Th1Th17, and Th1 lineages, respectively. CCR4+CCR6+ and CXCR3+CCR6+ T cells expressed the HIV coreceptors CCR5 and CXCR4 and were permissive to R5 and X4 HIV replication. CCR4+CCR6− T cells expressed CXCR4 but not CCR5 and were permissive to X4 HIV only. CXCR3+CCR6− T cells expressed CCR5 and CXCR4 but were relatively resistant to R5 and X4 HIV in vitro. Total CCR6+ T cells compared with CCR6− T cells harbored higher levels of integrated HIV DNA in treatment-naive HIV-infected subjects. The frequency of total CCR6+ T cells and those of CCR4+CCR6+ and CXCR3+CCR6+ T cells were diminished in chronically infected HIV-positive subjects, despite viral-suppressive therapy. A high-throughput analysis of cytokine profiles identified CXCR3+CCR6+ T cells as a major source of TNF-α and CCL20 and demonstrated a decreased TNF-α/IL-10 ratio in CXCR3+CCR6− T cells. Finally, CCR4+CCR6+ and CXCR3+CCR6+ T cells exhibited gut- and lymph node-homing potential. Thus, we identified CCR4+CCR6+ and CXCR3+CCR6+ T cells as highly permissive to HIV replication, with potential to infiltrate and recruit more CCR6+ T cells into anatomic sites of viral replication. It is necessary that new therapeutic strategies against HIV interfere with viral replication/persistence in discrete CCR6+ T cell subsets.
Persistence of HIV-1 in discrete uncharacterized fractions of CD4+ T cells is a major barrier in HIV eradication despite viral-suppressive antiretroviral therapy (ART) (1–3). Previous studies demonstrated that HIV infects CD4+ T cells specific for different Ags, including HIV (4), producing IFN-γ and IL-17 (5) and carrying markers of resting memory T cells (6, 7). Most recently, studies by Chomont et al. (8) identified central memory (CD45RA−CCR7+CD27+) and transitional memory (CD45RA−CCR7−CD27+) T cells as the main viral reservoirs in HIV-infected subjects with preserved and decreased CD4 counts, respectively, under viral-suppressive ART. Despite these recent advances, the phenotypic and functional characteristics of CD4+ T cells permissive versus resistant to HIV infection remain elusive.
Chemokine receptors were initially discovered because of their role in immune cell trafficking regulation (9). Recently, the imprinting for homing was linked to the state of activation, differentiation, and lineage commitment of CD4+ T cells (10). The chemokine receptor CCR7 identifies central memory T cells that exhibit a lymph node-homing potential and ability to differentiate into effector CCR7− T cells subsequently recruited into peripheral tissues (11). The chemokine receptor CXCR3 identifies pre-Th1 central memory T cells that produce IFN-γ and proliferate in response to CMV peptides (12). The chemokine receptor CCR4 identifies pre-Th2 central memory T cells that produce IL-4 and are specific for extracellular pathogens (12). More recent studies demonstrated that CCR6 (the receptor for CCL20 and β-defensins (13)) in combination with CCR4 and CXCR3 identifies two distinct subsets of T cells (14): Th17 (CCR4+CXCR3−CCR6+) producing IL-17 and specific for Candida albicans and Th1Th17 (CCR4− CXCR3+CCR6+) producing IL-17 and IFN-γ and specific for Mycobacterium tuberculosis (14). These studies linked the expression of CXCR3, CCR4, and CCR6 to the lineage commitment profile and the antigenic specificity of memory T cells. Of particular relevance for HIV pathogenesis, the HIV coreceptor CCR5 is highly expressed on central memory CXCR3+ T cells (12). Also, a subset of CCR6+ T cells is HIV-specific, depleted, or both during HIV disease progression (15). Furthermore, CCR6+ T cells likely contribute to HIV dissemination from the portal site of entry, as demonstrated by the use of microbicides interfering with the CCL20-mediated recruitment of T cells into vaginal mucosal sites (16). Together, these lines of evidence suggest that the chemokine receptors CXCR3, CCR4, and CCR6, which are differentially expressed on CD4+ T cells with Th1, Th2, Th17, and Th1T17 profiles (12, 14), might also identify subsets with distinct susceptibility to HIV infection and contribution to viral pathogenesis.
To gain insights into the phenotypic and functional characteristics of CD4+ T cell subsets permissive versus resistant to HIV, we investigated the susceptibility to HIV infection of T cell subsets with differential expression of the chemokine receptors CCR4, CXCR3, and CCR6. We demonstrated that CCR4+CCR6+, CCR4+ CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cell subsets expressed cytokines and transcription factors specific for Th17, Th2, Th1Th17, and Th1 lineages, respectively, and exhibited distinct susceptibility to R5 and X4 HIV replication in vitro. We further demonstrated that circulating CCR6+ T cells harbored the highest levels of integrated HIV DNA in treatment-naive HIV-infected subjects. Moreover, the frequency of CCR4+CCR6+, CXCR3+CCR6+, and CCR4+CCR6− but not CXCR3+CCR6− T cells was significantly diminished in the peripheral blood of HIV-infected individuals despite an efficient control of HIV replication and preserved CD4 counts under ART. In conclusion, we demonstrate a profound and previously unrecognized alteration of CD4+ T cell functional heterogeneity in the context of HIV pathogenesis and emphasize the need for the design of new therapeutic strategies to eradicate HIV by interfering with viral replication in discrete CCR6+ T cell subsets.
Materials and Methods
Subjects
Subjects infected with HIV-1 (n = 42) and uninfected donors (n = 19) were recruited at the McGill University Health Centre, Royal Victoria Hospital, and the Saint-Luc Hospital, Montréal, Québec, Canada, through the Fonds de la Recherche en Santé Québec/AIDS-Infectious Diseases Network (Québec, Canada). Informed consent and Internal Review Board approval were obtained for all of the participants. Table I and Table II summarize immunological, virological, and clinical data for recently HIV-infected treatment-naive (RI w/o ART) and chronically infected under long-term ART (CI on ART) subjects, respectively. Subjects treated with ART received various antiviral regimens containing a protease inhibitor, a non-nucleoside reverse transcriptase inhibitor, or three nucleoside reverse transcriptase inhibitors. Plasma viral load was measured using the Amplicor HIV-1 monitor ultrasensitive method (Roche, Basel, Switzerland). PBMCs (109–1010 cells) were collected from HIV-infected and uninfected individuals by leukapheresis, as previously reported (17). For specific experiments, 10 ml fresh blood was collected from uninfected donors.
Table I.
Clinical parameters of RI w/o ART subjects
| Patient ID | CD4 (cells/µl) |
CD8 (cells/µl) |
Viral Loada | Treatment | Time Since Infection (mo) | Time of Aviremia (mo) |
|---|---|---|---|---|---|---|
| 1 | 704 | 1,081 | 68,412 | None | 9 | 0 |
| 2 | 310 | 350 | 200,363 | None | 46 | 0 |
| 3 | 522 | 366 | 2,021 | None | 5 | 0 |
| 4 | 691 | 1,122 | 8,714 | None | 12 | 0 |
| 5 | 341 | 372 | 16,883 | None | 5 | 0 |
| 6 | 483 | 930 | 366,646 | None | 2 | 0 |
| 7 | 857 | 1,499 | 93,223 | None | 3 | 0 |
| 8 | 475 | 640 | 56,838 | None | 2 | 0 |
| 14 | 338 | 1,829 | 81,984 | None | 4 | 0 |
| 15 | 378 | 779 | 93,706 | None | 2 | 0 |
| 16 | 443 | 736 | 176,557 | None | 5 | 0 |
| 17 | 442 | 538 | 36,349 | None | 5 | 0 |
| 29 | 571 | 1,266 | 5,897 | None | 7 | 0 |
| 30 | 824 | 626 | 1,167,770 | None | 6 | 0 |
| 36 | 494 | 1,055 | 15,703 | None | 16 | 0 |
| 37 | 730 | 1,310 | 97,044 | None | 6 | 0 |
| 50 | 255 | 988 | 52,835 | None | 6 | 0 |
| 51 | 316 | 376 | 57,154 | None | 8 | 0 |
| 52 | 200 | 320 | 4,389 | None | 25 | 0 |
| 64 | 600 | 1,140 | 3,909 | None | 8 | 0 |
| 65 | 378 | 779 | 93,706 | None | 4 | 0 |
| Median | 475 | 779 | 56,996 | – | 6 | – |
HIV RNA copies per milliliter of plasma.
Table II.
Clinical parameters of CI on ART subjects
| Patient ID | CD4 (cells/µl) |
CD8 (cells/µl) |
Viral Loada | Treatment Regimen | Time Since Infection (mo) |
Time of Aviremia (mo) |
|---|---|---|---|---|---|---|
| 18 | 890 | 673 | <50 | AZT; 3TC; NEV | 57 | 42 |
| 19 | 463 | 757 | <50 | 3TC; EFV; ABA | 152 | 20 |
| 20 | 602 | 767 | <50 | 3TC; ABA; SAQ | 158 | 53 |
| 21 | 563 | 613 | <50 | IND; 3TC; AZT | 86 | 71 |
| 22 | 424 | 461 | <50 | 3TC; D4T; DEL | 84 | 46 |
| 23 | 731 | 413 | <50 | EFV; AZT; 3TC | 51 | 22 |
| 24 | 834 | 527 | <50 | NEV; ATA; TENO; RIT | 38 | 25 |
| 25 | 552 | 715 | <50 | D4T; ATA | 139 | 56 |
| 26 | 671 | 1,120 | <50 | 3TC; ABA; LOP; RIT | 242 | 64 |
| 27 | 510 | 765 | <50 | AZT; 3TC; RIT | 61 | 52 |
| 28 | 799 | 1,727 | <50 | 3TC; D4T; NEV | 62 | 33 |
| 42 | 501 | 278 | <50 | D4T; 3TC; IND | 90 | 87 |
| 43 | 344 | 642 | <50 | 3TC; D4T; NEV | 59 | 44 |
| 44 | 604 | 1,281 | <50 | IND; AZT; 3TC | 53 | 35 |
| 47 | 443 | 322 | <50 | RIT; AZT; 3TC; KAL | 18 | 12 |
| 56 | 599 | 923 | <50 | AZT; 3TC; EFV | 86 | 46 |
| 57 | 688 | 1,273 | <50 | AZT; 3TC; EFV | 100 | 59 |
| 58 | 434 | 583 | <50 | 3TC; EFV; ABA | 165 | 34 |
| 59 | 492 | 582 | <50 | RIT; ABC; 3TC; ATA | 170 | 66 |
| 60 | 529 | 690 | <50 | 3TC; D4T; DEL | 49 | 11 |
| 61 | 888 | 942 | <50 | NA | NA | NA |
| 62 | 836 | 1236 | 267 | NA | NA | NA |
| 63 | 883 | 333 | <50 | EFV; IND | 89 | 78 |
| Median | 599 | 690 | – | – | 86 | 46 |
HIV RNA copies per milliliter of plasma.
3TC, Lamivudine; ABA/ABC, Abacavir; ATA, Atazanavir; AZT, Zidovudine; D4T, Stavudine; DEL, Delavirdine; EFV, Efavirenz; IND, Indinavir; KAL, Kaletra; LOP, Lopinavir; NA, not available; NEV, Nevirapine; RIT, Ritonavir; SAQ, Saqui-navir; TENO, Tenofovir.
Abs and polychromatic flow cytometry analysis
Fluorochrome-conjugated Abs used for polychromatic flow cytometry analysis were CD3-Pacific blue (UCHT1), CD4-Alexa Fluor 700 (RPA-T4), CD45RA-allophycocyanin/Cy7 (HI100), CCR4-PE/Cy7 (1G1), CXCR3-PE/Cy5 (1C6), CCR5-FITC (2D7), CCR5-PE (2D7), CCR6-PE (11A9), and CXCR4-PE (12G5) (BD Pharmingen, San Diego, CA), CXCR4-FITC (12G5) and CCR7-FITC (150503) (R&D Systems, Minneapolis, MN), α4-FITC (44H6), β7-FITC (FIB504), CD56-FITC (MEM188), and IL-17 PE (64DEC17) (eBioscience, San Diego, CA), CD14-FITC (My4) and HIV-p24-FITC (FH190-1-1) (Beckman Coulter, Fullerton, CA), CD8-FITC (BW135/80), CD19-FITC (LT19), and IFN-γ-PE (45–15) (Miltenyi Biotec, Auburn, CA). Cells were analyzed by FACS using the BD LSRII cy-tometer and BD Diva software (BD Biosciences, San Jose, CA).
MACS and FACS cell sorting
The PBMCs were isolated from fresh blood or leukapheresis by Ficoll-Paque centrifugation (18, 19). The CD4+ T cells were sorted from PBMCs by negative selection using magnetic beads (MACS; Miltenyi Biotec), with a purity >95%, as determined by triple staining with CD3, CD4, and CD8 Abs and FACS analysis. Then, CCR4+CCR6+, CCR4+CCR6−, CXCR3+ CCR6+, and CXCR3+CCR6− T cell subsets were sorted by FACS (BD FACSAria; BD Biosciences) upon staining with two different mixtures: 1) CD4-FITC, CD45RA-allophycocyanin/Cy7, CCR4-PE/Cy7, CXCR3-PE/ Cy5, and CCR6-PE for HIV-infected subjects where the sorting gate was set on CD4+CD45RA− T cells and 2) CD8-FITC, CD14-FITC, CD56-FITC, CD19-FITC, CD45RA-allophycocyanin/Cy7, CCR4-PE/Cy7, CXCR3-PE/ Cy5, and CCR6-PE for uninfected controls, where the sorting gate was set on FITC−CD45RA− T cells to exclude CD8+ T cells, monocytes, NK cells, and B cells, respectively. Sorted T cell subsets were on average >95% pure as determined by postsorting FACS analysis (Supplemental Fig. 1).
Quantitative SYBR Green real-time RT-PCR
Total RNA was isolated from sorted T cell subsets by RNeasy columns (Qiagen, Valencia, CA). The quality of RNA was assessed by Bioanalyzer (Agilent, Palo Alto, CA), and the quantity was measured by Nanodrop (Thermo Scientific, Wilmington, DE). One step SYBR Green real-time RT-PCR (Qiagen) was carried out in a LightCycler (Roche) according to the manufacturer’s recommendations. Primers spanning one or multiple exons were purchased from Qiagen (i.e., rorc, gata3, T-box expressed in T cells(t-bet), and foxp3 QuantiTect primer sets). Briefly, 5 ng total RNAwas reverse-transcribed in 20 µl 1× SYBR Green mix (Qiagen) containing 0.5 µM primers. Agarose gel electrophoresis was used to determine the size of the amplification products (100–200 bp) and allowed cDNA purification (QIAquick Gel Extraction Kit; Qiagen) for standard curve preparation. The absolute quantification of target gene expression was performed using a 10-fold serial dilution of purified RT-PCR products (i.e., 200, 20,2,0.2, and 0.02 fg cDNA), as previously described by us and others (20, 21). Melting curve analysis was performed after each real-time amplification and revealed the uniformity of thermal dissociation profile per amplification product. Samples without template or without reverse transcriptase were used as negative controls. The concentration of each gene was normalized to the 28S rRNA levels (RRN28S: forward, 5′-CGAGATTCCCACTGTCCCTA-3′; reverse, 5′-GGGGCCTCCCACTTATTCTA-3′) as an internal control (21). Each RT-PCR reaction was performed in duplicate.
HIV infection and quantification of viral replication
R5 (NL4.3BaL) and X4 (NL4.3) HIV stocks were produced by transfection of 293 T cells with the appropriate plasmids using Fugene6 (Roche) (22). Viral stocks were quantified by a HIV p24 ELISA assay (8) and titrated for infectiousness on CD4+CCR5+CXCR4+ MAGI cells and primary CD3/ CD28-activated CD4+ T cells. Sorted CD4+ T cell subsets were activated for 3 d with immobilized CD3 and soluble CD28 Abs (1 µg/ml) and then exposed to HIV (50 ng HIV p24 for 106 cells corresponding to a multiplicity of infection of ~0.01 for 106 cells) for 3 h at 37°C. Unbound HIV was removed by extensive washing, and cells were cultured at a concentration of 106 cells per milliliter in RPMI 1640 with 10% FBS and IL-2 (5 ng/ml; R&D Systems). Cell culture supernatants were harvested every 3 d, and HIV replication was measured by HIV p24 ELISA. In parallel, cells were harvested at days 3 or 12 postinfection, or both, and cell lysates were used for real-time RT-PCR quantification of HIV DNA.
Real-time RT-PCR quantification of HIV DNA
The quantification of integrated and total HIV DNA was performed, as previously described (8, 23). Briefly, T cells were digested in a proteinase K buffer (Invitrogen, Burlington, Ontario, Canada), and 105 cells/15 µl ly-sate were used per amplification. Integrated HIV DNA was amplified first (12 cycles) using two outward-facing Alu primers and one HIV long terminal repeat (LTR) primer tagged with a λ sequence, and the CD3 gene was amplified in the same reaction (8). The HIV and CD3 amplicons were then amplified in separate reactions (LightCycler; Roche). The HIV DNA was amplified using a λ-specific primer and an inner LTR primer in the presence of two fluorescent probes specific for HIV LTR (23). The CD3 DNA was amplified using inner primers and two fluorescent probes specific for CD3 (8). Amplification reactions were carried out with Jumpstart Taq Ready Mix (Sigma-Aldrich, St. Louis, MO) and Taq Polymerase (Invitrogen). The ACH2 cells carrying one copy of integrated HIV DNA per cell (The National Institutes of Health AIDS reagent program) were used as a standard curve (8). A similar method was used to quantify total HIV DNA with the appropriate primers for HIV Gag and LTR (8).
Cytokine screening and quantification
Culture supernatants were screened for the expression of 120 soluble factors using the Cytokine Antibody Arrays VI and VII (RayBiotec, Norcross, GA). Cytokine levels were further quantified by ELISA assays specific for IL-2, IL-10 (BD Pharmingen), TNF-α, CCL3, CCL20 (R&D Systems), IL-5, IL-17, and IFN-γ (eBioscience). CCL5 levels were quantified by Cytometric Bead Array (BD Pharmingen).
Statistical analysis
Statistical significance between groups (p values < 0.05 were considered significant) was calculated using the Mann-Whitney U test (comparison of unpaired samples) and Wilcoxon signed-rank test or paired t test (comparison of paired samples) as specified in the figure legends. Associations among study variables were assessed by Spearman correlation and linear regression. All of the statistical analyses were performed using Prism 5 software (GraphPad, San Diego, CA).
Results
CCR4, CXCR3, and CCR6 identify CD4+ T cell subsets with distinct lineage commitment and expression of the HIV coreceptor CCR5
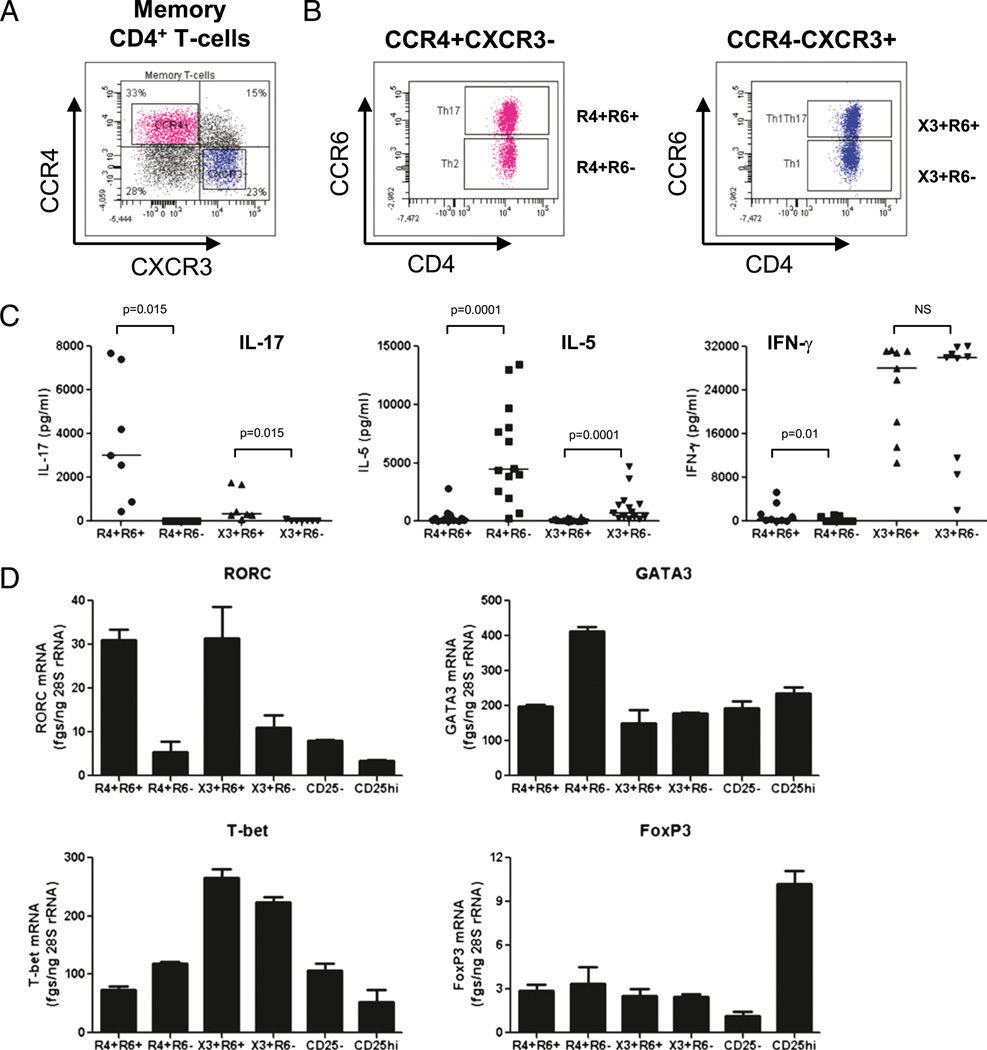
Previous studies demonstrated that CCR4 (R4), CXCR3 (X3), and CCR6 (R6) are surface markers for functionally distinct memory CD4+ T cell subsets: Th17 (CCR4+CCR6+), Th2 (CCR4+CCR6−), Th1Th17 (CXCR3+CCR6+), and Th1 (CXCR3+CCR6−) (14). Whether CCR4, CXCR3, and CCR6 identify subsets with distinct susceptibility to HIV infection remains unknown. Memory T cells were identified as cells lacking the expression of naive T cell CD45RA, as previously described (11, 14, 24). In this study, we used polychromatic flow cytometry to sort memory (CD45RA−) CD4+ T cell subsets with differential expression of CCR4, CXCR3, and CCR6 using a gating strategy depicted in Fig. 1A and 1B. The CCR4 and CXCR3 expression distinguished four memory subsets, including CCR4+CXCR3− and CCR4−CXCR3+ subsets (Fig. 1A). The expression of CCR6 further identified CCR4+ CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cell subsets (Fig. 1B). Highly pure T cell subsets (Supplemental Fig. 1) were assessed for the expression of lineage-specific cyto-kines and transcription factors upon CD3/CD28 triggering in vitro. The CCR4+CCR6+ T cells produced IL-17 and expressed the mRNA for the Th17-specific transcription factor RORC. The CCR4+CCR6− T cells produced IL-5 and expressed the mRNA for the Th2-specific transcription factor GATA3. The CXCR3+ CCR6+ T cells produced IFN-γ and IL-17 and the mRNA for the Th1- and Th17-specific transcription factors T-bet and RORC, respectively. The CXCR3+CCR6− T cells produced IFN-γ and expressed the mRNA for the Th1-specific transcription factor T-bet (Fig. 1C, 1D). These results are consistent with previous reports (12, 14). The expression of the mRNA for the regulatory T cell (Treg)-specific transcription factor FoxP3 was relatively low in the four T cell subsets compared with that in the CD25high Tregs (Fig. 1D). Thus, CCR4+CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cell subsets were enriched in markers specific for Th17, Th2, Th1Th17, and Th1 lineages, respectively, but did not express the Treg marker FoxP3.
FIGURE 1.
The chemokine receptors CCR4, CXCR3, and CCR6 identify CD4+ T cells with distinct Th17, Th2, Th1Th17, and Th1 profiles. PBMCs from uninfected individuals were stained with CD3, CD4, CD45RA, CCR4, CXCR3, and CCR6 Abs and then analyzed by polychromatic flow cytometry. A, CCR4 and CXCR3 expression identified four subsets of memory (CD45RA−) CD4+ T cells, including CCR4+CXCR3− and CCR4− CXCR3+ subsets. B, CCR6 expression distinguished four cell subsets within the CCR4+CXCR3− and CCR4−CXCR3+ T cell subsets. Results in A and B are representative of experiments performed with cells from >10 different donors. C, D, T cells identified in B were sorted by flow cytometry and then stimulated via CD3/CD28 for 3 d. C, Cytokine production in culture supernatants was quantified by ELISA. D, Shown is real-time RT-PCR quantification of lineage-specific transcription factors. Results in C were obtained with cells from n = 7 (IL-17), n = 14 (IL-5), and n = 9 (IFN-γ) different donors. Wilcoxon signed-rank test p values are indicated in the figure. Horizontal lines indicate median values. Results in D are from one experiment representative of experiments performed with cells from n = 4 (for Th17, Th2, Th1Th17, and Th1) and n = 2 different donors (for CD25+ and CD25−CD4+ T cells) (mean ± SD of duplicate wells).
To determine whether CCR4, CXCR3, and CCR6 identify subsets with distinct susceptibility to HIV infection, the expression of the HIV coreceptors CCR5 and CXCR4(25–27)was quantified on T cells from the fresh blood of uninfected individuals. The expression of CCR5 was the highest on CXCR3+CCR6+ T cells, moderate on CCR4+ CCR6+ and CXCR3+CCR6− T cells, and low to undetectable on CCR4+CCR6− T cells (Fig. 2A, 2B). In contrast, the HIV coreceptor CXCR4 was expressed at similar levels on the four T cell subsets (Fig. 2C). Thus, CCR4+CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cells differentially expressed the HIV coreceptor CCR5 and similarly expressed CXCR4, thereby suggesting a distinct susceptibility of these T cell subsets to R5 HIV infection.
FIGURE 2.
The HIV coreceptor CCR5 is differentially expressed on CCR4+CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cells. A– C, The expression of the HIV coreceptors CCR5 and CXCR4 was analyzed in CCR4+CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cells gated as in Fig. 1A and 1B. A, Shown are histograms of CCR5 and CXCR4 expression in T cell subsets from one representative donor; B, statistical analysis of CCR5 expression (percentage and mean fluorescence intensity) in T cell subsets from n = 13 different donors; and C, statistical analysis of CXCR4 expression (percentage and mean fluorescence intensity) in T cells from n = 7 different donors. Wilcoxon signed-rank test p values are indicated in the figure. Horizontal lines indicate median values.
CCR4+CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+ CCR6− T cells differentially replicate R5 and X4 HIV
We investigated the susceptibility of these four T cell subsets to R5 and X4 HIV replication in vitro. High levels of R5 HIV replication were detected in CCR4+CCR6+ and CXCR3+CCR6+ T cells, whereas R5 HIV replication was low to undetectable in CCR4+ CCR6− and CXCR3+CCR6− T cells up to 12 d postinfection (Fig. 3A). Levels of integrated and total R5 HIV DNA were relatively high in CCR4+CCR6+ and CXCR3+CCR6+ T cells but low in CCR4+ CCR6− and CXCR3+CCR6− T cells at day 3 postinfection (Fig. 3B). X4 HIV replicated at relatively high levels in CCR4+CCR6+ and CXCR3+CCR6+ T cells and at low to undetectable levels in CXCR3+ CCR6− T cells (Fig. 3C). Levels of integrated and total X4 HIV DNA were higher in CCR4+CCR6+ and CXCR3+CCR6+ T cells compared with those in CXCR3+CCR6− T cells at day 3 post-infection (Fig. 3D). Consistent with their expression of CXCR4, CCR4+CCR6− T cells were highly permissive to infection with X4 HIV (Fig. 3C, 3D). Of particular interest, CXCR3+CCR6− T cells were relatively resistant to both R5 and X4 HIV replication (Fig. 3A-D), and levels of integrated R5 and X4 HIV DNA were significantly lower compared with those of CXCR3+CCR6+ T cells at days 3 and 12 postinfection (Fig. 3E, 3F) (paired t test p values < 0.05, CXCR3+CCR6+ versus CXCR3+CCR6− T cells). This difference was observed despite the fact that CXCR3+CCR6− T cells expressed moderate levels of CCR5 and CXCR4 ex vivo (Fig. 2). Also, there was no difference in CCR5 and CXCR4 expression between CXCR3+CCR6− and CXCR3+CCR6+ T cells at day 3 post-stimulation (Supplemental Fig. 2). Moreover, T cell proliferation in culture was similar in the four T cell subsets as shown by a CFSE proliferation assay (Supplemental Fig. 3). In other studies, CXCR3+ CCR6− and CXCR3+CCR6+ T cells were infected with two other HIV molecular clones (i.e., R5 YU2 and X4 NDK strains) and results confirmed preferential HIV replication in CXCR3+CCR6+ T cells (data not shown). These results demonstrate the following: 1) CCR4+ CCR6+ and CXCR3+CCR6+ T cells are highly permissive to R5 and X4 HIV replication, 2) CCR4+CCR6− T cells are permissive to X4 but not R5 HIV strains, and 3) CXCR3+CCR6− T cells are relatively resistant to both R5 and X4 HIV replication in vitro. Considering the critical role of R5 HIV strains during natural HIV transmission (1), CCR4+CCR6+ and CXCR3+CCR6+ T cells may contribute significantly to HIV disease pathogenesis in vivo.
FIGURE 3.
CCR4+CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cells exhibit differential susceptibility to R5 and X4 HIV replication. FACS sorted T cell subsets were stimulated via CD3/CD28 for 3 d and exposedtoR5 (NL4.3BaL)orX4 (NL4.3) HIV strains for3 hat37°C. Unbound virus was removed by extensive washing, and cells were cultured at a concentration of 106 cells per milliliter in RPMI 1640 with 10% FBS and IL-2 (5 ng/ml). A, C, Supernatants were harvested every 3 d postinfection, and levels of HIV p24 were quantified by ELISA. B, D, E, F, Integrated and total HIV DNA levels were quantified by real-time PCR at days 3and12 postinfection. Shown are (A–D) HIV-p24 levels or HIV DNA copy numbers from one experiment representative of results obtained with cells from more than three different donors (mean ± SD of triplicate wells) and (E, F) relative integrated HIV DNA levels in CXCR3+ CCR6+ and CXCR3+CCR6− T cell subsets from three or four different donors. (E, F) Values above bars are integrated HIV DNA copy numbers per 106 cells in CXCR3+CCR6+ T cells.
CCR6+ T cells harbor high levels of integrated HIV DNA, and their frequencyis diminished inHIV-infected subjects under ART
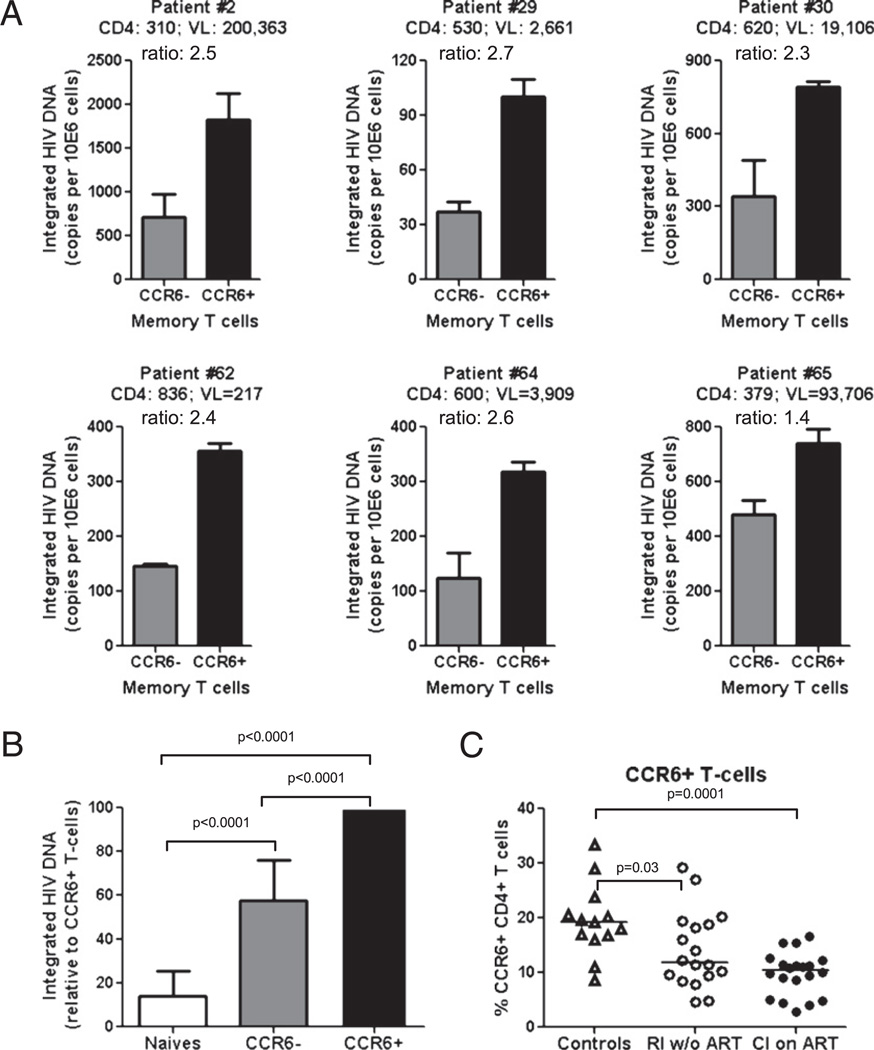
Previous studies demonstrated that CCR6+ T cells disappeared from the peripheral blood of HIV-infected patients as the disease progressed (15). Depletion of CCR6+ T cells in HIV-infected subjects (15) may be a consequence of their selective infection by HIV in vivo. To test this hypothesis, we quantified integrated HIV DNA levels in memory CCR6+ and CCR6− T cells and analyzed the frequency of these cells in HIV-infected and uninfected individuals. Two HIV-infected patient cohorts were available for this study: 1) RI w/o ART (n = 19) (Table I) and 2) CI on ART (n = 23) (Table II). Levels of integrated HIV DNA were quantified by realtime PCR in matched naive (CD45RA+) and memory (CD45RA−) CCR6+ and CCR6− CD4+ T cell subsets from six RI w/o ART subjects (Fig. 4A, 4B). In six out of six HIV-infected subjects tested, levels of integrated HIV DNA were higher in memory CCR6+ compared with those in CCR6− T cells (Fig. 4A). Moreover, integrated HIV DNA levels were significantly higher in memory CCR6+ compared with those in naive CD4+ T cells (Fig. 4B). Increased levels of integrated HIV DNA in memory CCR6+ T cells coincided with a significant decrease in the frequency of CCR6+ T cells in the peripheral blood of HIV-infected patients, both RI w/o ART and CI on ART, as compared with that in un-infected controls (Fig. 4C). These results demonstrate for the first time that circulating memory CCR6+ T cells are notable cellular sites for HIV integration in vivo and suggest that depletion of these cells may be related in part to their increased susceptibility to HIV replication.
FIGURE 4.
CCR6+ T cells harbor relatively high levels of integrated HIV DNA, and their frequency is diminished in HIV-infected subjects. A, B, Matched naive (CD45RA+) and memory (CD45RA−) CCR6+ and CCR6− T cell subsets were sorted by MACS and FACS from PBMCs of viremic treatment-naive HIV-infected subjects (Table I). Levels of integrated HIV DNA were quantified by real-time nested PCR. Shown are (A) integrated HIV DNA copy numbers per 106 CCR6+ and CCR6− T cells (mean ± SD of triplicate wells) and (B) relative integrated HIV DNA levels in naive and memory CCR6+ and CCR6− T cells (mean ± SD, n = 6). CD4 counts (cells per microliter), plasma viral loads (HIV RNA copies per milliliter), and paired t test p values are indicated in the figure. C, The frequency of memory CCR6+ T cells was analyzed in recently RI w/o ART (n = 18) and CI on ART (n = 20) individuals (Tables I and II) as compared with that in uninfected subjects (n = 13). Mann-Whitney U test p values are indicated in the figures. Horizontal lines indicate median values.
Frequency of CCR4+CCR6+ and CXCR3+CCR6+ T cells is diminished in HIV-infected subjects under viral-suppressive therapy
To investigate a potential link between HIV infection in vitro and depletion in vivo, the frequencies of CCR4+CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− Tcellswere analyzedin RI w/o ARTand CI on ART HIV-infected subjects (Table I, II) and uninfected controls. The frequencies of CCR4+CCR6+ and CXCR3+CCR6+T cell subsets were slightly but significantly decreased in CI on ART but not RI w/o ART HIV-infected subjects compared with those in controls (Fig. 5A), indicating that this frequency alteration occurs only later during disease progression despite viral-suppressive therapy. In addition, a longitudinal follow-up in four CI on ART subjects demonstrated persistent reduced frequencies of circulating CCR4+CCR6+ and CXCR3+CCR6+ T cell subsets despite efficient control of HIV replication and CD4 counts within a normal range (≥500 cells per micro-liter) under ART (Supplemental Fig. 4). However, the frequency of CXCR3+CCR6− T cells was significantly increased in both RI w/o ART and CI on ART HIV-infected subjects compared with those in controls (Fig. 5A) and the four CI on ART subjects followed up longitudinally (Supplemental Fig. 4), which was consistent with the relative resistance of these cells to HIV infection in vitro (Fig. 3). Unexpectedly, the frequency of CCR4+CCR6− T cells was significantly decreased in RI w/o ART HIV-infected subjects compared with that in controls but increased compared with that in CI on ART subjects (Fig. 5A). CCR4+CCR6− and CXCR3+CCR6− T cell counts positively correlated with CD4 counts in CI on ART HIV-positive subjects, whereas there was a weak or not significant correlation between CD4 counts and the number of circulating CCR4+CCR6+ and CXCR3+ CCR6+ T cells (Fig. 5B). Thus, ART is associated with a tendency for normalization in the frequencies of CCR4+CCR6− and CXCR3+ CCR6− T cells but not CCR4+CCR6+ and CXCR3+CCR6+ T cells.
FIGURE 5.
The frequency of CCR4+CCR6+ and CXCR3+CCR6+ T cell subsets but not CXCR3+CCR6− T cell subsets is diminished in HIV-infected subjects under ART. A, The frequency of CCR4+CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cell subsets within the total memory fraction (CD45RA−) was analyzed in RI w/o ART (n = 18) and CI on ART (n = 20) HIV-infected subjects (Tables I and II) as compared with that in uninfected subjects (n = 13). B, Shown are the correlations among the CCR4+CCR6+, CCR4+ CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cell counts and CD4 counts in CI on ART individuals (n = 20) (Table II). Spearman correlation p and r values and linear regression r2 values are indicated in the figure. C, The relative frequency of CCR4+CCR6+ (Th17 profile) and CXCR3+CCR6+ T cells (Th1Th17 profile) versus CXCR3+CCR6− T cells (Th1 profile) (percentage/percentage) was calculated in RI w/o ART and CI on ART HIV-infected subjects as compared with that in uninfected subjects. Mann-Whitney U test p values are indicated in the figure. Horizontal lines indicate median values.
Very recent studies reported a Th17/Th1 switch during HIV/SIV infection (5, 28). Consistent with these studies, the ratios between CCR4+CCR6+ (Th17 profile) and CXCR3+CCR6− (Th1 profile) T cells and between CXCR3+CCR6+ (Th1Th17 profile) and CXCR3+CCR6− T cells demonstrated a preponderance of the Th1 profile in both RI w/o ART and CI on ART HIV-infected subjects compared with that in controls (Fig. 5B). Together, these results demonstrate that the frequency of circulating CCR4+CCR6+ and CXCR3+CCR6+ T cell subsets is diminished in the peripheral blood of CI subjects, despite an efficient control of HIV replication and preservation of CD4 counts under ART. Depletion of CCR4+CCR6+ and CXCR3+CCR6+ T cell subsets is likely due to their increased susceptibility to HIV infection. In contrast, depletion of CCR4+CCR6− T cells, which are infected with X4 but not R5 HIV in vitro, might be the consequence of a bystander mechanism of CD4+ T cell depletion in HIV-infected subjects, as previously documented (1, 29, 30).
CCR4+CCR6+, CCR4+CCR6−, CXCR3+CCR6+, and CXCR3+ CCR6− T cells exhibit differential cytokine/chemokine profiles
To further characterize the potential roles of these four T cell subsets in HIV pathogenesis, cytokine/chemokine profiles were investigated. Culture supernatants were screened for the expression of 60 soluble factors using the Cytokine Antibody Array VI (RayBiotec) and demonstrated differential expression of IL-10, IL-5, CCL20/MIP-3α, CCL5/RANTES, and TNF-α (Fig. 6A). IL-10 was highly produced by CXCR3+CCR6− T cells, IL-5 was selectively produced by the CCR4+ CCR6− T cells, and TNF-α and CCL20 were produced at higher levels in CXCR3+CCR6+T cells compared with those in the other three T cell subsets (Fig. 6A). The CCR5 binding chemokine CCL5 was expressed at high levels in CXCR3+CCR6− and CXCR3+CCR6+ T cells and low levels in CCR4+CCR6+ and CCR4+CCR6− T cells. Screening for 60 other soluble factors using the Cytokine Antibody Array VII demonstrated high production of the CCR5 ligands CCL3 and CCL4 by CXCR3+CCR6+ and CXCR3+CCR6− T cells, whereas IL-8 was preferentially expressed by CCR4+CCR6+ T cells (data not shown). Levels of IL-10, TNF-α, CCL20, IL-2, CCL3, and CCL5 were further quantified by ELISA or Cytometric Bead Array (Fig. 6B).
FIGURE 6.
CCR4+CCR6+, CCR4+ CCR6−, CXCR3+CCR6+, and CXCR3+ CCR6− T cell subsets exhibit differential cytokine/chemokine profiles. Memory CCR4+CCR6+, CCR4+CCR6−, CXCR3+ CCR6+, and CXCR3+CCR6− T cells were stimulated via CD3/CD28 for 3 d. A, Culture supernatants were screened for 60 soluble factors using the Cytokine Antibody Array VI (RayBiotec). Shown are results from one experiment representative of experiments performed with cells from two different donors. B, Levels of IL-10 (n = 11), TNF-α (n = 14), CCL20/MIP-3a (n = 14), CCL3/ MIP-1α (n = 6), and IL-2 (n = 12) were quantified by ELISA, whereas levels of CCL5/RANTES (n = 2) were quantified in cell supernatants by Cytometric Bead Array (mean ± SD). C, Shown are TNF-α/IL-10 ratios in CXCR3+CCR6+ and CXCR3+ CCR6− T cell subsets. Wilcoxon signed-rank test p values are indicated in the figure. Horizontal lines indicate median values.
The production of IL-10 (a cytokine produced by different cells subsets including effector CD4+ T cells (31, 32) and a potent inhibitor of HIV replication (33–37)) was higher in CXCR3+CCR6+ and CXCR3+CCR6− T cells compared with that in CCR4+CCR6+ and CCR4+CCR6− T cells (Fig. 6B). Only in 5 out of 11 donors IL-10 levels were significantly higherinCXCR3+CCR6− T cells compared with those in CXCR3+CCR6+ T cells (Supplemental Fig. 5). The production of TNF-α [a proinflammatory cytokine that enhances HIV replication (38)] was the highest in CXCR3+CCR6+ T cells (Fig. 6B). Of interest, the TNF-α/IL-10 ratio was significantly higher in CXCR3+CCR6+ T cells compared with that in CXCR3+CCR6− T cells (Fig. 6C), suggesting that the balance between TNF-α and IL-10 may control the degree of permissiveness to HIV replication in these cells. CCL20 (a Th17 marker (39) and a CCR6 ligand (16, 40)) was produced at high and moderate levels by CXCR3+CCR6+ and CCR4+CCR6+ T cells, respectively, but not by CCR4+CCR6− and CXCR3+CCR6− T cells (Fig. 6B), suggesting the ability of CXCR3+ CCR6+ and CCR4+CCR6+ T cells to attract other CCR6+ T cells to sites of inflammation. CCR5 binding chemokines CCL3, CCL4, and CCL5 were produced at similarly high levels by CXCR3+CCR6− and CXCR3+CCR6+ T cells but at low to undetectable levels by CCR4+ CCR6+ and CCR4+CCR6− T cells (Fig. 6B; data not shown). Finally, IL-2 production was similarly high in CXCR3+CCR6+ and CXCR3+ CCR6− T cells, moderate in CCR4+CCR6− T cells, and low to un-detectable in CCR4+CCR6+ T cells (Fig. 6B). Together, these results demonstrate distinct cytokine profiles in T cells with differential expression of CCR4, CXCR3, and CCR6 (Supplemental Fig. 5) with relevance for their role in HIV pathogenesis. These results suggest that high levels of TNF-α, CCL20, or both by CXCR3+CCR6+ and CCR4+CCR6+ T cells may contribute to the increased ability of these cells to support HIV replication and to attract other CCR6+ T cells at sites of HIV replication in vivo. In contrast, the relative resistance of CXCR3+CCR6− T cells to HIV replication might be linked to a decreased TNF-α/IL-10 ratio, a “self protection” mechanism against R5 HIV entry via autocrine production of CCR5 ligands, or both (41, 42).
CCR4+CCR6+ and CXCR3+CCR6+ subsets exhibit gut- and lymph node-homing potential
The gut and vaginal mucosa are major sites for HIV replication (43, 44). The CCR6+ T cells are attracted to these sites (40, 45) and play a critical role in HIV mucosal transmission (16, 46). In addition, recruitment of T cells into lymph nodes via CCR7 (47) significantly contributes to HIV pathogenesis (48). To further characterize the trafficking potential of CCR4+CCR6+, CCR4+ CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cells, the expression of the gut-homing α4β7 integrin (49) and the lymph node-homing chemokine receptor CCR7 (47) was analyzed on CD4+ T cell subsets from uninfected individuals. The α4 chain was similarly expressed at >95% on the four cell subsets (data not shown), whereas the β7 chain was expressed at relatively high levels on CXCR3+CCR6+ T cells, moderate levels on CCR4+ CCR6+ and CXCR3+CCR6− T cells, and low levels on CCR4+ CCR6− T cells (Fig. 7A). CCR7 was expressed on the four cell subsets, with the highest levels detected on CCR4+CCR6− and CXCR3+CCR6− T cells (Fig. 7B). Collectively these results suggest that a large fraction of CXCR3+CCR6+ T cells (median of 20%) have the potential to be recruited into the gut and vaginal mucosa via α4β7 and that a considerable fraction of each of the four subsets (median >37%) are CCR7+ central memory T cells with the potential be recruited into lymph nodes, which are major sites for HIV replication in vivo (43, 46).
FIGURE 7.
CCR4+CCR6+ and CXCR3+CCR6+ T cell subsets exhibit gut- and lymph node-homing potential. Memory CCR4+CCR6+, CCR4+ CCR6−, CXCR3+CCR6+, and CXCR3+CCR6− T cells from uninfected individuals were analyzed for the expression of (A) the integrin β7 and (B) CCR7. Shown are expression values in n = 13 HIV-uninfected subjects. Wilcoxon signed-rank test p values are indicated in the figure. Horizontal lines indicate median values.
Discussion
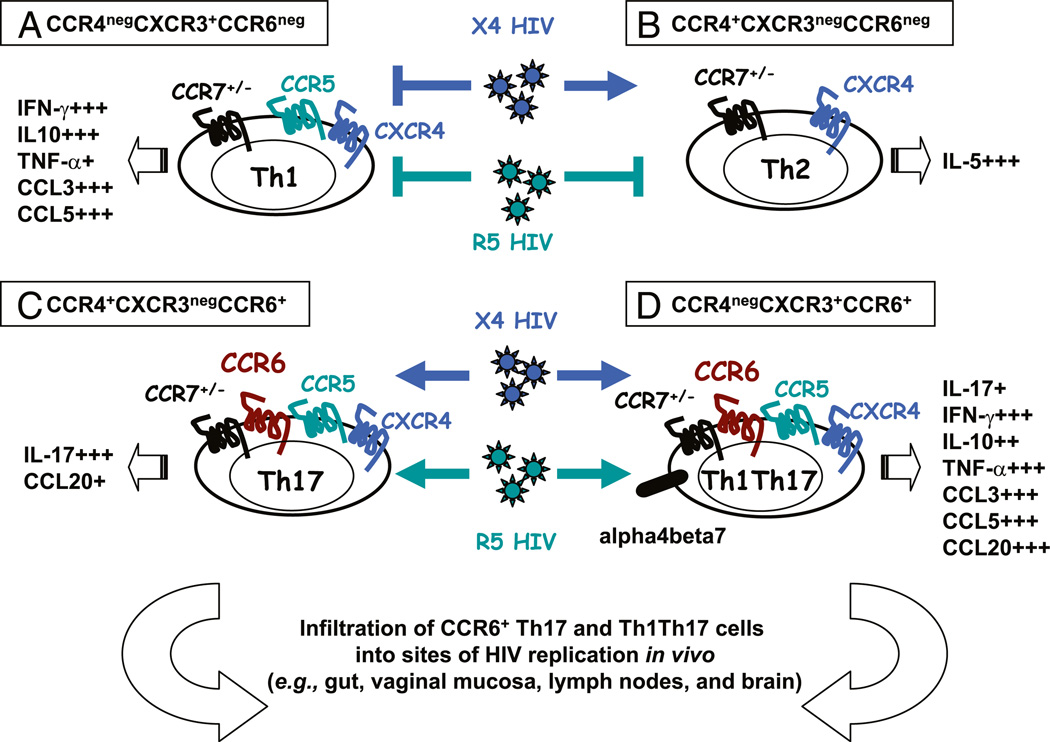
In this study, we revealed the distinct susceptibility to HIV infection of four CD4+ T cell subsets recently identified based on their differential expression of the homing receptors CCR4, CXCR3, and CCR6 and lineage-specific markers (Fig. 8). We demonstrated that CCR4+CCR6+ (Th17 profile) and CXCR3+CCR6+ T cells (Th1Th17 profile) were highly permissive to R5 and X4 HIV infection, that CCR4+CCR6− T cells (Th2 profile) were susceptible to X4 HIV replication only, and that CXCR3+CCR6− T cells (Th1 profile) were relatively resistant to both R5 and X4 HIV replication in vitro. Circulating CCR6+ T cells harbored the highest levels of integrated HIV DNA in treatment-naive HIV-infected subjects. A high-throughput analysis of cytokine profiles identified CXCR3+CCR6+ T cells as a major source of TNF-α and CCL20 and demonstrated a low TNF-α/IL-10 ratio in CXCR3+CCR6− T cells. Finally, we showed that CCR4+CCR6+ and CXCR3+ CCR6+ T cells exhibited gut- and lymph node-homing potential. Collectively, our study identified circulating CCR4+CCR6+ and CXCR3+CCR6+ T cells as highly permissive and CXCR3+CCR6− T cells as relatively resistant to HIV infection and demonstrated that the frequency of these subsets was significantly altered in HIV-infected subjects despite viral-suppressive ART.
FIGURE 8.
Proposed model for the contribution of CCR4+CCR6+ and CXCR3+CCR6+ T cell subsets to HIV pathogenesis. A, CXCR3+CCR6− T cells (Th1 profile) are resistant to R5 and X4 HIV replication. B, CCR4+CCR6− T cells (Th2 profile) are permissive to X4 HIV only. C, CCR4+CCR6+ (Th17 profile) and (D) CXCR3+CCR6+ T cell subsets (Th1Th17 profile) are highly permissive to R5 and X4 HIV strains and have the potential to be recruited into the gut via the α4β7 integrin (49) and CCR6 (45), the lymph nodes via CCR7 (47), and the brain via CCR6 (71). They produce the CCR6 ligand CCL20, a chemokine critical for HIV dissemination from the site of portal entry (16). In addition, CXCR3+CCR6+ T cells are a major source of TNF-α, a proinflammatory cytokine previously linked to the positive regulation of HIV replication (38). We propose a model in which CCR4+CCR6+ Th17 and CXCR3+CCR6+ Th1Th17 T cells significantly contribute to HIV-1 pathogenesis by their ability to promote viral replication and attract more CCR6+ T cells at sites of HIV replication in vivo.
Recent studies identified the chemokine receptors CCR4, CXCR3, and CCR6 as surface markers for CD4+ T cells with distinct cytokine profiles and lineage-specific transcription factor expression (12, 14, 50–52) and so established a link between CD4+ T cell trafficking potential and immunologic function. In the current study, we confirmed first that 1) CCR4+CCR6+ T cells produced IL-17 and expressed the Th17-specific transcription factor RORC, 2) CCR4+CCR6− T cells produced IL-5 and expressed the Th2-specific transcription factor GATA3, 3) CXCR3+ CCR6− T cells produced IFN-γ and expressed the Th1-specific transcription factor T-bet, and 4) CXCR3+CCR6+ T cells produced IL-17 and IFN-γ and expressed the transcription factors T-bet and RORC. Our findings, together with those published by other groups (14, 50–52), support the idea that chemokine receptors are surface markers for CD4+ T cell subsets with distinct lineage-specific transcriptional programs.
Studies by us and others demonstrated that CCR4 expression is relatively high on T cells infected with X4 HIV in vitro (22) and that CXCR3+ T cells preferentially express the HIV coreceptor CCR5 (12). In this study, we report that CCR5 is expressed at high levels on CXCR3+CCR6+ T cells, moderate levels on CCR4+ CCR6+ and CXCR3+CCR6− T cells, and low to undetectable levels on CCR4+CCR6− T cells. In contrast, CXCR4, the second major HIV coreceptor (25–27), was similarly expressed on these four subsets. Expression of CCR5 and CXCR4 on CCR4+CCR6+ and CXCR3+CCR6+ T cells was associated with permissiveness of these cells to R5 and X4 HIV replication in vitro, whereas CXCR4 but not CCR5 expression on CCR4+CCR6− T cells was in line with their permissiveness to X4 but not R5 HIV strains. These results are consistent with some but not all of the previous studies performed on Th2 clones (42, 53). Of particular interest, we identified CXCR3+CCR6− T cells as relatively resistant to R5 and X4 HIV replication in vitro despite expression of both HIV coreceptors. A report identified a CCR5+CCR7−CD45RO− CD45RA+ T cell subset resistant to R5 but not to X4 HIV replication due to a postentry restriction mechanism (54). CCR5+ CCR7−CD45RO−CD45RA+ T cells are phenotypically distinct from CXCR3+CCR6− T cells, and to our best knowledge, our work is the first characterization of a primary CCR5+CXCR4+ T cell subset resistant to both R5 and X4 HIV infection. Infection by R5 HIV strains is a critical step in primary infection (44, 48, 55), whereas X4 strains emerge later during disease progression (1). Thus, CCR4+CCR6− T cells may contribute to the emergence of X4 HIV strains, whereas CCR4+CCR6+ and CXCR3+CCR6+ T cells may be major players during HIV infection in vivo because they are permissive to both R5 and X4 HIV strains.
The CCR6-CCL20 axis is essential for the maintenance of mucosal homeostasis (40, 56) and contributes to HIV/SIV path-ogenesis (16, 28, 40, 45, 56–58). CCR6 might also mediate migration of the CCR4+CCR6+ and CXCR3+CCR6+ T cells into different peripheral tissues expressing CCL20, including the gut and vaginal mucosa, which are major sites for HIV replication in vivo (43, 59). In addition to expressing CCR6, we found that CCR4+CCR6+ and CXCR3+CCR6+ T cells selectively produce the CCR6 ligand CCL20, previously identified as a Th17 marker (39). Furthermore, we showed that CXCR3+CCR6+, CCR4+ CCR6+, and CXCR3+CCR6− T cells expressed high to moderate levels of the α4β7 integrin, which in addition to mediating gut-trafficking (60) binds R5 HIV gp120 (61). The expression of CCR5 and α4β7 integrin on CXCR3+CCR6+ T cells, and to a lesser extent on CCR4+CCR6+ T cells, may render these cells extremely prone to R5 HIV binding and subsequent replication. Thus, CCR4+CCR6+ and CXCR3+CCR6+ T cells, by their permissiveness to HIV infection and ability to produce CCL20, have the potential to recruit more CCR6+ T cells at anatomic sites of HIV replication (Fig. 8).
We demonstrated that CCR6+ T cells compared with CCR6− T cells harbored higher levels of integrated HIV DNA in HIV-infected subjects. In addition, we found that the frequency of CCR6+ T cells was significantly reduced in HIV-infected subjects, RI w/o ART and CI on ART subjects compared with uninfected individuals. This is consistent with a previous report that CCR6+ T cells disappeared from the peripheral blood of HIV-infected patients as HIV disease progressed and underwent apoptosis in the spleen (15). The observation that CCL20 levels in the gut mucosa diminish significantly upon SIV infection (28, 58) suggests that CCR6+ T cells may not infiltrate these sites via CCR6-CCL20 interaction in HIV-infected subjects. However, we cannot exclude the possibility that CCR6+ T cells infiltrate in other tissues via CCL20-independent mechanisms. The finding that total CCR6+ T cells but not CCR4+CCR6+ and CXCR3+CCR6+ T cell subsets were decreased in frequency in RI w/o ART patients compared with controls highlights the possibility that other CCR6+ T cell subsets (i.e., the yet uncharacterized CCR4− CXCR3− CCR6+ or CCR4+CXCR3+CCR6+ T cell subsets) are subject to depletion during HIV primary infection. However, a decreased frequency of circulating CCR4+CCR6+ and CXCR3+CCR6+ T cells is observed in CI subjects as reflected by our transversal and a longitudinal study. This may explain deficient immunity against M. tuberculosis (62) and C. albicans during HIV infection (63), because these T cell subsets are specific for these two pathogens (14). Also, both RI and CI HIV-positive subjects express significantly lower CCR4+CCR6+ (Th17 profile) versus CXCR3+CCR6− (Th1 profile) and CXCR3+CCR6+ (Th1Th17 profile) versus CXCR3+ CCR6− (Th1 profile) ratios when compared with controls, thus supporting earlier findings that CD4+ T cells in HIV-infected patients are skewed toward a Th1 phenotype to the detriment of Th17 cells (5, 28). A skewed Th17/Th1 response may lead to microbial translocation, which is a cause of chronic immune activation in HIV-infected patients (43, 59, 64).
The frequency of circulating CCR4+CCR6− T cells was rapidly and notably decreased in HIV-infected subjects despite the fact that these cells were only permissive to X4 HIV strains that emerge later during disease progression (65). This phenomenon might be explained by either a bystander killing of CD4+ T cells in HIV-infected subjects (1, 29, 30) or a redistribution of these cells in peripheral tissues. Finally, we found an increased frequency of CXCR3+ CCR6− T cells in HIV-infected subjects compared with controls; this is consistent with their resistance to R5 and X4 HIV replication in vitro. Whether CXCR3+CCR6− T cells play a beneficial or a deleterious role in HIV pathogenesis remains unknown. Overall, our findings reveal a profound alteration of CD4+ T cell heterogeneity in HIV-infected subjects despite viral-suppressive ART that might impact on the quality of T cell responses against HIV.
Successful HIV replication requires a large number of host genes (66), with cytokines and chemokines regulating the state of T cell susceptibility to HIV infection. Proinflammatory cytokines such as TNF-α trigger nuclear translocation of NF-κB, which is critical for the initiation of HIV LTR transcription (38, 67). In contrast, the immunosuppressive cytokine IL-10 negatively regulates HIV replication by interfering with CCR5 and CXCR4 expression and cell responsiveness to TCR triggering (33–36). The present study demonstrated that IFN-γ, IL-10, TNF-α, IL-2, CCL3, and CCL5 were mainly produced by CXCR3+CCR6+ and CXCR3+CCR6− T cells, whereas CCR4+CCR6+ and CCR4+CCR6− T cells were sources of IL-17 and IL-5, respectively (Supplemental Fig. 5). Of particular interest, CXCR3+CCR6+ T cells were identified in this study as a major source of TNF-α, with a TNF-α/IL-10 ratio significantly higher compared with that of CXCR3+CCR6− T cells. Thus, permissiveness to HIV replication in CXCR3+ CCR6+ versus CXCR3+CCR6− T cells might be controlled at least in part by the TNF-α/IL-10 balance. Other studies described a synergy between TNF-α and IL-10 (68), but its relevance for HIV replication in vivo remains unknown. Originally considered a Th2 cytokine, IL-10 is produced by a large number of cells, including Tregs (69) and Th1 and Th17 cells (31, 32, 69). The CXCR3+CCR6+ and CXCR3+CCR6− T cells lacked the expression of FoxP3 (14, 70), a transcription factor specific for Tregs. Therefore CXCR3+CCR6+ and CXCR3+CCR6− T cells resemble recently described Th1/IL-10 cells (31, 32, 69).
The CCR5 binding chemokines prevent R5 HIV gp120 binding on CCR5, therefore limiting subsequent HIV entry and replication in T cells (25, 41). Although both CXCR3+CCR6+ and CXCR3+ CCR6− T cells produce CCR5 ligands, the existence of a preferential block in CCR5-mediated HIV entry (41) in CXCR3+CCR6− T cells cannot be excluded and needs to be investigated at the single-cell level. Of particular interest, CXCR3+CCR6+ and CXCR3+CCR6− T cells produced similar levels of IL-2 and exhibited a similar ability to proliferate, thus excluding the possibility that limited HIV replication in CXCR3+CCR6− T cells is related to deficient TCR engagement.
In summary, by using CCR4, CXCR3, and CCR6 as surface markers, we identified four primary CD4+ T cell subsets as either permissive or resistant to HIV infection (Fig. 8) and revealed a profound and previously unrecognized alteration in CD4+ T cell heterogeneity in HIV-infected subjects despite undetectable viral loads and preserved CD4 cell counts under viral-suppressive ART. The CCR4+CCR6+ and CXCR3+CCR6+ T cells have the potential to be recruited into the gut and vaginal mucosa as well as the brain via a CCR6-CCL20–dependent mechanism (16, 45, 71) and thus might significantly contribute to HIV dissemination and persistence by attracting other CCR6+ T cells at sites of viral replication in vivo. Our results open the path for future studies on the identification of new molecular determinants of HIV restriction in CXCR3+CCR6− T cells and may suggest new therapeutic strategies aimed at HIV eradication by interfering with HIV replication in CCR4+CCR6+ and CXCR3+CCR6+ T cells.
Supplementary Material
Acknowledgments
We thank Sylvain Gimmig and Laurence Lejeune (Flow Cytometry Core Facility, Research Centre, Centre Hospitalier de l’Université de Montréal, Saint-Luc Hospital, Montréal, Quebec, Canada) for expert technical support with polychromatic flow cytometry analysis and sorting, Anne Vassal for help with ethical approvals and informed consents, Mario Le-gault, Maryse Lainesse, and Véronique Lafontaine for the management of HIV-infected patient cohorts, and Dr. Aiken and Dr. Gabuzda for their gift of HIV plasmids. We also thank Dr. Gabuzda, Dr. Hocini, Dr. Shoukry, Dr. Landry, Heather Wilson, and Heather Yampolsky for critical reading of the manuscript and valuable discussions. Finally, we acknowledge HIV-in-fected and uninfected donors for their gift of leukapheresis.
This work was supported in part by grants to P.A. from the Canadian Institutes of Health Research (CIHR) (MOP-82849), Fondation du Centre Hospitalier de l’Université de Montréal, Fonds de la Recherche en Santé Québec (FRSQ), the French National Institute of Health and Medical Research (INSERM), National Agency for AIDS Research (ANRS), Agence Nationale de Recherche sur le SIDA, and Fondation de France. P.A. was supported by New Investigator awards from FRSQ and INSERM. P.M., N.C., E.A.S., and M.E.F were supported by postdoctoral fellowships from ANRS, Fondation de France, FRSQ, The Foundation for AIDS Research, and CIHR, respectively. Core facilities were supported by the Fondation du Centre Hospitalier de l’Université de Montreal and the FRSQ AIDS-Infectious Diseases Network.
Abbreviations used in this paper
- ART
antiretroviral therapy
- CI on ART
chronically infected under long-term antiretroviral therapy
- LTR
long terminal repeat
- NA
not available
- RI w/o ART
recently HIV-infected treatment-naïve
- T-bet
T-box expressed in T cells
- Treg
regulatory T cell
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 2.Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 3.Geeraert L, Kraus G, Pomerantz RJ. Hide-and-seek: the challenge of viral persistence in HIV-1 infection. Annu. Rev. Med. 2008;59:487–501. doi: 10.1146/annurev.med.59.062806.123001. [DOI] [PubMed] [Google Scholar]
- 4.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 5.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 2000;12:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 10.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2001;2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 11.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 12.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies Pre-T helper (Th)1, Pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 2004;200:725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schröder JM, Wang JM, Howard OM, Oppenheim JJ. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 15.Lécureuil C, Combadiére B, Mazoyer E, Bonduelle O, Samri A, Autran B, Debré P, Combadiére C. Trapping and apoptosis of novel subsets of memory T lymphocytes expressing CCR6 in the spleen of HIV-infected patients. Blood. 2007;109:3649–3657. doi: 10.1182/blood-2006-01-035717. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulassel MR, Spurll G, Rouleau D, Tremblay C, Edwardes M, Sekaly RP, Lalonde R, Routy JP. Changes in immunological and virological parameters in HIV-1 infected subjects following leukapheresis. J. Clin. Apher. 2003;18:55–60. doi: 10.1002/jca.10051. [DOI] [PubMed] [Google Scholar]
- 18.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ancuta P, Kunstman KJ, Autissier P, Zaman T, Stone D, Wolinsky SM, Gabuzda D. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology. 2006;344:267–276. doi: 10.1016/j.virol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Ancuta P, Liu KY, Misra V, Wacleche VS, Gosselin A, Zhou X, Gabuzda D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16- monocyte subsets. BMC Genomics. 2009;10:403. doi: 10.1186/1471-2164-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 22.Ancuta P, Autissier P, Wurcel A, Zaman T, Stone D, Gabuzda D. CD16+ monocyte-derived macrophages activate resting T cells for HIV infection by producing CCR3 and CCR4 ligands. J. Immunol. 2006;176:5760–5771. doi: 10.4049/jimmunol.176.10.5760. [DOI] [PubMed] [Google Scholar]
- 23.Brussel A, Sonigo P. Analysis of early human immunodeficiency virus type 1 DNA synthesis by use of a new sensitive assay for quantifying integrated provirus. J. Virol. 2003;77:10119–10124. doi: 10.1128/JVI.77.18.10119-10124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 25.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1a, MIP-1b receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 26.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 27.Berger EA, Doms RW, Fenyö EM, Korber BT, Littman DR, Moore JP, Sattentau QJ, Schuitemaker H, Sodroski J, Weiss RA. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 28.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieillard V, Habib RE, Brochard P, Delache B, Bovendo HF, Calvo J, Morin J, Picq I, Martinon F, Vaslin B, et al. CCR5 or CXCR4 use influences the relationship between CD4 cell depletion, NKp44L expression and NK cytotoxicity in SHIV-infected macaques. AIDS. 2008;22:185–192. doi: 10.1097/QAD.0b013e3282f35551. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 31.Häringer B, Lozza L, Steckel B, Geginat J. Identification and characterization of IL-10/IFN-γ-producing effector-like T cells with regulatory function in human blood. J. Exp. Med. 2009;206:1009–1017. doi: 10.1084/jem.20082238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J. Exp. Med. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patterson BK, Czerniewski M, Andersson J, Sullivan Y, Su F, Jiyamapa D, Burki Z, Landay A. Regulation of CCR5 and CXCR4 expression by type 1 and type 2 cytokines: CCR5 expression is downregulated by IL-10 in CD4-positive lymphocytes. Clin. Immunol. 1999;91:254–262. doi: 10.1006/clim.1999.4713. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Angel JB, Daftarian MP, Parato K, Cameron WD, Filion L, Diaz-Mitoma F. Differential production of IL-10 by T cells and monocytes of HIV-infected individuals: association of IL-10 production with CD28-mediated immune responsiveness. Clin. Exp. Immunol. 1998;114:78–86. doi: 10.1046/j.1365-2249.1998.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jinquan T, Quan S, Jacobi HH, Madsen HO, Glue C, Skov PS, Malling HJ, Poulsen LK. CXC chemokine receptor 4 expression and stromal cell-derived factor-1α-induced chemotaxis in CD4+ T lymphocytes are regulated by interleukin-4 and interleukin-10. Immunology. 2000;99:402–410. doi: 10.1046/j.1365-2567.2000.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bento CA, Hygino J, Andrade RM, Saramago CS, Silva RG, Silva AA, Linhares UC, Brindeiro R, Tanuri A, Rosenzwajg M, et al. IL-10-secreting T cells from HIV-infected pregnant women downregulate HIV-1 replication: effect enhanced by antiretroviral treatment. AIDS. 2009;23:9–18. doi: 10.1097/QAD.0b013e328317461e. [DOI] [PubMed] [Google Scholar]
- 37.Weissman D, Poli G, Fauci AS. Interleukin 10 blocks HIV replication in macrophages by inhibiting the autocrine loop of tumor necrosis factor a and interleukin 6 induction of virus. AIDS Res. Hum. Retroviruses. 1994;10:1199–1206. doi: 10.1089/aid.1994.10.1199. [DOI] [PubMed] [Google Scholar]
- 38.Herbein G, Khan KA. Is HIV infection a TNF receptor signalling-driven disease? Trends Immunol. 2008;29:61–67. doi: 10.1016/j.it.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 40.Williams IR. CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann. N. Y. Acad. Sci. 2006;1072:52–61. doi: 10.1196/annals.1326.036. [DOI] [PubMed] [Google Scholar]
- 41.Guan Y, Abdelwahab S, Kamin-Lewis R, DeVico AL, Lewis GK. Self-protection of individual CD4+ T cells against R5 HIV-1 infection by the synthesis of anti-viral CCR5 ligands. PLoS One. 2008;3:e3481. doi: 10.1371/journal.pone.0003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moonis M, Lee B, Bailer RT, Luo Q, Montaner LJ. CCR5 and CXCR4 expression correlated with X4 and R5 HIV-1 infection yet not sustained replication in Th1 and Th2 cells. AIDS. 2001;15:1941–1949. doi: 10.1097/00002030-200110190-00005. [DOI] [PubMed] [Google Scholar]
- 43.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lackner AA, Veazey RS. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu. Rev. Med. 2007;58:461–476. doi: 10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veazey RS, Lackner AA. Getting to the guts of HIV pathogenesis. J. Exp. Med. 2004;200:697–700. doi: 10.1084/jem.20041464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sallusto F, Mackay CR. Chemoattractants and their receptors in homeostasis and inflammation. Curr. Opin. Immunol. 2004;16:724–731. doi: 10.1016/j.coi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 50.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 52.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat. Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 53.Vicenzi E, Panina-Bodignon P, Vallanti G, Di Lucia P, Poli G. Restricted replication of primary HIV-1 isolates using both CCR5 and CXCR4 in Th2 but not in Th1 CD4+ T cells. J. Leukoc. Biol. 2002;72:913–920. [PubMed] [Google Scholar]
- 54.Oswald-Richter K, Grill SM, Leelawong M, Tseng M, Kalams SA, Hulgan T, Haas DW, Unutmaz D. Identification of a CCR5-expressing T cell subset that is resistant to R5-tropic HIV infection. PLoS Pathog. 2007;3:e58. doi: 10.1371/journal.ppat.0030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westphal S, Lu¨gering A, von Wedel J, von Eiff C, Maaser C, Spahn T, Heusipp G, Schmidt MA, Herbst H, Williams IR, et al. Resistance of chemokine receptor 6-deficient mice to Yersinia enterocolitica infection: evidence of defective M-cell formation in vivo. Am. J. Pathol. 2008;172:671–680. doi: 10.2353/ajpath.2008.070393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-κB-dependent signaling pathway. J. Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 58.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 60.Mora JR, von Andrian UH. Retinoic acid: an educational “vitamin elixir” for gut-seeking T cells. Immunity. 2004;21:458–460. doi: 10.1016/j.immuni.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 62.Geldmacher C, Schuetz A, Ngwenyama N, Casazza JP, Sanga E, Saathoff E, Boehme C, Geis S, Maboko L, Singh M, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J. Infect. Dis. 2008;198:1590–1598. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Repentigny L, Lewandowski D, Jolicoeur P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin. Microbiol. Rev. 2004;17:729–759. doi: 10.1128/CMR.17.4.729-759.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 65.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goff SP. Knockdown screens to knockout HIV-1. Cell. 2008;135:417–420. doi: 10.1016/j.cell.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poli G, Fauci AS. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res. Hum. Retroviruses. 1992;8:191–197. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- 68.Rabbi MF, Finnegan A, Al-Harthi L, Song S, Roebuck KA. Interleukin-10 enhances tumor necrosis factor-α activation of HIV-1 transcription in latently infected T cells. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998;19:321–331. doi: 10.1097/00042560-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 69.Jankovic D, Trinchieri G. IL-10 or not IL-10: that is the question. Nat. Immunol. 2007;8:1281–1283. doi: 10.1038/ni1207-1281. [DOI] [PubMed] [Google Scholar]
- 70.Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr. Opin. Immunol. 2009;21:146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.