Abstract
Immunotherapeutic approaches to the treatment of advanced melanoma have relied on strategies that augment the responsiveness of endogenous tumor-specific T cell populations (e.g., CTLA-4 blockade-mediated checkpoint inhibition) or introduce exogenously-prepared tumor-specific T cell populations (e.g., adoptive cell transfer). Although both approaches have shown considerable promise, response rates to these therapies remain suboptimal. We hypothesized that a combinatorial approach to immunotherapy using both CTLA-4 blockade and non-lymphodepletional adoptive cell transfer could offer additive therapeutic benefit. C57BL/6 mice were inoculated with syngeneic B16F10 melanoma tumors transfected to express low levels of the lymphocytic choriomeningitis virus peptide GP33 (B16GP33), and treated with no immunotherapy, CTLA-4 blockade, adoptive cell transfer, or combination immunotherapy of CTLA-4 blockade with adoptive cell transfer. Combination immunotherapy resulted in optimal control of B16GP33 melanoma tumors. Combination immunotherapy promoted a stronger local immune response reflected by enhanced tumor-infiltrating lymphocyte populations, as well as a stronger systemic immune responses reflected by more potent tumor antigen-specific T cell activity in splenocytes. In addition, whereas both CTLA-4 blockade and combination immunotherapy were able to promote long-term immunity against B16GP33 tumors, only combination immunotherapy was capable of promoting immunity against parental B16F10 tumors as well. Our findings suggest that a combinatorial approach using CTLA-4 blockade with non-lymphodepletional adoptive cell transfer may promote additive endogenous and exogenous T cell activities that enable greater therapeutic efficacy in the treatment of melanoma.
Keywords: immunotherapy, CTLA-4, adoptive cell transfer, T cell, melanoma, cancer
Introduction
The potential immunogenicity of melanoma has motivated great interest in immune-based therapies for patients with advanced forms of disease. Indeed, recent investigational efforts have begun to realize some of the enormous potential of melanoma immunotherapy. One approach has been to exogenously engineer populations of melanoma-specific T cells intended to induce immunological regression of established tumors. Experimental strategies of adoptive cell transfer (ACT) utilize melanoma-specific CD8+ cytotoxic T lymphocytes (CTL) harvested from tumor-infiltrating lymphocytes (TIL); CTL are expanded and activated ex vivo, then infused into patients following aggressive lymphodepletion. Clinical trials of ACT have documented profound and durable treatment responses in patients who have been refractory to more traditional modalities of therapy (1-4). Another approach has been to augment endogenous melanoma-specific immune responses by blocking specific immunological checkpoints that typically downregulate T cell responsiveness. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is an inhibitory receptor expressed on activated T cells that, when engaged, functions to inhibit excessive T cell activation. Recently, enhancement of endogenous T cell function through CTLA-4 blockade has been shown to prolong survival for patients with advanced, metastatic melanoma (5,6).
Although both of these strategies have proven capable of unprecedented benefits, both are hampered by potential immunological risks (3-8). Perhaps more significantly, although treatment successes can be dramatic, the overall efficacies of both remain suboptimal, with a majority of treated patients having no demonstrable response to treatment (1-5). In this study, we examined the potential immunological interaction that could take place between CTLA-4 blockade and ACT strategies. Specifically, we used a murine model of melanoma ACT previously established in our laboratory (9) to test whether CTLA-4 blockade could augment the efficacy of non-lymphodepletion ACT, and to determine if any observed augmentation was due to the potentiation of exogenously-derived populations of adoptively transferred melanoma-specific CTLs, endogenous melanoma-specific T cell responses, or both.
Methods
Mice
Seven-to eight-week-old female Ly5.2+/C57BL/6 and Ly5.1+/B6.SJL mice were purchased from Taconic (Hudson, NY) and maintained in pathogen-free conditions. All animal work was performed in strict accordance with the guidelines of the University of Wisconsin and William S. Middleton Memorial VA Hospital Animal Care and Use Committees.
Tumor cell lines and virus
B16F10, a poorly immunogenic melanoma cell line derived from C57BL/6 mice, was maintained in RPMI-1640 medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum, 100U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine (Life Technologies, Inc., Grand Island, NY). The B16GP33 cell line was prepared as previously described (10,11). Briefly, B16F10 cells were transfected with a plasmid containing genes for the class I MHC-restricted LCMV surface glycoprotein GP33 and G418 resistance, and the resulting stably transfected cell line was selected by G418 resistance. B16GP33 clones expressing very low levels of GP33 and resulting in poorly immunogenic in vivo tumor growth were selected as previously described (10). Single inocula of 106 B16F10 or B16GP33 cells suspended in serum-free RPMI1640 media were injected subcutaneously into C57BL/6 mice. Mice were infected with 2×105 PFU of the Armstrong strain of LCMV by intraperitoneal injection.
CTLA-4 blockade and adoptive cell transfer
CTLA-4 blockade was performed by treating tumor-bearing mice with intraperitoneal injections of 200 μg anti-CTLA-4 mAb (or isotype control mAb) (R&D Systems, Minneapolis, MN) on days 2, 5, and 8 after B16GP33 tumor inoculation. Adoptive cell transfer as performed as previously described (9). Briefly, splenocytes were harvested from Ly5.1+ B6.SJL mice 8 days after LCMV infection, then enriched for CD8 expression using magnetic bead separation columns (Miltenyi, Auburn, CA). Flow cytometry was used to quantify CD8+ GP33-specific T cell populations. Tumor-bearing mice were treated with intravenous injections of 104 CD8+GP33-specific T cells (or serum-free media) on day 1 after B16GP33 tumor inoculation.
Flow cytometry
Flow cytometric analysis of tumor-infiltrating lymphocytes was performed on day 9 or 14 after B16GP33 tumor inoculation. Single cell suspensions of tumor-infiltrating lymphocytes were prepared by homogenizing explanted melanoma tumors and isolating lymphocytes over a Ficoll-Histopaque gradient (Sigma-Aldrich, St. Louis, MO). Lymphocytes were stained with APC-labeled MHC class I (Db) tetramers loaded with GP33, PErCp-labeled anti-CD8, PE-labeled anti-Ly5.1, and FITC-labeled anti-CD44 antibodies. Flow cytometric analysis of splenocytes was performed on day 9 or 14 after B16GP33 tumor inoculation using methods previously described (9-12). Briefly, freshly harvested splenocytes (106 cells/well) were stimulated with (or, as a negative control, without) GP33 at a concentration of 0.1 μg/mL in the presence of brefeldin A and human recombinant IL-2 (10 U/well) at 37°C for 5 hours in flat-bottomed 96-well plates. Cells were stained with FITC-labeled anti-CD8 antibody, then permeabilized and stained with APC-labeled anti-IFNγ antibody using the Cytofix/Cytoperm kit (BD Biosciences-Pharmingen, San Diego, CA). Stained cells were acquired on a FACSCalibur flow cytometer (BD Biosciences-Pharmingen, San Diego, CA) and resulting data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). Unless otherwise specified, all reagents and antibodies were purchased from BD Biosciences-Pharmingen.
Statistical analysis
Experimental data were analyzed using SAS statistical software version 9.2 (Cary, NC). Groups were compared using a repeated measures analysis of variance (ANOVA) with pair-wise comparisons performed using Fisher’s protected least significant difference tests. All data were log-transformed prior to analysis in order to better meet the assumptions of ANOVA. All p-values reported are two-sided, and significance was defined as p<0.05. All error bars in graphical representations of data indicate standard errors of the mean.
Results
Combination of CTLA-4 blockade and ACT promotes optimal control of melanoma tumors
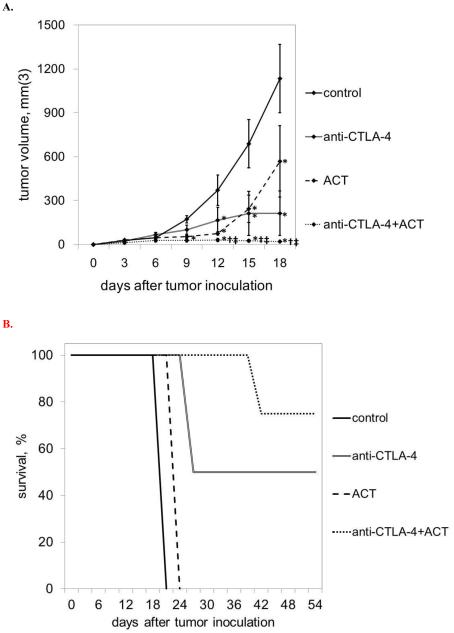
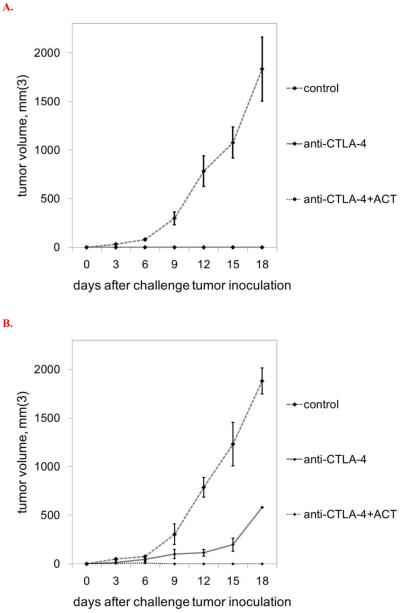
In order to compare the efficacy of CTLA-4 blockade monotherapy, ACT monotherapy, and combination immunotherapy, immunocompetent Ly5.2+/C57BL/6 mice were inoculated with flank subcutaneous injections of 106 B16GP33 melanoma tumors on day 0, and four treatment groups were compared. Control mice received no therapy. Mice in the CTLA-4 blockade therapy group received intraperitoneal injections of 200 μg anti-CTLA-4 mAb on days 2, 5, and 8. Mice in the ACT group received intravenous injections of 5×104 CD8+/GP33-specific CTLs (derived from Ly5.1+/C57BL/6 mice 8 days after LCMV infection) on day 1. Mice in the combination therapy group received both treatment regimens. Tumor measurements recorded at three day intervals are shown in Figure 2. Different patterns of tumor growth were observed in the four groups, tumor control was strongest in mice receiving combination CTLA-4 blockade plus ACT. Exponential tumor growth was observed in all control group mice. Among mice treated with CTLA-4 blockade only, initial non-exponential tumor growth was observed for the first 12-15 days, followed by durable growth arrest. Tumor growth was severely inhibited in mice treated with ACT for the first 12 days, after which exponential tumor growth was eventually seen. Very minimal tumor growth was observed in the combination therapy group, with most tumors exhibiting complete and durable regression.
Figure 2. Combination immunotherapy promotes optimal control of melanoma tumors.
A) Exponential B16GP33 tumor growth was observed in control mice. CTLA-4 blockade resulted in no initial inhibition of B16GP33 tumor growth followed by durable arrest of tumors beginning on day 12 after tumor inoculation. ACT resulted in early inhibition of B16GP33 tumor growth followed by exponential tumor growth beginning on day 12. Combination immunotherapy resulted in durable and optimal inhibition of B16GP33 tumor growth. (B) Kaplan-Meier survival curves of mice in the four treatment groups indicate that whereas control mice and mice treated with ACT alone met criteria for euthanasia by day 18, half of mice treated with CTLA-4 and the majority of mice treated with CTLA-4 and ACT experienced long-term survival for up to 8 weeks. This experiment (n=4 mice per group) was performed four times with similar results. (* p<0.05 compared with control group; † p<0.05 compared with CTLA-4 blockade group; ‡ p < 0.05 compared with ACT group.)
Combination immunotherapy promotes optimal tumor infiltration of CD8+ T cells
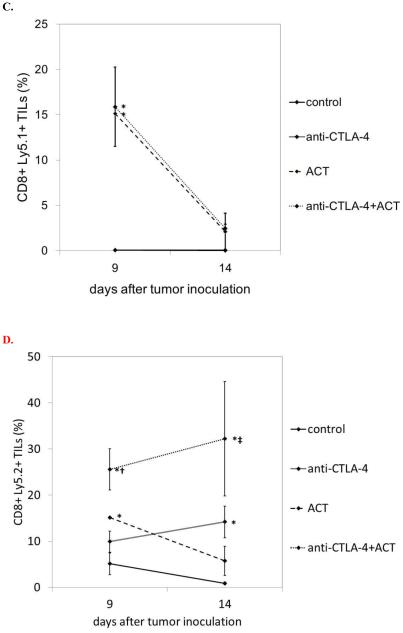
Intratumoral infiltration of CD8+ T cells has been shown to correlate with immunotherapeutic efficacy. In order to compare the induction of tumor-infiltrating lymphocyte (TIL) populations in the various treatment groups, mice were sacrificed at two time points (days 9 and 14) for TIL analysis (Figure 3A,B). An inverse correlation was observed between CD8+ TIL and tumor volume. A small population of CD8+ T cells was seen in control mice at day 9, and this regressed significantly by day 14. In contrast, CD8+ T cells persisted in stable and slightly increased numbers from day 9 to 14 in response to CTLA-4 blockade. Following ACT, a very large influx of CD8+ T cells was seen on day 9; on day 14, by which time tumor growth was exponential, the presence of CD8+ T cells was minimal. Importantly, the combination of CTLA-4 blockade and ACT resulted in largely additive numbers of CD8+ T cells on day 9, and large numbers of infiltrating CD8+ T cells persisted on day 14.
Figure 3. Combination immunotherapy promotes optimal tumor infiltration of CD8+ T cells.
Tumor infiltrating lymphocytes populations were evaluated by flow cytometry on days 9 and 14 after B16GP33 tumor inoculation. When total CD8+ T cells were evaluated (representative individual data shown in A, group data shown in B), control mice showed an initial small infiltration on day 9 that largely dissipated by day 14. CTLA-4 blockade resulted in an early infiltration on day 9 that persisted through day 14. ACT resulted in a strong early infiltration on day 9 that largely dissipated by day 14. Combination immunotherapy resulted in a strong early infiltration on day 9 that was stronger than that seen in control mice and after CTLA-4 blockade; this infiltration persisted on day 14 to levels that were stronger than those seen in control, CTLA-4 blockade, and ACT mice. (C) When only Ly5.1+/CD8+ T cells (derived from Ly5.1+ donor mice during ACT) were evaluated, no infiltration was seen in mice that did not received ACT (control and CTLA-4 blockade). In mice that did receive ACT, a brisk infiltration of exogenously-derived Ly5.1+/CD8+ T cells seen on day 9 was largely lost by day 14. No differences in the levels of exogenously-derived Ly5.1+/CD8+ T cells were observed between mice treated with CTLA-4 blockade alone and combination immunotherapy. (D) When only Ly5.2+/CD8+ T cells (derived from the tumor-bearing mouse and not from the adoptively transferred cells) were evaluated, ACT resulted in a slight increase in intratumoral T cell infiltration on day 9 compared with controls, but this effect was short-lived. In contrast, CTLA-4 blockade resulted in a gradual increase in endogenously-derived T cell infiltration, and the magnitude of this infiltration was largely additive in mice treated with combination immunotherapy. This experiment (n=4 mice per group) was performed three times with similar results. (* p<0.05 compared with control group; † p<0.05 compared with CTLA-4 blockade group; ‡ p < 0.05 compared with ACT group.)
GP33-specific CD8+ T cells were derived from Ly5.1+/C57BL/6 mice; adoptive transfer of these cells into Ly5.2+/C57BL6 mice permitted flow cytometric segregation of exogenous (Ly5.1+ donor-derived) and endogenous (Ly5.2+ tumor-bearing mouse-derived) CD8+ T cells (Figure 3C,D). As expected, when CD8+ TILs were examined for Ly5.1+ vs. Ly5.2+ expression, no exogenous CD8+ TILs were observed in control and CTLA-4 blockade mice that did not receive adoptive transfer. The addition of CTLA-4 blockade did not augment the infiltration of exogenous Ly5.1+/CD8+ T cells; indeed, the increased number of CD8+ T cells within tumors was due to enhanced infiltration of endogenous Ly5.2+/CD8+ T cells. In addition, very few Ly5.1+/CD8+ T cells were present on day 14; the vast majority of CD8+ TILs at that point were endogenous Ly5.2+/CD8+ T cells. Adoptive transfer appeared to promote early infiltration of endogenous Ly5.2+/CD8+ T cells into tumor, but this effect was relatively short-lived; in contrast, CTLA-4 blockade resulted in early intratumoral infiltration of endogenous Ly5.2+/CD8+ T cells that persisted on day 14.
Combination immunotherapy promotes systemic T cell responsiveness to melanoma antigen
Splenocytes were harvested on days 9 and 14 and stimulated in vitro with GP33 peptide (or no peptide for negative controls) for 5 hours in the presence of IL-2 and brefeldin A. GP33-responsive CD8+ T cells were identified by flow cytometry based on intracellular expression of IFNγ (Figure 4). In contrast to control mice, whose splenocytes exhibited no CD8+ T cell responsiveness to GP33 stimulation, mice treated with CTLA-4 blockade or ACT alone exhibited small populations of GP33-responsive T cells. However, the combination of CTLA-4 blockade and ACT resulted in largely additive numbers of GP33-specific CD8+ T cells in the spleen.
Figure 4. Combination immunotherapy promotes systemic T cell responsiveness to melanoma antigen.
Splenocytes were evaluated by flow cytometry on day 14 after B16GP33 tumor inoculation. Splenocytes were stimulated for 5 hours in vitro with the LCMV peptide GP33 (or no peptide) in the presence of IL-2 and brefeldin A, after which intracellular expression of IFNγ was measured. Although levels of GP33-induced IFNγ+ CD8+ T cells were higher after CTLA-4 blockade and ACT as compared with control mice, these levels were not significantly higher than background (no peptide stimulation) in these groups. In contrast, significantly higher levels of GP33-induced IFNγ expression were observed after combination immunotherapy, and these levels were significantly above background. (Representative individual data shown in A, group data shown in B.) This experiment was also performed on day 9 after B16GP33 tumor inoculation with similar results (data not shown). This experiment (n=3-4 mice per group) was performed two times with similar results. (* p<0.05 compared with control group; † p<0.05 compared with CTLA-4 blockade group; ‡ p < 0.05 compared with ACT group; x p<0.05 compared with background control.)
Combination immunotherapy improves the balance of CD8+ effector : CD4+ regulatory TILs
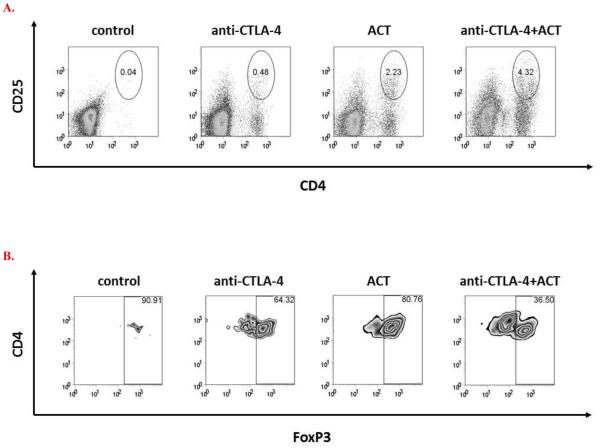
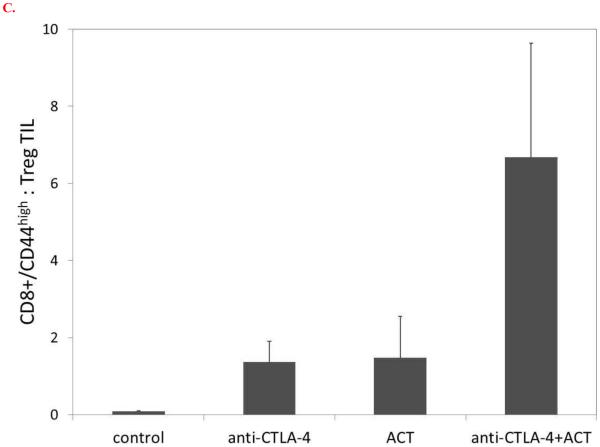
Tumors explanted on day 14 were analyzed for infiltration of CD4+CD25+FoxP3+ regulatory T cells (Treg) (Figure 5). In comparison to control mice, treatment with CTLA-4 blockade or ACT alone resulted in a relative influx of Treg. In contrast, relatively smaller populations of infiltrating Treg were observed in mice treated with the combination of CTLA-4 blockade and ACT. When analyzed as a ratio of CD8+/CD44high effector : Treg, the infiltration of activated CD8+ T cells in mice receiving CTLA-4 or ACT monotherapy was largely offset by the infiltration of Treg; in contrast, the ratio of CD8+/CD44high effector : Treg appeared to be optimal in mice receiving combination immunotherapy.
Figure 5. Combination immunotherapy improves the balance of CD8+ effector : CD4+ regulatory TILs.
Tumor infiltrating lymphocytes were evaluated by flow cytometry on day 14 after B16GP33 tumor inoculation for levels of CD8+ and CD4+ T cells. CTLA-4 blockade and ACT resulted in the induction of higher levels of CD4+CD25+FoxP3+ T cells compared with control mice. (Representative individual data of CD4+CD25+ T cells shown in A, representative individual data of intracellular FoxP3 expression gated on CD4+CD25+ T cell populations shown in B.) (C) When represented as ratios of activated CD8+/CD44high effector T cells to CD4+ regulatory T cells, a trend toward a favorable tumor infiltrating T cell profile was observed after combination immunotherapy (p=0.078 vs. control). This experiment (n=3-4 mice per group) was performed two times with similar results.
Combination immunotherapy promotes optimal immunity against recurrent melanoma tumors
As outlined in Figure 2B, some mice treated with CTLA-4 blockade and the combination of CTLA-4 blockade and ACT exhibited minimal to no tumors for up to 8 weeks. Selected mice in both treatment groups were challenged with contralateral flank injections of both B16GP33 tumors and parental B16F10 tumors (identical to B16GP33 with the absence of GP33 peptide expression) > 30 days after completion of all therapy. Mice treated with both CTLA-4 blockade and combination immunotherapy remained immune to B16GP33 as evidenced by the absence of B16GP33 challenge tumor growth (Figure 6A). In contrast, although mice treated with CTLA-4 blockade alone demonstrated delayed growth of B16F10 tumors as compared with naïve mice that had not received any previous immunotherapy, no growth of parental B16F10 tumors was observed in mice treated with combination immunotherapy (Figure 6B).
Figure 6. Combination immunotherapy promotes optimal immunity against recurrent melanoma tumors.
Some of the mice treated with CTLA-4 blockade exhibited persistent B16GP33 tumors that showed no progression, and all of the mice treated with combination immunotherapy exhibited minimal to no visible B16GP33 tumors with no progression. (A) When these selected mice were challenged with contralateral injections of B16GP33 tumors > 30 days after initial B16GP33 inoculation, no tumor growth was observed in mice that had initially been treated with CTLA-4 blockade or combination immunotherapy, indicating successful induction of immunity to B16GP33. As a reference, B16GP33 tumor growth in naïve mice that had not received any previous tumor injections or immunotherapy is also shown. (B) When these selected mice were challenged with contralateral injections of B16F10 tumors (identical to B16GP3 tumors with the exception of absent GP33 expression), only mice that had initially been treated with combination immunotherapy exhibited immunity to B16F10. As a reference, B16F10 tumor growth in naïve mice that had not received any previous tumor injections or immunotherapy is also shown. This experiment (n=3 mice per group) was performed two times with similar results. († p<0.05 compared with CTLA-4 blockade group.)
Discussion
Efforts to use melanoma-reactive T cells to treat melanoma have focused on approaches that either employ exogenously-prepared cells or mobilize endogenous populations of cells. ACT strategies that seek to introduce exogenously-prepared melanoma-specific CD8+ T cells from TIL have been associated with response rates as high as 50%, and are occasionally capable of inducing remarkably durable remission (1-3). Moreover, ACT strategies allow for the use of ex vivo cell stimulation protocols to maximize the function of cancer-specific T cells (13-15). However, successful ACT protocols rely on aggressive lymphoablation of endogenous immune cells to maximize in vivo persistence of adoptively transferred cells, and this often leads to significant treatment-related morbidity (3,4). CTLA-4 blockade has been proven to improve survival for patients with metastatic melanoma, and is also capable of promoting durable arrest of tumor growth (5,6). However, CTLA-4 blockade-mediated enhancement of endogenous immune cells can also promote untoward immune hyperresponsiveness resulting in dangerous manifestations of autoimmunity (7,8). In this study, we examined the interaction between CTLA-4 blockade and non-lymphodepleting ACT to determine if a combinatorial approach to immunotherapy would be of potential benefit.
Previous studies have suggested the potential benefit of a combinatorial approach to melanoma immunotherapy using CTLA-4 blockade. Li and co-authors used a murine model of prostate tumor antigen vaccine therapy to demonstrate that CTLA-4 blockade can work synergistically with vaccine-based immunotherapy to inhibit tumor growth and promote long-term protection from challenge tumors (16). Watanabe and colleagues recently used a murine model of ACT using lymphocytes stimulated in vitro with irradiated methylcholanthrene A-induced sarcoma cells in the presence of anti-CTLA-4 mAb and anti-OX40 to show that the ability of ACT to inhibit sarcoma growth in vivo was enhanced by pre-transfer, in vitro antibody-mediated depletion of CD25 and OX40 plus CTLA-4 blockade (17). This analysis suggested that the combination of all three antibodies was needed to have therapeutic effect. However, because antibody-mediated depletion and blockade was only administered to the adoptively transferred cells, this study did not evaluate the interaction between adoptively transferred and endogenous lymphocytes. Shin and colleagues genetically modified B16F10 melanoma-specific Pmel-1 cells to express a CTLA-4-CD28 chimera that eliminated CTLA-4-mediated negative signaling (18). When these transduced Pmel-1 CD8+ T cells were adoptively transferred into melanoma-bearing mice following lymphodepletion, a modest improvement in immunotherapeutic efficacy was seen compared with control, non-transduced CD8+ T cells. Similarly, Berrien-Elliott and colleagues recently used a non-lymphodepletion murine model to show that a combination of anti-CTLA-4, anti-PDL-1, and anti-LAG3 checkpoint inhibition blockade was necessary to improve the survival and differentiation of adoptively transferred T cells (19). These findings suggest that alteration of CTLA-4-mediated signaling may enhance the efficacy of adoptively transferred melanoma-specific T cells. However, the mechanism by which this salutary influence is exerted remains unclear. In addition, the design of these studies did not expose endogenous T cells to CTLA-4 blockade.
There have been preliminary human studies indicating the potential promise of CTLA-4 blockade-based combinatorial immunotherapy. A phase I clinical trial combining dendritic cell vaccines loaded with the melanoma antigen MART-1 with the anti-CTLA-4 mAb tremelimumab for 16 patients with metastatic melanoma identified two partial responses and two complete responses (20). Although it is difficult to ascertain the degree to which these results represent measurable improvements beyond that which would be expected with CTLA-4 blockade alone, it is striking to note that, when used in conjunction with operative metastasectomy, all four patients with objective responses were without evidence of disease for 28 to 59 months at last follow-up. A recent clinical trial of 57 patients treated with ACT of unselected TIL stimulated with high-dose IL-2 following lymphodepleting conditioning included 32 patients who had also received ipilimumab either before or after ACT (21). Subset analysis of this group of patients identified no obvious additive benefit associated with receipt of both ACT and CTLA-4 blockade. It is interesting to speculate that the absence of a measurably cooperative interaction may be due to the use of lymphodepletion of endogenous T cell populations. However, patients only received ACT after ipilimumab if they had not responded to ipilimumab, and only received ipilimumab after ACT if they failed ACT, raising the possibility that these patients may have been unresponsive to one of the therapies.
Using our simplified model of ACT, we observed that the combination of CTLA-4 blockade and ACT resulted in significantly better control of melanoma tumor growth than could be achieved with either monotherapy alone. In the absence of pre-ACT lymphodepletion, the salutary effect of CTLA-4 blockade in the combinatorial approach to immunotherapy was not from enhanced survival or expansion of adoptively transferred exogenous CD8+ T cells, but from augmented infiltration of endogenous CD8+ T cells. Indeed, the TIL profile resulting from combination immunotherapy appeared to be an additive result of the same early but transient trafficking of exogenously-derived melanoma-specific T cells seen after ACT monotherapy, plus the influx and persistence of endogenous CD8+ T cells seen after CTLA-4 monotherapy. This additive effect may have been reflected in the kinetics of tumor growth observed in the various treatment groups. Whereas ACT resulted in an initial resistance to tumor growth that was eventually overcome, CTLA-4 blockade initially resulted in a relatively moderate inhibition of tumor growth followed by durable arrest of late tumor growth. This additive effect was seen not only locally within the tumor, but systemically as well, reflected by the ability of splenocytes to respond to melanoma antigen with IFNγ production.
In addition to these additive benefits, combination immunotherapy appeared to induce immunological changes that were qualitatively different from those seen after monotherapy. Combination immunotherapy appeared to overcome the influx of Treg seen within tumors following monotherapy, possibly creating a qualitatively favorable milieu for infiltrating effector CTLs. The ratio of activated CD8: Treg infiltration was somewhat higher after combinatorial immunotherapy with ACT and CTLA-4 blockade, although this difference was not statistically significant (p=0.078). This may have been due to the paradoxical upregulation of intratumoral Treg populations we observed in response to CTLA-4 blockade. Interestingly, this pattern is concordant with recent observations that CTLA-4 blockade not only promotes Treg infiltration, but that the presence of intratumoral Treg may actually be prognostically favorable in melanoma patients treated with ipilimumab (22,23).
The favorable profile of intratumoral and peripheral T cell responses observed after combination immunotherapy was not only associated with optimal control of tumor growth, but with optimal tumor immunity as well. Whereas both CTLA-4 blockade and combination immunotherapy were capable of promoting durable immunity to the original B16GP33 melanoma, combination immunotherapy also resulted in greater immune protection against the parental B16F10 melanoma. This observation suggests that the addition of ACT strengthened the potenticy of antigenically-broad immune responses directed not only at the GP33 antigen, but at other melanoma antigens as well.
The potential benefit of combining CTLA-4 blockade with ACT may be complementary rather than synergistic. We did not identify any potentiation of the survival or tumoral infiltration of adoptively transferred melanoma-specific T cells resulting from CTLA-4 blockade. Rather, the salutary impact of CTLA-4 blockade was largely confined to endogenous lymphocyte populations, whose ability to traffic into tumors was augmented. Thus, whereas ACT induces an initial, transient tumor-specific T cell response, CTLA-4 blockade engenders a more gradual but durable tumor-specific T cell response.
Our study has important limitations. We employed a simplified model of ACT previously developed in our laboratory to compare the therapeutic efficacy of tumor-specific CD8+ T cells in various levels of differentiation. Although this model uses an artificial tumor model, we employed a B16GP33 melanoma cell line that expresses very low levels of GP33 peptide, resulting in a poorly immunogenic tumor with growth kinetics that are largely indistinguishable from those of parental B16F10 tumors (10). Moreover, we used a dose of adoptively transferred tumor-specific CD8+ T cells that we have previously found to result in suboptimal control of B16GP33 tumor growth (data not shown), maximizing our opportunity to observe an interaction with CTLA-4 blockade immunotherapy. In addition, we limited our investigation to a model that involved early administration of immunotherapy (ACT on day 1 and CTLA-4 blockade beginning on day 2). Ongoing work in our laboratory will determine if the therapeutic cooperation we observed between ACT and checkpoint blockade in this very preclinical model will also be observed in a model of TIL-based ACT that will more rigorously approximate ACT strategies that have been used for patients with advanced melanoma.
In conclusion, we suggest that the combination of checkpoint inhibition with CTLA-4 blockade plus ACT may represent a paradigm of cancer immunotherapy deserving of further preclinical evaluation. Our findings indicate that the combination of an early infiltration of exogenously-derived, adoptively transferred T cells (via ACT) with a late infiltration of endogenously-derived T cells (via CTLA-4 blockade) promotes the induction of optimal tumor control, systemic anti-tumor T cell responsiveness, and immunity.
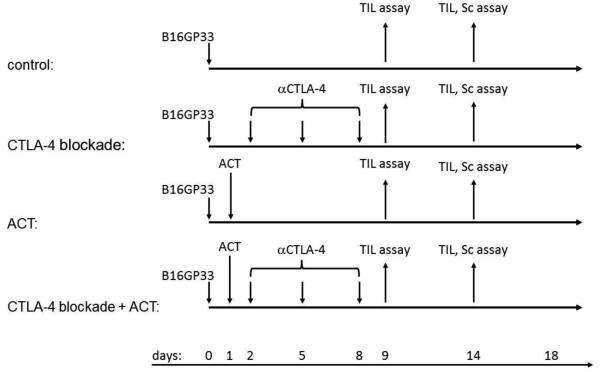
Figure 1. Experimental schema.
Ly5.2+/C57BL/6 mice were inoculated subcutaneously with 106 B16GP33 melanoma cells on day 0. Mice received no immunotherapy (control), CTLA-4 blockade, adoptive cell transfer (ACT), or combination immunotherapy of CTLA-4 blockade plus ACT. CTLA-4 blockade was administered by three intraperitoneal injections of 200 mg anti-CTLA-4 mAb on days 2, 5, and 8. GP33-specific T cells were harvested from Ly5.1+/B6.SJL mice 8 days after LCMV injection. ACT was administered by one intravenous injection of 104 Ly5.1+/CD8+/GP33-specific T cells on day 1. On days 9 or 14 after B16GP33 tumor inoculation, mice were euthanized for TIL and/or splenocyte analysis. Tumors were measured every three days.
Acknowledgements
This work was supported by grant support from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Science Research and Development Service, Merit Review Award (1I01BX001619-01A1), Career Development Award (CDA-2), American College of Surgeons Faculty Research Fellowship, and Central Surgical Association Foundation Grant to CSC, and by support from NIH Grant AI48785 to MS.
Footnotes
Disclosure/disclaimer of potential conflicts of interest
There are no potential conflicts of interest; the contents of this work do not represent the view of the Department of Veterans Affairs on the United States Government.
References
- 1.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, Yang JC, Yolles P, Larson SM, Rosenberg SA. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989;7:250–261. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanan JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg F, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012:2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675–1682. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 8.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18:733–743. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wentworth L, Meyers JV, Alam S, et al. Memory T cells are uniquely resistant to melanoma-induced suppression. Cancer Immunol Immunother. 2013;62(1):149–159. doi: 10.1007/s00262-012-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russ AJ, Wentworth L, Xu K, et al. Suppression of T-cell expansion by melanoma is exerted on resting cells. Ann Surg Oncol. 2011;18(13):3848–3857. doi: 10.1245/s10434-011-1667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russ AJ, Xu K, Wentworth L, et al. Melanoma-induced suppression of tumor antigen-specific T cell expansion is comparable to suppression of global T cell expansion. Cell Immunol. 2011;271(1):104–109. doi: 10.1016/j.cellimm.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 13.Parviz M, Chin CS, Graham LJ, et al. Successful adoptive immunotherapy with vaccine-sensitized T cells, despite no effect with vaccination alone in a weakly immunogenic tumor model. Cancer Immunol Immunother. 2003;52:739–750. doi: 10.1007/s00262-003-0405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le HK, Graham L, Miller CH, et al. Incubation of antigen-sensitized T lymphocytes activated with bryostatin 1 + ionomycin in IL-7 + IL-15 increases yield of cells capable of inducing regression of melanoma metastases compared to culture in IL-2. Cancer Immunol Immunother. 2009;58:1565–1576. doi: 10.1007/s00262-009-0666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller CH, Graham L, Bear HD. Phenotype, functions and fate of adoptively transferred tumor-draining lymphocytes activated ex vivo in mice with an aggressive weakly immunogenic mammary carcinoma. BMC Immunol. 2010;11:54. doi: 10.1186/1471-2172-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Qin H, Li X, et al. Synergistic antitumor effect of chemotactic-prostate tumor-associated antigen gene-modified tumor cell vaccine and anti-CTLA-4 mAb in murine tumor model. Immunol Lett. 2007;113:90–98. doi: 10.1016/j.imlet.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe A, Hara M, Chosa E, et al. Combination of adoptive cell transfer and antibody injection can eradicate established tumors in mice – an in vivo study using anti-OX40 mAb, anti-CD25 mAb and anti-CTLA4 mAb. Immunopharm Immunotoxicol. 2010;32(2):238–245. doi: 10.3109/08923970903222355. [DOI] [PubMed] [Google Scholar]
- 18.Shin JH, Park HB, Oh YM, et al. Positive conversion of negative signaling of CTLA-4 potentiates antitumor efficacy of adoptive T-cell therapy in murine tumor models. Blood. 2012;119(24):5678–5687. doi: 10.1182/blood-2011-09-380519. [DOI] [PubMed] [Google Scholar]
- 19.Berrien-Elliott MM, Jackson SR, Meyer JM, et al. Durable adoptive immunotherapy for leukemia produced by manipulation of multiple regulatory pathways of CD8+ T-cell tolerance. Cancer Res. 2012;73(2):605–616. doi: 10.1158/0008-5472.CAN-12-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribas A, Comin-Anduix B, Chmielowski B, et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15:6267–6276. doi: 10.1158/1078-0432.CCR-09-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besser MJ, Shapira-Frommer R, Itzhaki O, et al. Adoptive transfer of tumor infiltrating lymphocytes in metastatic melanoma patients: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19:4792–4800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 22.Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarhini AA, Edington H, Butterfield LH, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One. 2014;9:e87705. doi: 10.1371/journal.pone.0087705. [DOI] [PMC free article] [PubMed] [Google Scholar]